Abstract
Background: Several studies reported an increased cardiovascular (CV) risk in Cushing’s syndrome (CS). We performed a meta-analysis on the impact of CS on major markers of atherosclerosis.
Methods: Studies on intima-media thickness (IMT), carotid plaques prevalence, and flow-mediated dilation (FMD) in CS patients and controls were searched in the PubMed, Web of Science, Scopus, and EMBASE. Differences between cases and controls were expressed as mean difference (MD) with 95% confidence intervals (95%CI) for continuous variables, and as Odds Ratio (OR) with 95%CI for dichotomous variables.
Results: Fourteen studies (332 CS, 462 controls) were included. Compared with controls, CS patients showed higher IMT (MD: 0.20 mm; 95% CI: 0.12, 0.28; p < .001), increased prevalence of carotid plaques (OR: 8.85, 95%CI: 4.09, 19.14; p < .001), and lower FMD (MD: −2.65%; 95% CI: −3.65, −1.65; p < .001). Difference in IMT and in the prevalence of carotid plaques was confirmed also in patients with CS remission (MD: 0.24 mm; 95% CI: 0.07, 0.40; p = .005 and OR: 9.88, 95%CI: 2.69, 36.3; p < 0.001, respectively). Regression models showed that age, diabetes, obesity, ACTH-dependent CS, serum and urinary cortisol levels impacted on the observed difference in IMT.
Conclusions: CS is significantly associated with markers of subclinical atherosclerosis and CV risk. These findings could help establish more specific CV prevention strategies in this clinical setting.
A series of studies reported an increased cardiovascular risk in patients with Cushing’s syndrome (CS).
In the present meta-analysis we demonstrated that CS is associated with an increased intima-media thickness, higher prevalence of carotid plaques, and lower flow-mediated dilation as compared with controls.
These data consistently suggest the need for a strict monitoring of early signs of subclinical atherosclerosis in CS patients
Key messages
Introduction
Cushing’s syndrome (CS) results from chronic exposure to an excess of cortisol. Endogenous CS can be due to adrenocorticotropic hormone (ACTH)-dependent (about 80%) and ACTH-independent (about 20%) causes. Among ACTH-dependent forms, pituitary corticotroph adenoma is most common, out numbering extrapituitary ACTH-secreting tumors or tumors that secrete corticotropin-releasing hormone (CRH) (i.e., neuroendocrine tumors, medullary thyroid carcinoma, and phaeochromocytoma). ACTH-independent forms are mainly caused by primary unilateral adrenal adenomas or carcinomas. Rarely, CS is caused by primary bilateral macronodular adrenal hyperplasia or primary pigmented nodular adrenocortical disease and its non-pigmented variant, isolated micronodular adrenocortical disease (Citation1).
It has been reported that cortisol excess is associated with increased cardiovascular (CV) risk and adverse CV events. Indeed, CS either due to endogenous or exogenous glucocorticoid excess has been associated with an increased CV risk due to direct and indirect effects of these hormones. In particular, the chronic cortisol hypersecretion is associated with central obesity, hypertension, insulin resistance and/or impairment of glucose tolerance, hyperlipidemia, and prothrombotic state (Citation2). All these abnormalities are well-recognized CV risk factors (Citation2,Citation3). As a consequence, patients with CS showed a higher rate of CV complications (myocardial infarction, congestive heart failure, stroke) with a four-fold higher CV mortality rate than the age- and gender-matched normal subjects (Citation2,Citation3).
Carotid intima-media thickness (IMT) assessment is a non-invasive imaging test for subclinical atherosclerosis (Citation4), and it has been widely accepted as one of the strongest predictors of major CV events (Citation5). Similarly, flow-mediated dilation (FMD) and nitrate-mediated dilation (NMD), widely accepted as accurate and non-invasive methods to assess endothelial function in humans (Citation6), are considered surrogate markers of subclinical atherosclerosis and independent predictors of CV events (Citation7). Thus, these CV risk markers provide important prognostic data over and above traditional CV risk factors. During recent years, a series of studies reported accelerated atherosclerosis (Citation8,Citation9) and impaired endothelial function (Citation10) in patients with CS. However, this evidence has been challenged by other studies (Citation11,Citation12) and no meta-analytical data providing an overall information about this issue are currently available. In order to provide a comprehensive overview of the relationship between CS and subclinical atherosclerosis, we performed a systematic review with meta-analysis of literature studies evaluating the impact of CS on major markers of CV risk.
Materials and methods
A protocol for this review was prospectively developed, detailing the specific objectives, the criteria for study selection, the approach to assess study quality, the outcomes, and the statistical methods.
Search strategy
To identify all available studies, a detailed search pertaining to CS and the markers of CV risk (i.e., IMT, carotid plaques, FMD, NMD) was conducted according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Citation13). A systematic search was performed in the electronic databases (PubMed, Web of Science, Scopus, EMBASE), using the following search terms in all possible combinations: Cushing disease, CS, hypercortisolism, IMT, carotid plaques, atherosclerosis, atherosclerotic, vascular, CV, FMD, endothelium-dependent dilation, NMD, endothelium-independent dilation, endothelial function, and endothelial dysfunction. The last search was performed on 7 September 2016. The search strategy was developed without any language or publication year restriction.
In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, study authors were contacted by e-mail to try to retrieve original data. Two independent authors (RL and PA) analyzed each article and performed the data extraction independently. In case of disagreement, a third investigator was consulted (MNDDM). Discrepancies were resolved by consensus. Selection results showed a high inter-reader agreement (κ = 0.98) and have been reported according to the PRISMA flowchart (Supplementary Figure S1).
Data extraction and quality assessment
According to the pre-specified protocol, all studies evaluating the impact of CS on the markers of CV risk were included. Case-reports, case-series without a control group, reviews, and animal studies were excluded. To be included in the analysis, a study had to provide values (mean and standard deviation) of at least one variable among common carotid artery IMT (CCA-IMT), prevalence of carotid plaques, or brachial artery FMD and NMD. In each study, data regarding sample size, major clinical and demographic variables, values of IMT, FMD, NMD, and the prevalence of carotid plaques in CS patients and controls were extracted. Both studies on active CS and after CS remission were included.
Given the characteristics of the included studies, the evaluation of methodological quality of each study was performed with the Newcastle–Ottowa Scale (NOS), which is specifically developed to assess quality of non-randomized observational studies (Citation14). The scoring system encompasses three major domains (selection, comparability, exposure) and a resulting score range between 0 and 8, a higher score representing a better methodological quality. Results of the NOS quality assessment are reported in Supplementary Table S1.
Statistical analysis and risk of bias assessment
Statistical analysis was carried out using Review Manager [Version 5.2, The Cochrane Collaboration, Copenhagen, Denmark] provided by The Cochrane Collaboration. Differences among cases and controls were expressed as mean difference (MD) with pertinent 95% confidence intervals (95%CI) for continuous variables, and as Odds Ratio (OR) with pertinent 95%CI for dichotomous variables. IMT has been expressed in millimeters (mm), FMD and NMD as percentage (%), and the prevalence of carotid plaques as row number.
The overall effect was tested using Z scores and significance was set at p < .05. Statistical heterogeneity between studies was assessed with chi square Cochran’s Q test and with I2 statistic, which measures the inconsistency across study results and describes the proportion of total variation in study estimates, that is due to heterogeneity rather than sampling error. In detail, I2 values of 0% indicate no heterogeneity, 25% low, 25–50% moderate, and 50% high heterogeneity (Citation15). Publication bias was assessed by the Egger’s test and represented graphically by funnel plots of the standard difference in means versus the standard error. Visual inspection of funnel plot asymmetry was performed to address for possible small-study effect, and Egger’s test was used to assess publication bias, over and above any subjective evaluation. A p < .10 was considered statistically significant (Citation16). In case of a significant publication bias, the Duval and Tweedie’s trim and fill method with the random-effect model was used to allow for the estimation of an adjusted effect size (Citation17). In order to be as conservative as possible, the random-effect method was used for all analyses to take into account the variability among included studies.
Sensitivity analyses
We repeated analyses by including only the studies judged as “high quality” according to NOS (i.e., NOS ≥ to the median value found among included studies). A further sensitivity analysis has been performed after excluding studies enrolling patients with subclinical CS. In addition, a separate analysis was performed only including studies enrolling BMI-matched controls.
Meta regression analyses
We hypothesized that differences among included studies may be affected by demographic variables (mean age, male gender), clinical data (ACTH-dependence, serum and urinary cortisol levels), and the coexistence of traditional CV risk factors (hypertension, smoking habit, diabetes mellitus, obesity, hyperlipidemia, HOMA index). To assess the possible effect of such variables in explaining different results observed across studies, we planned to perform meta-regression analyses after implementing a regression model with difference in IMT, FMD, and presence of carotid plaques as dependent variables (y) and the above mentioned co-variates as independent variables (x). This analysis was performed with Comprehensive Meta-analysis, Version 2 [Biostat, Englewood NJ (2005)].
Results
After excluding duplicate results, the search retrieved 413 articles. Of these studies, 330 were excluded because they were off the topic after scanning the title and/or the abstract, 57 were excluded because they were reviews/comments/case reports or they lacked data of interest. Twelve studies were excluded after full-length paper evaluation (Supplementary Figure S1). In one of the studies the on-line full-length version was not available, but data could be extracted from the abstract.
Thus, 14 articles (on 332 CS patients and 462 controls) were included in the final analysis (Citation8–12,Citation18–26). In detail, 12 studies (Citation8–12,Citation18,Citation20–23,Citation25,Citation26) (on 257 CS cases and 389 controls) were on patients with active CS, of which 10 (Citation8–11,Citation20–23,Citation25,Citation26) with data on IMT (on 221 CS cases and 350 controls), 5 (Citation8,Citation9,Citation12,Citation20,Citation21) reporting on the prevalence of carotid plaques (on 125 CS cases and 206 controls), and three studies (Citation10,Citation11,Citation18) on FMD (on 65 CS patients and 68 controls). In addition, five studies (Citation12,Citation19,Citation20,Citation22,Citation24) (on 148 CS cases and 160 controls) reported data on CS patients after disease remission (median: 4.2 years, range 1–13.6 years), of which four (Citation19,Citation20,Citation22,Citation24) with data on IMT (on 121 CS cases and 143 controls) and three (Citation12,Citation19,Citation20) reporting on the prevalence of carotid plaques (on 67 CS cases and 79 controls).
Only one study (Citation11) reported data on NMD in patients with active CS, thus, this outcome was not included in the meta-analytical evaluation.
Study characteristics
All included studies had a case–control design. Major characteristics of populations are shown in .
Table 1. Demographic and clinical data of patients with CS and controls in included studies.
The number of CS patients varied from 5 to 58, the mean age from 14.3 to 60 years, and the prevalence of male gender from 0 to 45.5%. The presence of hypertension was reported by 0–90% of patients, smoking habit by 0–16.7%, diabetes mellitus by 0–57%, obesity by 20–40%, and hyperlipidemia by 20.7–71.4%. Mean body mass index (BMI) varied from 23.7 to 32.3 kg/m2. Mean values of total cholesterol (TC) ranged from 5.16 to 6.20 mmol/l, of LDL-cholesterol (LDLc) from 2.84 to 4.35 mmol/l, of HDL-cholesterol (HDLc) from 1.0 to 1.58 mmol/l, and of triglycerides (TGs) from 1.28 to 2.59 mmol/l. The prevalence of patients with ACTH-dependent CS ranged from 0 to 100%.
Active CS
In 10 studies (Citation8–11,Citation20–23,Citation25,Citation26), we found a significantly higher IMT in 221 CS patients than 350 controls (MD: 0.20 mm; 95% CI: 0.12, 0.28; p < .001, , Panel A). The heterogeneity among studies was significant (I2 = 96%; p < .001) and it was not reduced after excluding one study at time. Interestingly, same results were obtained after excluding studies (Citation21,Citation23,Citation26) including patients with subclinical CS (MD: 0.22 mm; 95% CI: 0.08, 0.37; p = .003, I2: 97%, p < .001).
Figure 1. CCA-IMT (Panel A) and prevalence of carotid plaques (Panel B) in patients with active CS and controls.
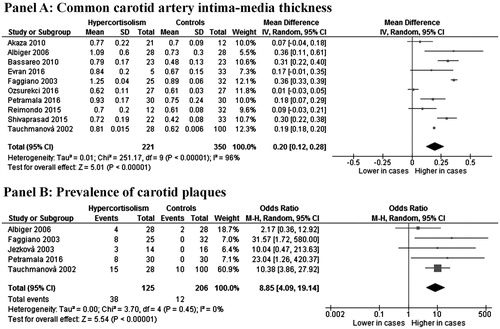
A total of five studies (Citation8,Citation9,Citation12,Citation20,Citation21) showed an increased prevalence of carotid plaques in 125 CS patients as compared with 206 controls (30.4% vs 5.8%), with a corresponding OR of 8.85 (95%CI: 4.09, 19.14; p < .001, , Panel B). No heterogeneity among studies was found (I2 = 0%; p = .45). Interestingly, same results were obtained after excluding the study (Citation21) including patients with subclinical CS (OR: 7.70; 95%CI: 1.93, 30.81; p = .004, I2: 16%, p = .31).
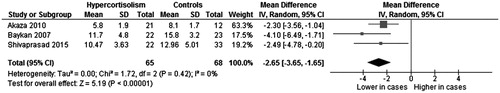
Three studies (Citation10,Citation11,Citation18), evaluating a total of 65 CS cases and 68 controls, showed a significantly lower FMD in CS subjects as compared with controls (MD: −2.65%; 95%CI: −3.65, −1.65; p < .001, ), without heterogeneity among studies (I2 = 0%; p = .42).
CS after remission
Four studies (Citation19,Citation20,Citation22,Citation24), on a total of 121 CS patients after disease remission and 143 controls, showed a significantly higher IMT in cases than in controls (MD: 0.24 mm; 95% CI: 0.07, 0.40; p = .005, , Panel A). Significant heterogeneity among studies was found (I2 = 98%; p < .001).
Figure 3. CCA-IMT (Panel A) and prevalence of carotid plaques (Panel B) in patients with CS after remission and controls.
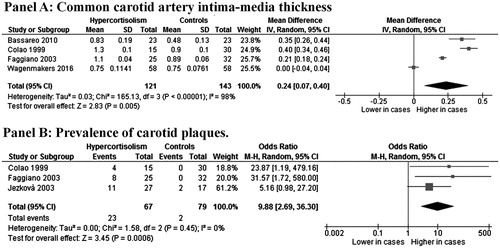
An increased prevalence of carotid plaques was found in three studies (Citation12,Citation19,Citation20) on a total of 67 CS cases and 79 controls (34.3% vs 2.5%), with a corresponding OR of 9.88 (95% CI: 2.69, 36.3; p < .001, , Panel B) and with no heterogeneity among studies (I2 = 0%; p = .45).
Sensitivity analyses
The NOS for quality assessment of included studies showed a median value of 5 (Supplementary Table S1). Thus, 13 of the included studies (Citation8–11,Citation18–26) were considered as “high quality” (NOS ≥5), whereas of one study (Citation12) data of interest were extracted by the abstract, thus no quality assessment could be performed. Of interest, after excluding this study, all results were entirely confirmed (). Similarly, results were confirmed when only including studies (Citation9,Citation19–21,Citation24,Citation25) enrolling BMI-matched controls (Supplementary Table S2).
Table 2. Sensitivity analyses on “high quality” studies (i.e., Newcastle–Ottowa scale ≥5).
Publication bias
Because it is recognized that publication bias can affect the results of meta-analyses, we attempted to assess this potential bias using funnel plots analysis. Funnel plots of effect size versus standard error for studies evaluating IMT, prevalence of carotid plaques, and FMD in patients with active CS were rather symmetrical, suggesting the absence of publication bias and of small-study effect (Supplementary Figure S2), confirmed by the Egger’s test (p = .760; p = .765 and p = .521, respectively).
Similarly, studies on IMT and on the prevalence of carotid plaques in patients with CS after remission were not affected by publication bias (Egger’s p = .547 and p = .259, respectively, Supplementary Figure S3).
Meta-regression analyses
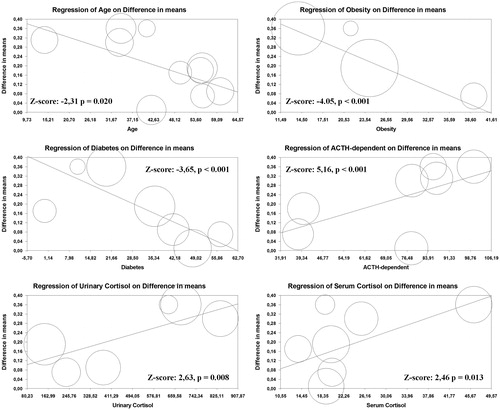
Regression models () showed that an increasing age (Z = −2.31; p = .02), the presence of diabetes (Z = −3.65; p < .001), and obesity (Z = −4.05; p < .001) were inversely associated with the difference in CCA-IMT between CS patients and controls. In contrast, increasing serum cortisol levels (Z-score: 2.46, p = .013), free cortisol urinary levels (Z-score: 2.63, p = .008), and the prevalence of ACTH-dependent CS (Z = 5.16; p < .001) directly impacted on the observed difference in IMT. Interestingly, an inverse association between the time from remission and the difference in IMT between cases and controls was found (Z-score: −2.04, p = .040, Supplementary Figure S4). All the other demographic and clinical data did not impact on the evaluated outcomes.
Discussion
Results of the present meta-analysis consistently show that CS is associated with subclinical atherosclerosis and impaired endothelial function. In particular, we reported an increased IMT, higher prevalence of carotid plaques, and lower FMD in patients with CS as compared with controls. Our findings are strengthened by the sensitivity analysis on high-quality studies. Moreover, regression models were able to further refine results providing the evidence that age, diabetes, obesity but also serum and urinary cortisol levels may significantly impact on the evaluated outcomes. Interestingly, we also demonstrated that the difference in IMT and in the prevalence of carotid plaques was consistently confirmed both in CS patients with active disease and in patients after CS remission.
Our results are in line with some previously published studies reporting an increased risk of major CV events and CV death in CS patients (Citation2,Citation3). Indeed, it is well-recognized that endogenous glucocorticoids excess in CS patients is associated with several CV risk factors, including abdominal obesity, hypertension, diabetes/insulin resistance, and dyslipidemia (Citation2), leading to the development of atherosclerosis and increased prevalence of CV morbidity and mortality (Citation2,Citation3). In particular, vascular events are one of the major causes of death in untreated CS patients (Citation3). In order to provide a comprehensive overview of the relationship between CS and subclinical atherosclerosis, the major recognized markers of CV risk were taken into account in the current meta-analysis. In addition, we performed meta-regression analyses to evaluate whether clinical and demographic variables may impact on the observed results. As expected, regression models showed that results are conditioned by an increasing prevalence of traditional CV risk factors (i.e., obesity, age, and diabetes mellitus) that were associated with a lower difference in IMT between CS patients and controls. This finding is likely due to the pro-atherogenic effect of these CV risk factors that reduce the independent effect of CS on the difference in IMT values. In the attempt of adjusting for potential confunders, we repeated analyses only including studies (Citation9,Citation19–21,Citation24,Citation25) enrolling BMI-matched controls and results were entirely confirmed. In contrast, some other major CV risk factors, such as impaired fasting glucose and dyslypidemia, known to be frequently reported in patients with CS, did not seem to impact on the difference in markers of CV risk between CS patients and controls.
Thus, although CS patients often present central adiposity, high blood pressure, dyslypidemia, and alterations of glycemic omeostasis, the relationship between subclinical atherosclerosis and CS seems to be more complex and the presence of CV risk factors might not entirely explain the accelerated atherosclerotic process in this clinical setting. In fact, in several studies, endothelial function in CS patients resulted more impaired than in age- and gender-matched control subjects with comparable blood pressure, glycemic, and lipid profiles (Citation11,Citation18). Moreover, in our study regression models showed that serum cortisol and free cortisol urinary levels were directly associated with the observed difference in IMT, supporting an independent role for cortisol excess in the pathogenesis and progression of atherosclerosis and, in turn, of the CV risk.
Mechanisms by which glucocorticoids excess could induce endothelial dysfunction in CS are not yet completely understood. Both morphologic and functional alterations of vascular smooth muscle cells may lead to impaired vasoreactivity and predispose to atherosclerosis. Indeed, arterial wall damage was found to be more frequent in CS patients than in patients with essential hypertension (Citation9). Moreover, patients with CS showed impairment of several humoral markers of endothelial dysfunction (such as endothelin-1, homocysteine, vascular endothelial factor, adrenomedulin, and cell adhesion molecules), responsible for vascular endothelial and smooth muscle proliferation as well as fibrosis around vessels (Citation27). In particular, increased levels of endothelin-1, a potent vasoconstrictor with hypertensive, mitogenic, and atherogenic effects, were reported in patients with CS (Citation28). Moreover, vascular endothelial growth factor, a highly specific chemotactic and mitogenic factor for vascular endothelial cells, could be involved in the development and stabilization of atherosclerotic plaques by inducing neoangiogenesis (Citation29). In fact, higher vascular endothelial growth factor levels were found in patients with CS than the patients of essential hypertension (Citation30).
As to endothelial function, several experimental studies have shown that, cortisol could potentiate cardiac angiotensin II and noradrenaline responsiveness or stimulate the local renin–angiotensin system (Citation31). In addition, inhibition of vasodilator systems, including nitric oxide, kinin/kallikrein or prostacyclin and inhibition of peripheral catabolism of catecholamine, in particular of noradrenaline, may affect endothelial function (Citation32). In particular, cortisol excess could exert a direct effect on vascular endothelium through impairment of endothelial nitric oxide production and release. Indeed, it has been shown that glucocorticoids decrease endothelial nitric oxide synthase activity (Citation33), inhibit transmembrane transport of its substrate, arginine (Citation34), and decrease its cofactor, tetrahydrobiopterin (Citation35). Intriguingly, glucocorticoids have been shown to increase oxidative stress in humans by means of hydrogen peroxide and peroxynitrite production (Citation36). Overall, these data suggest that glucocorticoids excess of CS patients may play an important role in the development of endothelial dysfunction and subclinical atherosclerosis. The hypothesis of an independent pro-atherogenic role for cortisol excess is confirmed and extended by results of our study, suggesting that increasing serum cortisol and free cortisol urinary levels were associated with a higher difference in IMT between CS patients and controls. Furthermore, our data showed that an increasing prevalence of ACTH-dependent CS directly impacted on the observed difference in IMT between CS patients and controls suggesting a role of ACTH in determining vascular and endothelial impairment. In animal models, ACTH was shown to determine vascular injury (Citation37) and to enhance experimentally induced atherosclerosis (Citation38). Moreover, it has been hypothesized that ACTH can influence and potentiate differentiation of mesenchymal progenitor cells along with the osteochondrogenic lineages which, in turn, contributes to the progression of the calcified atherosclerotic plaques (Citation39).
An interesting finding is that the difference in IMT and in the prevalence of carotid plaques persists in CS patients after remission. Indeed, Faggiano et al. (Citation20) reported that, 1 year after hypercortisolism remission, the prevalence of the main cardiometabolic risk factors, although reduced compared with the active phase of the disease, was still significantly higher than that observed in the control population. This observation is confirmed also in the case of longer (≥5 years) disease remission (Citation19) and is in line with previous data demonstrating persistence of moderate hypertension after removal of adrenal cortisol-secreting tumors (Citation40). Interestingly, postoperative persistence of hypertension seems to correlate with entity and duration of hypertension during the active phase of hypercortisolism (Citation40). It is likely that patients with longer disease duration and higher cortisol levels may maintain an increased CV risk for a longer time after disease remission (Citation40). Confirming and extending this hypothesis, we documented an inverse association between the median time from remission and the difference in IMT between cases and controls, thus suggesting a progressive reduction in CV risk along with the increase in disease remission duration.
Overall, these data clearly show an increased CV risk in patients with CS and suggest the need for a strict monitoring of CV risk factors and of early signs of subclinical atherosclerosis in this clinical setting. Therefore, early detection of atherosclerosis and endothelial dysfunction in CS patients with measurement of IMT and FMD might be suggested for predicting CV events. The clinical relevance of these results and the need for a strict monitoring of subclinical signs of atherosclerosis in CS patients can be better understood if we consider that the risk of myocardial infarction increases of 43% every 0.163 mm increase in carotid IMT (Citation41,Citation42) while the risk of major CV events rises of about 12% each 1% decrease in FMD (Citation43).
Our results support the need for large long-term interventional trials with CV end-points to investigate whether disease remission obtained with appropriate surgical and/or medical treatments may modify the CV risk in CS patients.
Some potential limitations of our study need to be discussed. First, studies included in our meta-analysis have different inclusion and exclusion criteria and most of patients included in the analysis had concomitant CV risk factors (smoking, obesity, diabetes mellitus, hyperlipidemia). Since meta-analysis is performed on aggregate data and some missing information is present in each study, the multivariate approach allowed for the adjustment for some, but not all, potential confounders. Moreover, some studies specifically included subclinical CS. This might represent a relevant source of heterogeneity since subclinical and overt CS are two distinct clinical settings with different characteristics. However, some literature evidence (Citation44) suggested that even when clinical signs are not present, patients with hypercortisolism have an increased risk of CV events and mortality. Interestingly, further confirming this evidence, we have repeated all analyses after excluding studies on subclinical CS and all results were confirmed.
Thus, although results of meta-regression analyses were able to refine analyses by assessing the influence of most clinical and demographic variables on the observed results, caution is necessary in overall results interpretation. Second, heterogeneity among the studies evaluating the difference in IMT values was generally significant. Although it was not possible to conclusively ascertain sources of heterogeneity, the Egger’s test showed that absence of publication bias and all results were confirmed when analyzing high quality studies. Moreover, at variance with IMT, the measurement of FMD may be influenced by many confounding factors, significantly limiting reproducibility of endothelial dysfunction assessment (Citation45,Citation46) and, in turn, the relevance of results. We have also to consider that differences among assessment techniques and devices, as well as the lack of comparable age-adjusted normal values may limit the validity of these parameters as markers of early atherosclerosis. Thus, caution is necessary in overall results interpretation. Our analysis was specifically designed to evaluate markers of atherosclerosis and of endothelial function in CS patients. Although these are widely recognized as markers of CV risk, results of our study could be strengthen by the evaluation of CV events in this clinical setting. However, this outcome was not reported in included studies and evaluated in only few published studies (Citation5). Thus, further data on this specific topic derived from registries or from large cohort studies are needed to address this issue.
In conclusion, CS is significantly associated with subclinical atherosclerosis and, in turn, with an increased CV risk. Thus, patients with CS may benefit from a periodic assessment of surrogate markers of CV risk and this could help establish more specific CV prevention strategies for these patients.
ONLINE_SUPPLEMENTARY_MATERIAL.docx
Download MS Word (121.6 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015;386:913–27.
- Ferraù F, Korbonits M. Metabolic comorbidities in Cushing's syndrome. Eur J Endocrinol. 2015;173:M133–57.
- Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4:611–29.
- de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33–8.
- Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, Smit A, et al. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (carotid intima media thickness [IMT] and IMT-progression as predictors of vascular events in a high risk European population) study. J Am Coll Cardiol. 2012;60:1489–99.
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65.
- Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–51.
- Petramala L, Lorenzo D, Iannucci G, Concistré A, Zinnamosca L, Marinelli C, et al. Subclinical atherosclerosis in patients with Cushing syndrome: evaluation with carotid intima-media thickness and ankle-brachial index. Endocrinol Metab (Seoul). 2015;30:488–93.
- Albiger N, Testa RM, Almoto B, Ferrari M, Bilora F, Petrobelli F, et al. Patients with Cushing's syndrome have increased intimal media thickness at different vascular levels: comparison with a population matched for similar cardiovascular risk factors. Horm Metab Res. 2006;38:405–10.
- Shivaprasad K, Kumar M, Dutta D, Sinha B, Mondal SA, Maisnam I, et al. Increased soluble TNF receptor-1 and glutathione peroxidase may predict carotid intima media thickness in females with Cushing syndrome. Endocr Pract. 2015;21:286–95.
- Akaza I, Yoshimoto T, Tsuchiya K, Hirata Y. Endothelial dysfunction aassociated with hypercortisolism is reversible in Cushing's syndrome. Endocr J. 2010;57:245–52.
- Jezková J, Marek J, Prázný M, Krsek M, Malíckov´ K, Rosická M, et al. Effect of hypercortisolism on development of atherosclerotic changes in blood vessels. Vnitr Lek. 2003;49:656–67.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2014. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj 2003;327:557–60.
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–05.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
- Baykan M, Erem C, Gedikli O, Hacihasanoglu A, Erdogan T, Kocak M, et al. Impairment of flow-mediated vasodilatation of brachial artery in patients with Cushing's syndrome. Endocrine. 2007;31:300–04.
- Colao A, Pivonello R, Spiezia S, Faggiano A, Ferone D, Filippella M, et al. Persistence of increased cardiovascular risk in patients with Cushing's disease after five years of successful cure. J Clin Endocrinol Metab. 1999;84:2664–72.
- Faggiano A, Pivonello R, Spiezia S, De Martino MC, Filippella M, Di Somma C, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing's disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab. 2003;88:2527–33.
- Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87:4872–78.
- Bassareo PP, Fanos V, Zaffanello M, Mercuro G. Early markers of cardiovascular dysfunction in young girls affected by Cushing's syndrome before and after successful cure. J Pediatr Endocrinol Metab. 2010;23:627–35.
- Evran M, Akkuş G, Berk Bozdoğan İ, Gök M, Deniz A, Sert M, et al. Carotid intima-media thickness as the cardiometabolic risk indicator in patients with nonfunctional adrenal mass and metabolic syndrome screening. Med Sci Monit. 2016;22:991–97.
- Wagenmakers M, Roerink S, Schreuder T, Plantinga TS, Holewijn S, Thijssen D, et al. Vascular health in patients in remission of Cushing's syndrome is comparable to that in BMI-matched controls. J Clin Endocrinol Metab. 2016;jc20161674; Aug 23 [Epub ahead of print].
- Ozsurekci CG, Akturk M, Ozkan C, Gulbahar O, Altinova AE, Yalcin M, et al. Asymmetric dimethylarginine level and atherosclerosis markers in Cushing's syndrome. Endocr Pract. 2016;22:1088–95.
- Reimondo G, Allasino B, Coletta M, Pia A, Peraga G, Zaggia B, et al. Evaluation of midnight salivary cortisol as a predictor factor for common carotid arteries intima media thickness in patients with clinically inapparent adrenal adenomas. Int J Endocrinol. 2015;2015:674734.
- Ermetici F, Malavazos AE, Corbetta S, Eller-Vainicher C, Cannavò S, Corsi MM, et al. Soluble adhesion molecules levels in patients with Cushing's syndrome before and after cure. J Endocrinol Investig. 2008;31:389–92.
- Kirilov G, Tomova A, Dakovska L, Kumanov P, Shinkov A, Alexandrov AS. Elevated plasma endothelin as an additional cardiovascular risk factor in patients with Cushing's syndrome. Eur J Endocrinol. 2003;149:549–53.
- Cucina A, Borrelli V, Randone B, Coluccia P, Sapienza P, Cavallaro A. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J Surg Res. 2003;109:16–23.
- Zacharieva S, Atanassova I, Nachev E, Orbetzova M, Kirilov G, Kalinov K, et al. Markers of vascular function in hypertension due to Cushing's syndrome. Horm Metab Res. 2005;37:36–39.
- Sudhir K, Jennings GL, Esler MD, Korner PI, Blombery PA, Lambert GW, et al. Hydrocortisone-induced hypertension in humans: pressor responsiveness and sympathetic function. Hypertension. 1989;13:416–21.
- Isidori AM, Graziadio C, Paragliola RM, Cozzolino A, Ambrogio AG, Colao A, et al. The hypertension of Cushing's syndrome: controversies in the pathophysiology and focus on cardiovascular complications. J Hypertens. 2015;33:44–60.
- Wallerath T, Witte K, Schafer SC, Schwarz PM, Prellwitz W, Wohlfart P, et al. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc Natl Acad Sci USA. 1999;96:13357–62.
- Simmons WW, Ungureanu-Longrois D, Smith GK, Smith TW, Kelly RA. Glucocorticoids regulate inducible nitric oxide synthase by inhibiting tetrahydrobiopterin synthesis and L-arginine transport. J Biol Chem. 1996; 271:23928–37.
- Mitchell BM, Dorrance AM, Mack EA, Webb RC. Glucocorticoids decrease GTP cyclohydrolase and tetrahydrobiopterin-dependent vasorelaxation through glucocorticoid receptors. J Cardiovasc Pharmacol. 2004;43:8–13.
- Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, et al. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003;92:81–87.
- Stamler J, Pick R, Katz LN. Effects of cortisone, hydrocortisone and corticotropin on lipemia, glycemia and atherogenesis in cholesterol-fed chicks. Circulation. 1954;10:237–46.
- Rosenfeld S, Marmorston J, Sobel H, White AE. Enhancement of experimental atherosclerosis by ACTH in the dog. Proc Soc Exp Biol Med. 1960;103:83–86.
- Evans JF, Ragolia L. Systemic and local ACTH produced during inflammatory states promotes osteochondrogenic mesenchymal cell differentiation contributing to the pathologic progression of calcified atherosclerosis. Med Hypotheses. 2012;79:823–26.
- Suzuki T, Shibata H, Ando T, Kurihara I, Kobayashi S, Hayashi K, et al. Risk factors associated with persistent postoperative hypertension in Cushing's syndrome. Endocr Res. 2000;26:791–95.
- van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam study. Circulation. 2004;109:1089–94.
- Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, Di Minno MN. Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb Haemost. 2015;113:916–30.
- Shimbo D, Grahame-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di Tullio M, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007;192:197–203.
- Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing's syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2:396–405.
- Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, et al. Clinical assessment of endothelial function in patients with rheumatoid arthritis: a meta-analysis of literature studies. Eur J Intern Med. 2015;26:835–42.
- Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, et al. Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med. 2015;47:346–53.