Abstract
Introduction: The burden of stroke is increasing globally. Reports on seasonal variations in stroke occurrence are conflicting and long-term data are absent.
Methods: A retrospective cohort study using discharge registry data of all acute stroke admissions in Finland during 2004–2014 for patients ≥18 years age. A total of 97,018 admissions for ischemic stroke (IS) were included, 18,252 admissions for intracerebral hemorrhage (ICH) and 11,271 admissions for subarachnoid hemorrhage (SAH).
Results: The rate of IS admissions increased (p = 0.025) while SAH admission rate decreased (p < 0.0001), and ICH admission rate remained stable during the study period. The lowest seasonal admission rates were detected in summer and the highest in autumn for all stroke subtypes. Seasonal variation of IS was more pronounced in men (p = 0.020), while no sex difference was detected in ICH or SAH. The seasonal patterns of in-hospital mortality and length of stay (LOS) differed markedly by stroke subtype. Diagnoses of hypertension, atrial fibrillation, or diabetes showed no seasonality.
Conclusions: All major stroke subtypes occurred most commonly in autumn and most infrequently in summer. Seasonality of in-hospital mortality and length of hospital stay appears to vary by stroke subtype. The seasonal pattern of ischemic stroke occurrence appears to have changed during the past decades.
All major stroke subtypes (ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage) occurred most frequently in autumn and least frequently in summer.
Seasonal patterns of in-hospital mortality and length of stay differed markedly by stroke subtype.
The seasonal pattern of ischemic stroke occurrence in Finland seems to have changed compared to 1982–1992.
Key messages
Introduction
The global burden of disease is shifting towards ever larger proportion of death and disability being inflicted by non-communicable diseases such as stroke (Citation1). Probably because of improved therapy (Citation2,Citation3), age-standardized stroke mortality has declined (Citation4). However, the absolute number of stroke patients and disability-adjusted life-years lost continue to increase indicating a continuing need to further our understanding of stroke determinants and burden worldwide (Citation4). The classical risk factors of stroke, such as higher age, sex, smoking, and hypertension are well established, but factors that trigger acute stroke have been studied less. Alcohol abuse and infections are the best known stroke triggers (Citation5).
The frequency of clinical infections varies by season (Citation6). Moreover, stroke risk has been inversely associated with temperature (Citation7,Citation8). Most previous studies performed in populations of mostly Caucasian descent have, in general. found that lowest rate of yearly ischemic stroke (IS) occurs in summer but results of the season with the highest incidence are conflicting (Citation9–17). Furthermore, two large studies from the USA and one smaller from the UK found no seasonality in IS occurrence (Citation18–20). Seasonality of intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) have been studied less with conflicting findings in ICH and findings of no seasonality in SAH (Citation12–19). Besides comparing an earlier study performed in Athens with a later one performed in Northern Greece suggesting no change in stroke seasonality in Greece (Citation15,Citation17), there is no information as to whether the seasonal patterns of stroke incidence have changed within populations over time.
Data on the seasonality of stroke in Scandinavia is scarce (Citation12,Citation13). There is no recent data on the seasonal pattern of stroke occurrence in Finland and significant warming has occurred in recent decades (Citation21). Given the inverse correlation between temperature and risk of stroke (Citation7,Citation8) and the importance of this question in the era of global climate change, we took the opportunity to study seasonal trends of stroke occurrence nationwide in Finland during 2004–2014 using national, obligatory healthcare registry data.
Materials and methods
Data collection
The Care Register for Health Care (CRHC), a mandatory database for all public health care hospital discharges in Finland, was searched for all discharges from neurological, medical, surgical, neurosurgical, and intensive care units with ischemic stroke (ICD-10 code I63.XX), intracerebral haemorrhage (I61.XX) or subarachnoid haemorrhage (I60.XX) as the primary diagnosis between 1 January 2004 and 31 December 2014. The search included all five university hospitals and 15 non-university central hospitals on mainland Finland as only these hospitals provide acute stroke care. Hospital transfers were combined as one admission. Only patients ≥18 years of age were included. The background population at risk consisted of 46,648,979 person-years. Young patients were defined as aged 18-64 years on admission and old patients as at least 65 years of age on admission. Charlson Comorbidity Index (CCI) including age was calculated as previously described (Citation22). The study was approved by the National Institute for Health and Welfare of Finland (permissions no: THL/143/5.05.00/2015 and THL/1349/5.05.00/2015).
Statistical methods
Patient characteristics were analyzed with Chi-square or ANOVA tests as appropriate. Monthly trends in number of admissions, length of stay as beginning days (monthly mean), and in-hospital mortality were analyzed using linear regression. Seasons were defined as December, January, and February being winter, March, April, and May being spring, June, July, and August being summer and September, October, and November being autumn. Seasonal and monthly differences in number of daily admissions were analyzed using negative binomial regression with sex, study year, age (> 65 years or not), and sex * age included in the model. Difference in monthly number of days was taken into account by using logarithm of days in corresponding month as an offset parameter. In-hospital mortality was studied with the Cox regression. Linear regression was utilized for studying standardized length of stay (logarithmically transformed due to skewness). These regression models included CCI, sex, usage of thrombolytic treatment (IS), sex * CCI and study year (as strata in Cox modeling). Furthermore, logarithm of the number of IS/ICH/SAH admissions in treating hospital during the study period was used as an offset parameter in regression models to accommodate results for potential inter-hospital differences. When analyzing differences in length of stay, generalized estimating equations (GEE) to accompany repeated admissions from individual subjects were used. Results of the analyses are presented as relative risks (RR) or hazard ratios (HRs) with 95% confidence intervals (CIs) or β coefficients with SE. Statistical significance was inferred at pvalue <0.05. Incidence rates were standardized with European 2013 standard population and direct method. All analyses were conducted using SAS System for Windows, version 9.4 (SAS Institute Inc., Cary, NC).
Results
The study period included 97,018 (46.7% women) IS admissions with men being younger (mean age 69.1 years, SD 12.0) than women (mean age 74.7 years, SD 12.4, p <0.0001). The standardized incidence of IS was 227/100,000 person years. Over two-third (71,992 or 74.2%) of these admissions comprised of patients older than 65 years of age and 51.5% (p < 0.0001) of the patients in this group were women whereas majority of the patients in the younger age group were men (67.0%, p < 0.0001). Altogether 3518 (3.6%) IS patients died while in hospital. Mean CCI score of IS patients was 5.0 (SD 1.4) with no difference between seasons (p= 0.407). During the study period, the rate of IS hospitalizations showed an increasing trend (Supplemenatary Figure 1 and Table 1) whereas the rates of in-hospital mortality and length of stay (LOS) showed a declining trend (Supplementary Figures 2 and 3 and Table 1). Intravenous thrombolysis was administered to 2.65% of all IS patients with an increasing monthly trend from 2004 to 2014 (β = 0.007; SE = 0.002; p = 0.001). There was no seasonal variation in the proportions of IS patients with diagnosed hypertension (27.36%, p = 0.14), atrial fibrillation (17.20%, p = 0.067), or diabetes (8.22%, p = 0.13).
There were 18,252 admissions for ICH (45.7% women) of whom 3353 (18.4%) died while in hospital. The standardized incidence of ICH was 42/100,000 person years. Again, over two-third (68%) were over 65 years of age and 51.1% of these were women (p < 0.0001). Women (mean age 72.9 years, SD 13.1) were older than men (mean age 66.9 years, SD 12.9; p < 0.0001). There was no significant change in monthly ICH admission rates over the study period (Supplementary Figure 1 and Table 1) but in-hospital mortality and LOS decreased (Supplementary Figures 2 and 3 and Table 1). Mean CCI was 4.7 (SD 1.5) with no seasonal variation (p = 0.401). There was no seasonal variation in the proportions of ICH patients with diagnosed hypertension (27.97%, p = 0.97) or atrial fibrillation (7.64%, p = 0.25).
We found 11,271 admissions for SAH (57.0% women) with an in-hospital mortality of 12.6%. The standardized incidence of SAH was 25/100,000 person years. Only slightly over a quarter (27.2%) of the SAH patients were over 65 years of age (65.0% women; p < 0.0001). Women with SAH were older (mean age 57.9 years, SD 14.0) than men (mean age 54.8 years, SD 13.3; p < 0.0001). The admission rate (Supplementary Figure 1) and LOS (Supplementary Figure 2) for SAH showed decreasing trends over the study period but there was no change in trend of in-hospital mortality during study period (Supplementary Figure 3 and Table 1). No seasonal variation was observed in CCI (p = 0.352), with a mean of 3.3 (SD 1.4). There was no seasonal variation in the proportions of SAH patients with diagnosed hypertension (8.16%, p = 0.37) or atrial fibrillation (1.21%, p = 0.10).
Seasonal admission rates
The seasonal rate of IS admissions showed a roughly bimodal distribution with the highest rates observed for autumn and the lowest for summer (). Seasonal variation was more pronounced in men than in women (p = 0.0198), but there was no difference between young vs. older patients. The highest rate of daily admissions was observed for May and the lowest for August (p < 0.0001 for monthly variation) ().
Figure 1. Monthly variation of number of daily stroke admissions for ischemic stroke (IS) (A), intracerebral haemorrhage (ICH) (B), and subarachnoid hemorrhage (SAH) (C).
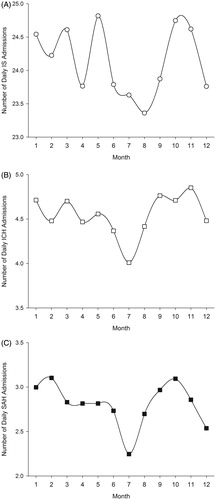
Table 1. Association of season and number of ischemic stroke (IS), intracerebral hemorrage (ICH) and subarachnoidal hemorrage (SAH) admissions.
The highest seasonal rate of ICH admissions was observed in autumn and the lowest in summer with 13.2% higher RR for admission during autumn (; p < 0.0001). There was no difference in seasonal ICH admission rates between sexes or young and old patients. The highest rate of daily ICH admissions was observed for November and the lowest for July (, p < 0.0001 for monthly variation).
Largest seasonal variation in admission frequency between seasons was observed in SAH with 17.5% higher relative risk for admission in autumn vs. summer (). Pattern of seasonal variation in SAH admission was similar to that of ICH. The seasonal admission rates for SAH did not differ between sexes or young vs. old patients. Highest monthly SAH admission rate occurred in October and lowest in July (, p < 0.0001 for monthly variation).
Seasonality of in-hospital mortality rates
Compared to IS admission rates, the seasonal pattern of IS in-hospital mortality showed a different pattern with the highest rate in winter whereas the lowest was observed in autumn (p = 0.010 for seasonal variation) (). All subgroups (men and women, young and old) showed the same in-hospital mortality pattern as the whole group overall. Mortality was higher in winter (HR 1.16; CI 1.05-1.27; p = 0.002) and spring (HR 1.14; CI 1.04-1.25; p = 0.007) than in autumn. Overall, the highest in-hospital mortality rate was observed for February (4.1%) and the lowest for August (3.2%) (, p = 0.072 for monthly variation).
Figure 2. Monthly variation of in-hospital mortality (%) for ischemic stroke (IS) (A), intracerebral hemorrhage (ICH) (B), and subarachnoid hemorrhage (SAH) (C).
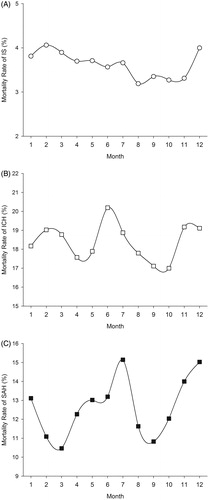
Table 2. Association of season and in-hospital mortality rate of ischemic stroke (IS), intracerebral hemorrage (ICH), and subarachnoidal hemorrage (SAH) admissions.
The pattern of ICH in-hospital mortality rates was opposite to seasonal admission pattern with the highest rate in summer and the lowest in autumn (). The highest monthly mortality rate was observed in June (20.2%) and the lowest in October (17.0%) (, p = 0.064 for monthly variation).
There was no difference in SAH in-hospital mortality between seasons (). Monthly mortality was highest in July (15.1%) and lowest in March (10.5%), (p = 0.3508 for monthly variation, ).
Seasonal variation in length of stay
LOS for IS showed yet a new pattern as the longest mean stays had been recorded for winter and the shortest for summer (). The pattern was similar in all subgroups (women and men, young and old). The longest lengths of stay were observed for February (mean 8.43 days) and the shortest for June (mean 7.48 days) (p < 0.0001 for monthly variation, ).
Figure 3. Monthly variation of length of hospital stay (days) for ischemic stroke (IS) (A), intracerebral hemorrhage (ICH) (B), and subrachnoid hemorrhage (SAH) (C).
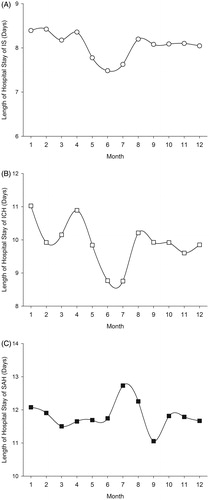
Table 3. Association of season and length of stay for ischemic stroke (IS), intracerebral hemorrage (ICH), and subarachnoidal hemorrage (SAH) admissions.
Among ICH patients, LOS varied significantly on monthly basis (p = 0.0021, ), but no significant seasonal variation was observed (). Longest admissions occurred in January (mean 11.02 days) and the shortest in June (mean 8.77 days). For SAH patients, the pattern of LOS was different than that of IS and ICH, with longest admissions in summer and shortest in autumn (; p = 0.0019 for seasonal variation). The longest monthly admissions for SAH were observed in July (12.73 days) and the shortest in September (11.05 days) (p = 0.0140 for monthly variation, ).
Discussion
This decade-long nationwide study with over 46 million observed person years found the highest rate of daily hospital admissions to occur in autumn and the lowest in summer for all main subtypes of stroke (IS, ICH, and SAH). Seasonal patterns of in-hospital mortality and length of hospital stay, where observed, differed between the subtypes. The sample size was the largest in mainly Caucasian populations to date for ICH and SAH and outside the USA for IS.
Our finding of the lowest seasonal frequency of IS admissions in summer is in line with most previous studies (Citation9,Citation10,Citation12–15). The highest seasonal frequency of IS admissions has been variably attributed to winter (Citation10,Citation12,Citation14), spring (Citation9,Citation15,Citation16), and in keeping with our result, autumn (Citation13). Interestingly, the only other study to find peak incidence rate in autumn was conducted very close to Finland, in neighboring Sweden. Otherwise, no geographic distribution is evident, which corresponds to the previous finding of no association between stroke seasonality and climate region (Citation16). Furthermore, as already stated, some studies from the USA and the UK have reported no seasonality (Citation18–20). This discrepancy may be related to ethnic differences as seasonal patterns of stroke differ between African Americans and Caucasians (Citation16) and Asian populations usually show quite different seasonal stroke patterns compared to Caucasians. One possible contributor to seasonal variation in stroke incidence is the distribution of public holidays and therefore rest and travel around the year. Differences in this distribution might also explain some of the discrepancies between results of studies performed in different populations.
Our results suggest that seasonal differences in IS occurrence may be more pronounced in men, while no sex differences were detected for ICH and SAH. Risk factor profiles are somewhat different between IS, ICH, and SAH and the hazard incurred by diabetes as well as smoking, for instance, on experiencing these types of stroke differ by sex (Citation23–25). Considering that stroke is more common among men, but more severe in women (Citation25) the sex-difference in IS seasonality might indicate more frequent admissions of less severely afflicted men in autumn. Furthermore, the co-occurrence of the highest frequencies of daily admissions and lowest in-hospital mortality rates for IS and ICH in our study suggests that the proportions of less severe cases of these stroke subtypes may increase in autumn in Finland. This would fit the pattern of lacunar strokes being more common in men than in women (Citation26). As we had, however, no specific data on stroke severity, this needs to be studied further.
Previously, 1982–1992 study from Finland reported highest IS incidence in winter compared to our finding of peak in autumn (Citation12). As the previous study was performed in three distinct regions and our study was based on nationwide data, the results may however not be entirely comparable. While our study cannot uncover any reasons for a possible relative decline of IS occurrence in winter, five possible explanations may be suggested. Firstly, the mean annual temperature has risen considerably in Finland (Citation21) with the most considerable increases seen for winters (Citation27). Given the inverse correlation between temperature and stroke incidence (Citation7,Citation8), this may partly explain our result. Second, air quality in Finland is at its worst in winter and spring months but overall has become markedly better in Finland during the last three decades (Citation28). Considering the inverse correlation between air quality and stroke risk (Citation29,Citation30), this could also explain some stroke risk reduction in winter. Third, infections are more prevalent in winter and act as important triggers of stroke (Citation5). Epidemic infections have been reduced by consistently extending the free seasonal influenza vaccination program during the study period (Citation31). Population wide Pneumococcal vaccination of newborn babies introduced as a part of the national vaccination program, and availability of vaccination to older children and adults, have reduced acute pneumococcal infections, most prevalent in the winter, also in older age groups, which may also lead to a decline in the number of acute cardiovascular complications (Citation32–36). Fourth, seasonal variation of stroke incidence may also have been affected by changes concerning social and working factors as, for instance, the cold exposure of workers has reduced from the level of over 40% of the workforce in 1984 to 30% in 2013 (Statistics Finland). Lastly, although the recorded sales of alcohol products in Finland have increased for all seasons when comparing 1982-1992 and 2004-2014, the increase has been most prominent for spring (THL). As alcohol abuse is a well-known trigger of stroke (Citation5), this may also contribute to our results.
While our observation of the seasonal pattern of ICH occurrence was similar to that reported in the previous Finnish study, our result of a seasonal pattern in SAH occurrence differed as the previous study found no seasonality in SAH incidence (Citation12). This may however be attributable to our study’s sample size being the largest ever to investigate SAH seasonality. The similarity of seasonal admission patterns for all stroke subtypes in our study suggests that, despite differences in the weight of risk factors (Citation37–40), very similar physiological processes drive the occurrence of all types of stroke. However, the divergence of LOS and mortality findings in seasonality underscores their well-known differences regarding phenotype and prognosis (Citation37–41). Interestingly, we observed no seasonal differences in CCI scores indicating that the variation in LOS and in-hospital mortality results from intrinsic features of the stroke subtypes and possibly from factors related to healthcare service provision. In line with a previous study that found seasonal variation in the incidence of first-ever ischemic stroke but not these patients’ stroke risk factors (Citation14), our results show no association between stroke seasonality and presence of hypertension, atrial fibrillation, or diabetes. This suggests that the seasonal incidence variation is not mediated by traditional stroke risk factors but rather triggering mechanisms that remain to be studied.
The increasing rate of IS admissions and decreasing rate of SAH admissions are in line with previous reports (Citation2,Citation42), but it remains unclear why this decline was not observed in ICH rates. The age-standardized incidence rate for IS largely corresponded to that reported earlier for Finland (Citation43). Although age-specific incidence rates of SAH are similar between our study (data not shown) and a recent report from Finland (Citation42), the differences in study design and inclusion of SAH patients from all age-groups in the previous study (Citation42) result in different overall SAH incidence estimates.
Our study is limited by usage of retrospective registry data. We did not had detailed risk factor or clinical data available. Data on precise symptom onset was also unavailable and we did not had data on patients who have not reached the hospital. We were also unable to differentiate between incident and repeated strokes but never thought this to be crucial for the study question. The major strengths of our study are the availability of complete national data spanning a decade from a register that has been proven valid (Citation44).
In conclusion, we found all major types of stroke to occur most frequently in autumn and most infrequently in summer in Finland. Furthermore, the seasonal pattern of ischemic stroke occurrence appears to have changed during the past decades. Seasonality of in-hospital mortality and duration of admissions varies by stroke subtype and proportion of less severe cases of IS and ICH may increase in autumn compared to other seasons.
SupplTable_1_Overall_trends_of_study_outcome_measures_during_the_study_period.docx
Download MS Word (13.3 KB)SupplFig3_FINAL.tif
Download TIFF Image (614.8 KB)SupplFig2_FINAL.tif
Download TIFF Image (611.7 KB)SupplFig1_FINAL.tif
Download TIFF Image (449.7 KB)Acknowledgements
We would like to thank Hanna Nohynek, MD, PhD, from THL for advice and providing vaccination information and Hilppa Gregow, PhD, and Heikki Lihavainen, PhD, from Finnish Meteorological Institute for providing advice and data on temperature and air quality.
Disclosure statement
Jussi O.T. Sipilä has received travel grants and congress fee covering (Orion Corporation, Abbvie, Lundbeck, Merck Serono, Sanquin) and holds shares (Orion Corporation).
Jori O. Ruuskanen, Tommi Kauko, Päivi Rautava, and Ville Kytö declare no conflicts of interest.
Funding
This work was supported by governmental VTR-funding of the hospital district of Southwestern Finland and grant funding of the Finnish Cardiac Society. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223.
- Meretoja A, Kaste M, Roine RO, Juntunen M, Linna M, Hillbom M, et al. Trends in treatment and outcome of stroke patients in Finland from 1999 to 2007. PERFECT Stroke, a nationwide register study. Ann Med. 2011;43:S22–S30.
- Appelros P, Jonsson F, Åsberg S, Asplund K, Glader EL, Åsberg KH, et al. Trends in stroke treatment and outcome between 1995 and 2010: observations from Riks-Stroke, the Swedish stroke register. Cerebrovasc Dis. 2014;37:22–9.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–54.
- Guiraud V, Amor MB, Mas JL, Touzé E. Triggers of ischemic stroke: a systematic review. Stroke. 2010;41:2669–77.
- Fisman DN. Seasonality of infectious diseases. Annu Rev Public Health. 2007;28:127–43.
- Lichtman JH, Leifheit-Limson EC, Jones SB, Wang Y, Goldstein LB. Average temperature, diurnal temperature variation, and stroke hospitalizations. J Stroke Cerebrovasc Dis. 2016;25:1489–94.
- Mostofsky E, Wilker EH, Schwartz J, Zanobetti A, Gold DR, Wellenius GA, et al. Short-term changes in ambient temperature and risk of ischemic stroke. Cerebrovasc Dis Extra. 2014;4:9–18.
- Mao Y, Schnytzer Y, Busija L, Churilov L, Davis S, Yan B. “MOONSTROKE”: lunar patterns of stroke occurrence combined with circadian and seasonal rhythmicity – a hospital based study. Chronobiol Int. 2015;32:881–8.
- Christensen AL, Rasmussen LH, Baker MG, Lip GY, Dethlefsen C, Larsen TB. Seasonality, incidence and prognosis in atrial fibrillation and stroke in Denmark and New Zealand. BMJ Open. 2012;2:e001210.
- Myint PK, Vowler SL, Woodhouse PR, Redmayne O, Fulcher RA. Winter excess in hospital admissions, in-patient mortality and length of acute hospital stay in stroke: a hospital database study over six seasonal years in Norfolk, UK. Neuroepidemiology. 2007;28:79–85.
- Jakovljević D, Salomaa V, Sivenius J, Tamminen M, Sarti C, Salmi K, et al. Seasonal variation in the occurrence of stroke in a Finnish adult population. The FINMONICA Stroke Register. Finnish Monitoring Trends and Determinants in Cardiovascular Disease. Stroke. 1996;27:1774–9.
- Khan FA, Engstrom G, Jerntorp I, Pessah-Rasmussen H, Janzon L. Seasonal patterns of incidence and case fatality of stroke in Malmo, Sweden: the STROMA study. Neuroepidemiology. 2005;24:26–31.
- Palm F, Dos Santos M, Urbanek C, Greulich M, Zimmer K, Safer A, et al. Stroke seasonality associations with subtype, etiology and laboratory results in the Ludwigshafen Stroke Study (LuSSt). Eur J Epidemiol. 2013;28:373–81.
- Karagiannis A, Tziomalos K, Mikhailidis DP, Semertzidis P, Kountana E, Kakafika AI, et al. Seasonal variation in the occurrence of stroke in Northern Greece: a 10 year study in 8204 patients. Neurol Res. 2010;32:326–31.
- Oberg AL, Ferguson JA, McIntyre LM, Horner RD. Incidence of stroke and season of the year: evidence of an association. Am. J. Epidemiol. 2000;152:558–64.
- Spengos K, Vemmos KN, Tsivgoulis G, Synetos A, Zakopoulos N, Zis VP, et al. Seasonal variation of hospital admissions caused by acute stroke in Athens, Greece. J Stroke Cerebrovasc Dis. 2003;12:93–6.
- Cowperthwaite MC, Burnett MG. An analysis of admissions from 155 United States hospitals to determine the influence of weather on stroke incidence. J Clin Neurosci. 2011;18:618–23.
- Rothwell PM1, Wroe SJ, Slattery J, Warlow CP. Is stroke incidence related to season or temperature? The Oxfordshire Community Stroke Project. Lancet. 1996;347:934–6.
- Kumar N, Venkatraman A, Garg N. Seasonality in acute ischemic stroke related hospitalizations and case fatality rate in the United States. Int J Cardiol. 2015;195:134–5.
- Mikkonen S, Laine M, Mäkelä HM, Gregow H, Tuomenvirta H, Lahtinen M, et al. Trends in the average temperature in Finland, 1847–2013. Stoch Environ Res Risk Assess. 2015;29:1521–9.
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9.
- Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917–32.
- Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Sex, smoking, and risk for subarachnoid hemorrhage. Stroke. 2016;47:1975–81.
- Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–90.
- Förster A, Gass A, Kern R, Wolf ME, Ottomeyer C, Zohsel K, et al. Gender differences in acute ischemic stroke: etiology, stroke patterns and response to thrombolysis. Stroke. 2009; 40:2428–399.
- Tietäväinen H, Tuomenvirta H, Venäläinen A. Annual and seasonal mean temperatures in Finland during the last 160 years based on gridded temperature data. Int J Climatol. 2010;30:2247–56.
- Air quality in Finland [Internet]. Helsinki: The Finnish Meteorological Institute. [cited 2016, Aug 15] Available from: http://www.ilmanlaatu.fi/ilmansaasteet/tietosivut/trendit.php.
- Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295. doi: 10.1136/bmj.h1295.
- Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-term exposure to particulate matter air pollution is a risk factor for stroke: meta-analytical evidence. Stroke. 2015;46:3058–66.
- Heikkinen T, Heinonen S. Effectiveness and safety of influenza vaccination in children: European perspective. Vaccine. 2011;29:7529–34.
- Palmu AA, Kilpi TM, Rinta-Kokko H, Nohynek H, Toropainen M, Nuorti JP, et al. Pneumococcal conjugate vaccine and clinically suspected invasive pneumococcal disease. Pediatrics. 2015;136:e22–7.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–9.
- Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med. 2014;2:387–94.
- Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101:1738–47.
- Vamos EP, Pape UJ, Curcin V, Harris MJ, Valabhji J, Majeed A, et al. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ. 2016;188:E342–51.
- Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke risk factors. Stroke. 1997;28:1507–17.
- Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–18.
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23.
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44.
- Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry. 2005;76:1534–8.
- Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87:1118–23. doi: 10.1212/WNL.0000000000003091.
- Pajunen P, Pääkkönen R, Laatikainen T, Hämäläinen H, Keskimäki I, Niemi M, et al. Aivohalvausten ilmaantuvuuden ja kuolleisuuden muutokset Suomessa vuosina 1991-2002. Suom Lääkäril. 2005;22:2437–42.
- Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–15.
