Abstract
Background: This study investigated whether levofloxacin-containing concomitant therapy can effectively eradicate Helicobacter pylori infection in patients with type 2 diabetes mellitus (T2DM).
Methods: A total of 797 T2DM patients were screened for anti-H. pylori IgG antibodies, and the presence of H. pylori infection was confirmed by 13C-urea breath test. We prospectively randomized 114 of these patients to receive either 10 d of levofloxacin-concomitant therapy (n = 55) or sequential therapy (n = 59). Antimicrobial resistance of H. pylori isolates collected from the patients with T2DM (n = 109) and dyspeptic controls without DM (n = 110) was determined using the E-test. This study was approved by our Institutional Review Board (A-BR-103-021).
Results: The H. pylori eradication rates with concomitant therapy were higher than sequential therapy in both intention-to-treat (96.4% versus 81.4%, p = 0.012) and per-protocol (100% versus 85.4%, p = 0.006) analysis. The adverse effects in both groups were similarly mild. In the patients who received sequential therapy, clarithromycin resistance was significantly associated with eradication failure (p = 0.02). There were no significant differences in the antibiotic-resistant rates to amoxicillin, clarithromycin, metronidazole, tetracycline, and levofloxacin between the patients with and without T2DM.
Conclusions: Ten days of levofloxacin-containing concomitant therapy is an effective and well-tolerated treatment to eradicate H. pylori infection for T2DM patients.
Ten days of levofloxacin-containing concomitant therapy is well tolerated and superior to clarithromycin-containing sequential therapy for first-line H. pylori eradication in patients with type 2 diabetes.
Clarithromycin resistance to H. pylori is the main factor associated with eradication failure in clarithromycin-containing sequential therapy in diabetic patients.
Key messages
Introduction
Helicobacter pylori infection plays a major role in human peptic ulcer disease, and the World Health Organization classifies H. pylori as a type 1 carcinogen for gastric cancer (Citation1,Citation2). The estimated incidence rates of peptic ulcers and gastric cancer are 10% and 1–3% after infection, respectively (Citation3,Citation4). In addition, the Maastricht IV Consensus suggested that H. pylori eradication can cure peptic ulcers and reduce the risk of gastric cancer development (Citation5).
Helicobacter pylori infection is also associated with an increase in insulin resistance and the development of diabetes mellitus (Citation6–8). The high levels of insulin and C-peptide resulting from insulin resistance can increase the risk of gastric cancer (Citation9), and an increased risk of gastric cancer has been reported in patients with diabetes in countries with a high prevalence of H. pylori (Citation10). A population-based cohort study using data from the universal health insurance program in Taiwan indicated that a longer duration of diabetes can result in a higher risk of developing gastric cancer (Citation11). Helicobacter pylori treatment may thus be helpful in reducing the risk of gastric cancer in patients with diabetes.
Clarithromycin-based triple therapy and sequential therapy are widely used as effective first-line regimens to eradicate H. pylori in areas with low rates (<15–20%) of clarithromycin resistance (Citation5). In Taiwan, 10 d of sequential therapy have been reported to be effective to eradicate H. pylori, with reported eradication rates of 78.2–87.2% and 81.9–91.6% in intention-to-treat and per-protocol analysis, respectively (Citation12–14). However, the H. pylori eradication rates of clarithromycin-based triple therapy and sequential therapy are poor in patients with diabetes (Citation15–17). As a result, there is a need for an effective first-line therapy rather than the standard sequential therapy for diabetic patients with H. pylori infection.
Levofloxacin-containing triple and non-bismuth quadruple (concomitant) therapy have been reported to be effective alternative first- or second-line regimens to achieve a favorable H. pylori eradication rate in the general population without diabetes (Citation5,Citation18). However, no previous study has investigated the application of levofloxacin for H. pylori eradication in diabetic patients. Therefore, the aim of this study was to investigate whether levofloxacin-containing concomitant therapy is superior to the standard clarithromycin-based sequential therapy in patients with type 2 diabetes mellitus (T2DM). In addition, we also compared the antimicrobial susceptibility of H. pylori isolates between T2DM patients and controls.
Research design and methods
Study population and design
We consecutively enrolled patients older than 20 years of age with T2DM from our out-patient clinic to receive screening for H. pylori infection after obtaining informed consent. The diagnosis of T2DM was based on the criteria of the American Diabetes Association (Citation19). The Ethics Committee of our Institutional Review Board approved this study (A-BR-103-021). All authors had access to the study data and reviewed and approved the final version of the manuscript. The schematic flow chart of the study design is shown in .
Figure 1. The consort diagram with the study case numbers in the enrollment, allocation, follow-up, and analysis are listed for the two study groups with type 2 diabetes (T2DM). UBT: urea breath test; EGD: esophageaogastroduodenal endoscopy; GFR: glomerular filtration rate; ITT: intention-to-treat; PP: per protocol.
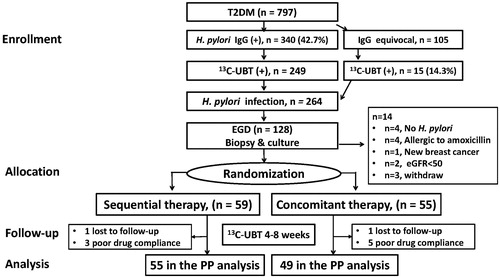
Sera from the screened patients were tested for anti-H. pylori IgG antibodies (HEL-p TESTTM II; AMRAD Biotech, Perth, Western Australia) using an enzyme-linked immunosorbent assay with sensitivity and specificity >90%. The patients with seropositive or borderline seropositive results were further confirmed by 13C-urea breath test as detailed in our previous study (Citation20). Esophagogastroduodenoscopy was then suggested for the patients with a positive 13C-urea breath test. During the endoscopic procedures, gastric biopsies including one piece for the rapid urease test, one for a bacterial culture and five for topographic gastric histology (two antrum, two corpus, and one cardia) were obtained. One experienced pathologist blinded to the clinical data reviewed the gastric histology, including acute inflammatory score (AIS, range 0–3), chronic inflammation score (CIS, range 0–3), H. pylori density (HPD, range 0–5), atrophic change (AT, range 0–3), and intestinal metaplasia (IM, range 0–3) according to the modified Updated Sydney System and our previous publication (Citation20–22). Total HPD, CIS, and AIS scores were calculated as the sum of scores of the antrum, corpus, and cardia (Citation22). We also collected H. pylori isolates from dyspeptic non-diabetic patients in our institute during the same study period to serve as controls to compare antimicrobial resistance between subjects with and without T2DM.
The following T2DM patients were excluded from the clinical trial for H. pylori eradication: those who were pregnant, or had critical conditions, previous failure of eradication therapy, contraindications for gastroduodenal endoscopy, bleeding tendency (those who used anti-platelets or anti-coagulants), and a history of allergy to pantoprazole, amoxicillin, clarithromycin, metronidazole, and levofloxacin. Patients with azotemia (creatinine >2.0 mg/dL), renal insufficiency (estimated glomerular filtration rate <50 ml/min/1.73 m2), undergoing dialysis for renal failure, and those with malignancies were also excluded.
Two T2DM treatment groups for H. pylori eradication
The T2DM patients confirmed to have H. pylori infection were prospectively randomized into two therapeutic groups. The clarithromycin-based sequential group received a 10-d regimen including pantoprazole 40 mg and amoxicillin 1000 mg twice daily for the first 5 d, followed by pantoprazole 40 mg, clarithromycin 500 mg, and metronidazole 500 mg twice daily for another 5 d. In the levofloxacin-based concomitant group, the 10-d regimen included pantoprazole 40 mg, amoxicillin 1000 mg, and metronidazole 500 mg twice daily plus levofloxacin 500 mg once daily. Adverse effects and good drug compliance (80% completion of the study regimen) were recorded in each patient. Successful H. pylori eradication was defined as a negative 13C-urea breath test result in the 6–8 weeks after therapy.
Sample size and statistical analysis
We estimated that there would be a 15% improvement in eradication rate between the levofloxacin-based concomitant therapy and sequential therapy in the T2DM patients based on previous studies. Therefore, the estimated eradication rate of the diabetic patients in the sequential therapy group was set as 85% (Citation12–17). With a two-sided α value of 0.05 and a power of 90% (β = 0.1), the total number of patients required was 113. The categorical data related to baseline characteristics and study end-points were analyzed using Pearson’s χ2 test or Fisher’s exact test. The continuous data were analyzed using an independent t test, and significant differences were defined as a two-tailed p value <0.05.
Results
Demographic features of the study subjects and follow-up
A total of 797 T2DM patients were screened for anti-H. pylori antibodies, of whom, 340 (43%) had seropositive results and 105 had equivocal seropositive results. All of these 445 patients received the 13C-urea breath test, with 264 having positive results. Of these 264 patients, 128 agreed to receive upper gastroduodenal endoscopy (). There were no significant differences in demographic data including age, sex, smoking status, alcohol drinking, insulin use, and levels of glycated hemoglobin A1c and fasting sugar between the patients who did and did not agree to undergo endoscopy (all p > 0.05) ().
Table 1. The demographic data and laboratory tests in the enrolled T2DM patients with and without esophagogastroduodenoscopy.
Exclusion and inclusion criteria for randomization to receive anti-H. pylori therapy
Of the 128 patients who received endoscopy, 14 were excluded (four confirmed without H. pylori infection after endoscopy, four allergic to amoxicillin, one with a newly developed malignancy, two with an estimated glomerular filtration rate <50 ml/min/1.73 m2, and three who withdrew from the study). The remaining 114 patients with H. pylori infection and T2DM were eligible for the study and randomly divided into either 10-d sequential therapy or 10-d levofloxacin-based concomitant therapy groups. The esophagogastroduodenoscopy findings of the 114 patients showed that 48 (42.1%) had peptic ulcers and the others had non-ulcer gastritis. The histology of the biopsy samples showed mean total CIS, AIS, and HPD scores of 7.88 ± 0.14, 4.39 ± 0.16, and 9.14 ± 0.27, respectively.
Similar demographic data, antimicrobial status of H. pylori isolates, and diabetic characteristics between the two treatment groups
There were no significant differences in gender, rate of peptic ulcers, alcohol drinking, smoking status, use of insulin, antibiotic resistance to clarithromycin, metronidazole, and levofloxacin, and duration of diabetes between the two study groups (p > 0.05, ). The only significant difference was a higher mean age in the sequential therapy group than in the levofloxacin-concomitant group (62.0 versus 57.7 years, p = 0.03). Furthermore, there were no significant differences in the histology of the biopsy samples evaluated according to the modified Updated Sydney System (total CIS, AIS, and HPD) between the two groups (all p ≥ 0.05, ). Moreover, there were no significant differences in fasting sugar, glycated hemoglobin A1c, and creatinine levels between the two study groups (all p > 0.05, ).
Table 2. The comparisons of the demographic and clinical features of the H. pylori-infected diabetic patients between sequential and LVX-concomitant groups.
Superior H. pylori eradication outcomes in the concomitant group
All of the patients were included for intention-to-treatment protocol (ITT) analysis; however, eight patients were excluded from per-protocol (PP) analysis: two were lost to follow-up (1 in the sequential group and 1 in the concomitant group), and six took less than 80% of the drug prescription (two in the sequential group, four in the concomitant group) and were defined as having poor drug compliance (). The H. pylori eradication rates were significantly higher in the concomitant group than in the sequential group in terms of both ITT analysis (96.4% versus 81.4%, RR: 5.1, 95% CI: 1.19-22.1, p = 0.012) and PP analysis (100% versus 85.4%, RR: 1.2, 95% CI: 1.05–1.31, p = 0.006) (). Of note, four patients who only took the regimen for 5–7 d in the concomitant group still achieved successful H. pylori eradication.
Table 3. The differences of the eradication rate and the adverse effects between sequential and LVX-concomitant therapy for the T2DM patients.
The frequency of the presence of any adverse effects was slightly higher in the concomitant group than in the sequential group (14.5% versus 8.5%); however, the difference was not significant (p = 0.31, ). Moreover, nearly all of the adverse effects were mild and did not require further clinical attention, except for one (1.8%) of the patients in the concomitant group who discontinued treatment because of multiple adverse effects.
Factors affecting eradication failure in sequential therapy
Ten (17.2%) of the 58 T2DM patients who received sequential therapy had failure to eradicate H. pylori. As shown in , there were no significant differences in age, sex, peptic ulcers, alcohol drinking, smoking status, duration of diabetes, drug compliance, use of insulin, diabetic-related laboratory data, and histology (total CIS, AIS, and HPD) between the subjects with failed and successful eradication (all p > 0.05). The presence of clarithromycin-resistant H. pylori infection was higher in the patients with failed sequential therapy than in those with successful H. pylori eradication (60% versus 9.7%, p = 0.02).
Table 4. The risk factor related to the eradication outcome of sequential therapy in T2DM patients.
No difference in antibiotic susceptibility to H. pylori between the patients with T2DM and the controls
Comparisons of demographic data of the patients with H. pylori with and without T2DM who were tested for antimicrobial susceptibility based on H. pylori isolates are shown in . Although the mean age was older in the patients with T2DM (59.7 versus 51.8 years, p < 0.01), there were no significant differences in female gender (44.0% versus 55.5%, p = 0.07) and the rate of peptic ulcer (44.0% versus 50.9%, p = 0.28). Of the 219 H. pylori isolates collected from both groups, none were resistant to amoxicillin or tetracycline. There were no significant differences in the antibiotic-resistant rates to levofloxacin (17.4% versus 20.0%, p = 0.63), clarithromycin (13.8% versus 9.1%, p = 0.28), and metronidazole (18.3% versus 23.6%, p = 0.34) between the two groups.
Table 5. The rates of antibiotic resistance of H. pylori isolated from the T2DM patients and non-diabetic controls.
Discussion
This randomized single-blind case-–control study is highly original and novel in demonstrating that 10 d of levofloxacin-based concomitant therapy can be highly effective and well tolerated in patients with T2DM to eradicate H. pylori. We also found that clarithromycin-resistant H. pylori infection was a risk factor for eradication failure in the T2DM patients receiving clarithromycin-based sequential therapy.
Helicobacter pylori infection has been associated with insulin resistance and the development of diabetes (Citation6–8). This may be because T2DM is more common in the elderly, in whom the reported seroprevalence of H. pylori ranges from 43% to 63% (Citation23–26). For patients with H. pylori infection in areas with high rates of gastric cancer, the risk of gastric cancer has been shown to increase with a longer duration of diabetes (Citation10,Citation11). These findings provide support for early screening and eradication of H. pylori infection in diabetic patients to decrease the risk of gastric cancer. However, even with earlier screening for H. pylori infection in diabetic patients, the current treatment options for H. pylori eradication have limited efficacy (Citation15–17). Therefore, we investigated whether 10 d of levofloxacin-based concomitant therapy is superior to the standard clarithromycin-based sequential therapy.
In this study, the H. pylori eradication rate in ITT analysis of 10-d sequential therapy for the patients with T2DM was 81.4%, which is slightly lower than the 87.2% in dyspeptic patients reported by Liou et al. (Citation13) also in Taiwan, who also reported a low (around 10%) clarithromycin-resistance rate to H. pylori infection. In antimicrobial analysis, we confirmed that clarithromycin-resistant H. pylori infection was a significant risk factor for failed sequential therapy in the patients with T2DM (). Therefore, 10-d standard sequential therapy should not be recommended for T2DM patients, especially in areas with high clarithromycin-resistant rates to H. pylori.
Levofloxacin-containing triple therapy can be an effective regimen for H. pylori eradication, how it is commonly used as the second-line therapy (Citation5,Citation15). Although non-bismuth concomitant therapy for 7–10 d has been shown to yield a high eradication rate by overcoming dual antimicrobial resistance, previous reports of such concomitant therapy have not included levofloxacin (Citation18,Citation27). Moreover, 14 d of bismuth-based quadruple treatment for H. pylori eradication in T2DM patients has been shown to result in a lower eradication rate than in non-diabetic controls (Citation28). We thus tested levofloxacin-based rather than bismuth-based concomitant therapy in this trial. Federico et al. reported that 5 d of levofloxacin-containing concomitant therapy was effective in eradicating H. pylori (ITT: 92.2%, PP: 96.5%) in the general population. However, the eradication rate was only 66.7% in those with levofloxacin-resistant infections (Citation29). Levofloxacin-containing eradication regimens have also been proven to be effective when administered in a sequential manner (Citation30). Our findings are novel in that we used levofloxacin in the 10-d concomitant therapy regimen and achieved a high H. pylori eradication rate in patients with T2DM in both ITT and PP analysis (). This new strategy not only overcame levofloxacin-resistant (21.6%) H. pylori isolates but also simplified the drug-taking procedure with two-step sequential therapy. This strategy was also effective for metronidazole-resistant (21.6%) and dual levofloxacin- and metronidazole-resistant (5.4%) H. pylori isolates in the patients with T2DM. The high efficacy of concomitant therapy with levofloxacin may due to the longer duration of therapy with one or all of the components (Citation27). The compliance was good and the side effects were in general mild in the T2DM patients receiving the 10-d levofloxacin-based concomitant therapy ().
To date, no published study has investigated the antimicrobial susceptibility of H. pylori isolates collected from diabetic patients. In the present report, we found no significant differences in resistance rates to amoxicillin, tetracycline, levofloxacin, metronidazole, and clarithromycin between the diabetic and non-diabetic patients. Our findings are highly original in that they indicate that clarithromycin-resistant H. pylori can reduce the eradication rate of sequential therapy in T2DM patients compared with non-diabetic dyspeptic patients (Citation5,Citation18,Citation27). To overcome clarithromycin-resistant infection, 14 d of clarithromycin-containing concomitant eradication therapy was shown to be a valid option to achieve a high eradication rate (ITT: 91.7%, PP: 96.1%) in a country with a high clarithromycin-resistant rate (23.5%) (Citation31).
Future studies are needed to elucidate whether a shorter duration of treatment is more cost effective while achieving the same H. pylori eradication rates in diabetic patients, as seen in several cases in our 10-d levofloxacin-concomitant therapy group who still achieved successful H. pylori eradication even after taking the regimen for less than 7 d. Moreover, as all of our study cases were adults with T2DM, the efficacy of levofloxacin-containing concomitant therapy should be further validated in patients with type I DM in pediatric clinics. The limitations of the current study include the relatively small size of the treated group and few variations in drug combinations, both of which should be addressed in future studies.
In conclusion, 10 d of levofloxacin-based concomitant therapy appeared to be superior to clarithromycin-based standard sequential therapy for H. pylori eradication in T2DM patients. It was well tolerated and highly effective as the first-line therapy for H. pylori eradication in T2DM patients.
Trial registration
Trial registration number: NCT02466919
Disclosure statement
None of the authors have any potential conflict of interest relevant to this article.
Additional information
Funding
References
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9.
- International Agency for Research of Cancer. Monographs on the evaluation of carcinogenic risks to humans (Vol. 60); Infection with Helicobacter pylori, pp. 177–240. Lyon: International Agency for Research of Cancer; 1994.
- Zhang RG, Duan GC, Fan QT, Chen SY. Role of Helicobacter pylori infection in pathogenesis of gastric carcinoma. World J Gastrointest Pathophysiol. 2016;7:97–107.
- Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461–73.
- Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection-the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646–64.
- Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter 2009;14:144–50.
- Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205:1195–202.
- Hsieh MC, Wang SS, Hsieh YT, Kuo FC, Soon MS, Wu DC. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur J Clin Invest. 2013;43:949–56.
- Hidaka A, Sasazuki S, Goto A, Sawada N, Shimazu T, Yamaji T, et al. Plasma insulin, C-peptide and blood glucose and the risk of gastric cancer: the Japan Public Health Center-based prospective study. Int J Cancer. 2015;136:1402–10.
- Marimuthu SP, Vijayaragavan P, Moysich KB, Jayaprakash V. Diabetes mellitus and gastric carcinoma: is there an association? J Carcinog. 2011;10:30.
- Chen YL, Cheng KC, Lai SW, Tsai IJ, Lin CC, Sung FC, et al. Diabetes and risk of subsequent gastric cancer: a population-based cohort study in Taiwan. Gastric Cancer 2013;16:389–96.
- Chen KY, Lin TJ, Lin CL, Lee HC, Wang CK, Wu DC. Hybrid vs sequential therapy for eradication of Helicobacter pylori in Taiwan: a prospective randomized trial. World J Gastroenterol. 2015;21:10435–42.
- Liou JM, Chen CC, Chang CY, Chen MJ, Chen CC, Fang YJ, et al. Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomized trial. Gut 2015 Sep 3; pii: gutjnl-2015-310142. [Epub ahead of print]. doi:10.1136/gutjnl-2015-310142.
- Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2013;381:205–13.
- Horikawa C, Kodama S, Fujihara K, Hirasawa R, Yachi Y, Suzuki A, et al. High risk of failing eradication of Helicobacter pylori in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2014;106:81–7.
- Demir M, Gokturk HS, Ozturk NA, Serin E, Yilmaz U. Efficacy of two different Helicobacter pylori eradication regimens in patients with type 2 diabetes and the effect of Helicobacter pylori eradication on dyspeptic symptoms in patients with diabetes: a randomized controlled study. Am J Med Sci. 2009;338:459–64.
- Ataseven H, Demir M, Gen R. Effect of sequential treatment as a first-line therapy for Helicobacter pylori eradication in patients with diabetes mellitus. South Med J. 2010;103:988–92.
- Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 2011;34:604–17.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care* 2010;33(Suppl 1):S62–S9.
- Yang YJ, Wu JJ, Sheu BS, Chen CR, Lu CC, Yang HB. H. pylori infection can change the intensity of gastric Lewis antigen expressions differently between adults and children. J Biomed Sci. 2008;15:29–36.
- Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney system: International Workshop on the Histology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
- Sheu BS, Odenbreit S, Hung KH, Liu CP, Sheu SM, Yang HB, et al. Interaction between host gastric Sialyl-Lewis X and H. pylori SabA enhances H. pylori density in patients lacking gastric Lewis B antigen. Am J Gastroenterol. 2006;101:36–44.
- Quatrini M, Boarino V, Ghidoni A, Baldassarri AR, Bianchi PA, Bardella MT. Helicobacter pylori prevalence in patients with diabetes and its relationship to dyspeptic symptoms. J Clin Gastroenterol. 2001;32:215–7.
- Kayar Y, Pamukçu Ö, Eroğlu H, Kalkan Erol K, Ilhan A, Kocaman O. Relationship between Helicobacter pylori infections in diabetic patients and inflammations, metabolic syndrome, and complications. Int J Chronic Dis. 2015;2015:290128.
- Pareek RP, Kannan M. Prevalence of H. pylori infection in type 2 diabetes mellitus patients in rural Rajasthan – a case control study. Int J Med Sci Clin Invent. 2014;1:1–14.
- Chen LW, Chien CY, Yang KJ, Kuo SF, Chen CH, Chien RN. Helicobacter pylori infection increases insulin resistance and metabolic syndrome in residents younger than 50 years old: a community-based study. PLoS One 2015;10:e0128671.
- Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.e1.
- Sapmaz F, Kalkan IH, Suslu I, Demirci H, Atasoy P, Guliter S. Lower plasma pantoprazole level predicts Helicobacter pylori treatment failure in patients with type 2 diabetes mellitus. J Dig Dis. 2015;16:531–6.
- Federico A, Nardone G, Gravina AG, Iovene MR, Miranda A, Compare D, et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology 2012;143:55–61.e1.
- Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A, et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut 2010;59:1465–70.
- Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013;145:121–8.e1.
