Abstract
Introduction: Diabetes-associated kidney disease is characterized by impairment of renal function and albuminuria. The aim of the present study was to assess whether sleep-disordered breathing is associated with decreased estimated glomerular filtration rate or increased urine-albumin-to-creatinine-ratio independently from known modulators of diabetes-associated kidney disease.
Material and methods: Estimated glomerular filtration rate and urine-albumin-to-creatinine-ratio were determined in the baseline survey of the DIACORE-SDB substudy, a prospectively planned study of Diabetes mellitus 2 patients. As a measure of the severity of sleep-disordered breathing, the apnea-hypopnea-index was assessed using a 2-channel ambulatory SDB-monitoring device.
Results: A total of 679 patients (mean age 66 years, men 61%, mean body-mass-index 31.2 kg/m2) were analyzed. In multivariable linear regression models adjusting for known modulators of diabetes-associated kidney disease, such as sex, age, body-mass-index, systolic blood pressure, duration of diabetes and HbA1c, apnea-hypopnea-index [beta-estimate –0.2 ml/min/1.73 m2, 95% CI (–0.3; –0.1), p = .004], duration of diabetes and age were associated with estimated glomerular filtration rate. Apnea-hypopnea-index [beta-estimate 0.01 mg/g, 95% CI (0.00; 0.02), p = .009], duration of diabetes, HbA1c and systolic blood pressure were associated with ln(urine-albumin-to-creatinine-ratio).
Conclusion: In patients with diabetes mellitus type 2, more severe sleep-disordered breathing is significantly associated with lower estimated glomerular filtration rate and increased albuminuria, independent of known modulators of diabetes-associated kidney disease.
Introduction
Diabetes-associated kidney disease (DKD) is the most common cause for chronic kidney disease and end-stage renal disease [Citation1]. DKD is characterized by decreasing glomerular filtration rate and albuminuria. International guidelines classify DKD into five stages. DKD at stages 4 and stage 5 is associated with increased mortality rates and accounted for an incremental cost increase of $33,131 and $106,975 ($US) per year relative to stage 1 or no chronic kidney disease, and constitutes a major health care burden in Europe and the US [Citation2,Citation3]. Results from the United States Renal Data System demonstrate poor rates for survival and freedom of hospital admission in patients with DKD [Citation4]. Because of the increasing prevalence of DM2, the prevalence and incidence of DKD continue to increase [Citation5].
Sleep-disordered breathing (SDB) is characterized by repetitive apneas, arousals from sleep and intermittent hypoxia. SDB causes elevation of renin and angiotensin [Citation6], sympathetic nervous system activation [Citation7], oxidative stress [Citation8], endothelial dysfunction [Citation9] and contributes to the development of hypertension [Citation10]. While the prevalence of moderate SDB (AHI >15/h) in the general population is 3–17% [Citation11], it is 29% in individuals with DM2 [Citation12] and 60% in populations with advanced chronic kidney disease [Citation13].
A link between SDB and DKD independent of severity and duration of DM2 has been observed in two smaller studies: Tahrani et al. [Citation14] found an association between obstructive sleep apnea and DKD in a study of 224 DM2 patients. Furukawa et al. [Citation15] described an association between oxygen desaturation index and microalbuminuria in 513 patients. However, these studies may have been limited by potential bias inherent to patient recruiting in special diabetes clinics or nephrology departments. Thus, the aim of the present study is to perform a systematic investigation of the association between parameters of SDB and DKD in a large representative survey of outpatients with DM2.
Materials and methods
Study design
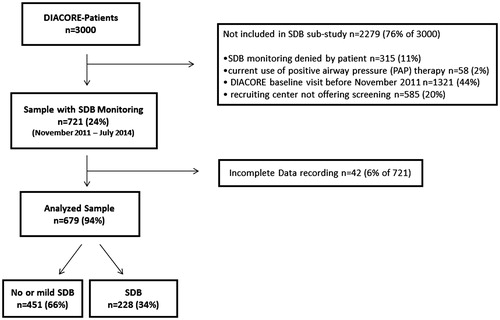
The investigated patients were participants of the DIACORE (DIAbetes COhoRtE) SDB substudy. DIACORE is designed as a two-center, prospectively planned study of DM2 patients of European descent, with a baseline survey conducted during 2010–2014 and recruitment and ascertainment described previously [Citation16]. Briefly, written invitations were mailed to all DM2 patients registered with five medical insurance companies in the respective year of the mailing, and to all DM2 outpatients of two diabetologists in Regensburg who had visited the offices within the last 6 months of the mailing. Further, invitations were sent to DM2 patients who had received inpatient treatment at the University Hospital Regensburg’s Internal Medicine Departments within 2 years prior to the mailing. Overall, 4226 patients contacted DIACORE, of which 1226 did not fulfill the in- and exclusion criteria. The status of diabetes was ascertained by assessing diabetes medication or by validating self-report. Patients were subjected to a standardized online questionnaire, blood sampling and physical examination at the two study centers. Among 1036 individuals invited to participate in the DIACORE SDB substudy, 721 individuals agreed to participate and were tested with a two-channel ambulatory SDB-monitoring device (Apnea Link®, ResMed, Australia, Sydney). In 679 patients (94% of the 721 tested), complete SDB parameters were recorded. The apparative monitoring for SDB was not performed in the Mannheim study center. A two-yearly follow-up is currently ongoing [Citation16]; for this investigation, the cross-sectional baseline dataset was used.
The protocol, the data protection strategy and the study procedures were approved by the Ethics Committees of participating institutions and are in accordance with the Declaration of Helsinki. Patients participate in DIACORE only after providing informed written consent. The study protocol has been described previously [Citation16].
Study population
All DM2 outpatients inhabiting the city and county of Regensburg or Speyer were eligible for DIACORE. Further inclusion criteria were the ability to fully understand the study information and to provide written informed consent, age ≥18 years and self-reported Caucasian ethnicity. Exclusion criteria were chronic renal replacement therapy (hemodialysis, peritoneal dialysis or transplantation), history of active malignancy within the last five years, autoimmune-disease potentially affecting kidney function, hemochromatosis, known pancreoprivic or self-reported type 1 diabetes mellitus, acute infection, fever, pregnancy and chronic viral hepatitis or HIV-infection. For the DIACORE SDB substudy, patients were included if they consented to perform SDB monitoring and excluded if they currently used positive airway pressure therapy.
Assessment of SDB
Nasal flow and pulse oximetry were measured using the ApneaLink device (ResMed, Australia, Sydney) that has been validated in several studies for monitoring of SDB [Citation17–19]. Trained study personnel instructed the participants about the use of the device in a standardized manner. By comparing ApneaLink to the gold standard polysomnography (PSG), studies have reported a sensitivity of 73–94% and a specificity of 85–95% using an AHI cut-off value of 15/h [Citation19,Citation20]. AHI, oxygen desaturation index, mean oxygen saturation and minimum SpO2 were documented. The default settings of the monitoring device were used for the definitions of apnea, hypopnea and desaturation: apnea was defined as a ≥80% decrease in airflow for ≥10 s; hypopnea was defined as a decrease in airflow by 50–80% versus baseline for ≥10 s followed by a ≥4% decrease in oxygen saturation. No or mild SDB was defined as AHI <15/h, moderate SDB as AHI ≥15/h to <30/h and severe SDB was defined as AHI ≥30/h. Periodic breathing (Cheyne–Stokes Respiration) was detected by automatic pattern recognition [Citation21]. Because of the missing chest band, a clear differentiation between types of SDB, such as obstructive and central sleep apnea, was not possible.
Additionally, subjective daytime sleepiness was assessed using the self-administered validated Epworth Sleepiness Scale. Individuals were asked to rate their likelihood of falling asleep in several common situations. Scores range from 0 (least sleepy) to 24 (sleepiest). Excessive daytime sleepiness is defined as a score of 11 or higher [Citation22].
Assessment of DKD
Diabetes-associated kidney disease was assessed using eGFR, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [Citation23]) and the urinary albumin-to-creatinine ratio (UACR) of a spot midstream urine sample, with dipstick testing on fresh urine used to exclude urinary tract infection. DKD was defined as the presence of eGFR <60 ml/min/1.73 m2 or an UACR >30 mg/g. Micro-albuminuria was defined as 30 mg/g < UACR ≤300 mg/g, and macroalbuminuria was defined as UACR >300 mg/g.
Statistical analysis
Descriptive data were presented as mean (±SD). Normally distributed values of baseline characteristics were evaluated with an ANOVA and post-hoc analysis (Bonferroni). Individuals with increasing severity of SDB (no/mild, moderate and severe) were compared according to their risk for eGFR <60 ml/min/1.73 m2 or UACR >30 mg/g by logistic regression analysis. Results were recorded as odds ratio and respective confidence intervals. Linear regression models were used to assess the association of AHI with eGFR and UACR. Known modulators for DKD such as sex, age, body-mass index (BMI), systolic blood pressure, duration of DM2 and HbA1c were included as covariates. Variables included in the linear regression models were either normally distributed or transformed prior to incorporation in the models (UACR). Results were given as beta estimates with 95% confidence interval; p values <.05 were considered significant. Data were analysed using the SPSS statistical software package (SPSS 20.0, IBM SPSS Statistics, Armonk, NY).
Results
Patient characteristics
Of the 721 DM2 patients monitored for SDB, 42 (6%) had incomplete or no recording of SDB parameters due to technical problems such as loss of the oximeter during sleep or failure to switch the device on at night. Thus, 679 patients were entered into the analysis (). Mean age was 65.6 years, 61% were men, patients were mostly obese (mean BMI 31.2 kg/m2), and average duration of DM2 was 10.2 years ().
Table 1. Clinical characteristics of the 679 analysed subjects at the DIACORE baseline visit.
Patient characteristics according to severity of SDB
Patients were classified into three groups of SDB-severity according to their AHI: 451 patients had no or mild SDB (AHI <15/h, 66%), 163 patients moderate SDB (AHI ≥15 to 29/h, 24%) and 65 patients severe SDB (AHI ≥30/h, 10%). Patients with higher AHI were predominantly men, older, more obese and had higher systolic blood pressure, higher waist-hip-ratio as well as a longer history of DM2 ().
Table 2. Clinical and SDB parameters of the 679 analysed subjects at the DIACORE baseline visit by SDB class.
Mean AHI of 6.7/h in the no or mild SDB group indicates a mild degree of SDB. An increase of oxygen desaturation index was observed by increasing severity of SDB. In addition, duration of peripheral SpO2 below 90% as well as mean SpO2 and minimum SpO2 differed significantly between the three groups (each p = .001). Periodic breathing was more frequent in patients with higher severity of SDB. Epworth Sleepiness Scale was similar between groups of SDB severity ().
Kidney function and albuminuria
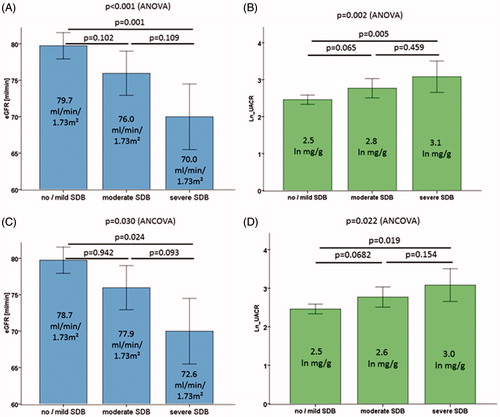
DM2 patients with no or only mild SDB had a significantly higher eGFR (79.7 ml/min/1.73 m2) than those with moderate SDB (76.0 ml/min/1.73 m2) or severe SDB (70.0 ml/min/1.73 m2; ). Further, UACR was higher in DM2 patients with more severe SDB: patients without or with mild SDB had a median albumin-creatinine-ratio (UACR) of 2.5 ln mg/g in their spot urine compared to 2.8 ln mg/g in moderate SDB and 3.1 ln mg/g in severe SDB ().
Figure 2. Kidney function measured as eGFR (A and C) and albuminuria measured as UACR (B and D, analysed on ln-scale) across SDB categories in 679 subjects at the DIACORE baseline visit. Bars indicate mean values by group and error bars indicate 95% confidence intervals. ANOVA p value was used for overall association and Bonferroni post-hoc test for pairwise comparisons (A and B). A generalized linear model (Analysis of covariance, ANCOVA) was used to compute adjusted mean eGFR and UACR for the categories of SDB (C and D, adjusted for age, BMI, systolic blood pressure, duration of DM2, HbA1c and AHI). eGFR: estimated glomerular filtration rate, UACR: urinary albumin-creatinine-ratio, SDB: sleep-disordered breathing.
Sleep-disordered breathing is associated with dichotomous endpoints defining DKD (eGFR ≤60 ml/min/1.73 m2 or UACR ≥30 mg/g): DM2 patients with no or mild SDB suffered significantly less frequently from DKD than those with moderate or severe SDB (31 vs. 40 vs. 57%, ). Both decreased eGFR and albuminuria were significantly more common in patient groups with moderate or severe SDB compared to no or mild SDB in a logistic regression model without adjustment ().
Table 3. Relationship between SDB and DKD in the total sample and by sex at the DIACORE baseline visit.
After adjusting for sex, age, BMI, systolic blood pressure, duration of DM2 and HbA1c, patients with severe SDB (AHI ≥30/h) have a significantly increased odds ratio for DKD (OR 1.62, 95% CI 1.21–2.17, p = .001), eGFR ≤60 ml/min/1.73 m2 (OR 1.59, 95% CI 1.12–2.24, p = .009) and for increased albuminuria defined as UACR ≥30 mg/g (OR 1.55, 95% CI 1.14–2.10, p = .005) compared to patients without or mild SDB. Association of severe SDB with DKD was not significantly different between men and women accounting for known modulators for DKD (interaction term, p = .723).
In univariable regression analysis, age, systolic blood pressure, duration of DM2, periodic breathing and AHI were significantly associated with lower eGFR. In multivariable regression analysis, age, duration of DM2 and AHI were independently associated with eGFR (). The same multivariable regression model adjusted for other SDB variables than AHI showed an independent association between eGFR and oxygen desaturation index, but not between eGFR and duration of peripheral SpO2 below 90% or mean SpO2.
Table 4. Modulators for eGFR in total sample, in 412 men and in 267 women.
Sex, age, BMI, systolic blood pressure, duration of DM2, HbA1c and AHI were associated with higher ln UACR in univariable regression analysis. In multivariable regression analysis, systolic blood pressure, duration of DM2, HbA1c and AHI were independent modulators of higher ln UACR (). The same multivariable regression model adjusted for other SDB variables than AHI showed an independent association between ln UACR and duration of peripheral SpO2 below 90%, but not between UACR and oxygen desaturation index or mean SpO2.
Table 5. Modulators for ln UACR in total sample, in 412 men and in 267 women.
If arteriopathy (defined as a history of coronary heart disease, peripheral arterial disease, transient ischemic attack or stroke) was added to the multivariable regression model, AHI was still independently associated with eGFR and ln UACR. Association of AHI with eGFR and ln UACR was not significantly different between men and women accounting for known modulators of DKD (interaction terms, p = .546 and p = .235, respectively).
Discussion
This report represents one of the largest studies investigating the association of SDB with DKD in outpatients with DM2 (), obtaining several key findings. First, with increasing severity of SDB, eGFR is lower and albuminuria higher, providing evidence of an association of SDB with DKD. Second, DM2 patients with severe SDB have a 2.94-fold increased odds ratio for having DKD compared to those without or mild SDB. Third, the significant association of AHI with measures of DKD is independent of important known modulators of DKD. This association was similar in men and women. Multivariable models using event-based measures of SDB (ODI and AHI) as well as hypoxia load-based measures of SDB (duration of peripheral SpO2 below 90% or mean SpO2) do not allow conclusions that one of those components results in the association of SDB and DKD.
Table 6. Cross-sectional studies looking for association between DKD and SDB.
This study extends previous findings from Tahrani et al. [Citation14], who described an association between obstructive sleep apnea and DKD, showing in longitudinal analysis that eGFR declined faster in patients with obstructive sleep apnea. However, the study from Tahrani et al. [Citation14] is smaller (n = 267) than DIACORE. Therefore, we could provide sex-specific estimates of the association of SDB and DKD. Second, the patients from Tahrani et al.’s [Citation14] study were recruited in special clinics attending secondary care units for diabetes, while the participants of the DIACORE study were invited mainly by their health insurance companies, and diabetes care of the DIACORE participants was predominantly provided by general practitioners. The DIACORE study represents a sample with a large range of diabetes severity and duration and is thus representative of a general type DM2 population. Third, DIACORE patients´ HbA1c was about 1.3% lower than those in the study by Tahrani et al. [Citation14], indicating either stricter blood glucose control or less severe DM2. Furthermore, DIACORE patients were about 10 years older and their systolic blood pressure was about 10 mmHg higher, which could be a sign of multimorbidity [Citation14].
Leong et al. [Citation24] described an association between higher AHI and lower eGFR in 90 diabetes patients with extreme obesity, but did not analyse UACR [Citation24]. In another cross-sectional study by Agrawal et al. [Citation25], 91 obese adults observed an independent association between SDB and higher serum creatinine, but not between SDB and albuminuria. Both studies were not comparable to an average diabetic population as mean BMI was 50.6 and 48.3 kg/m2, respectively, in the obstructive sleep apnea group, whereas mean BMI in the DIACORE SDB substudy was 31.2 kg/m2.
Several studies observed an independent association between UACR and SDB. Schober et al. [Citation26] found a higher prevalence for nephropathy in DM2 patients with SDB. A cross-sectional study by Furukawa et al. [Citation15] observed a sample of 513 patients with DM2 and found an association between nocturnal intermittent hypoxia and microalbuminuria, but only in the 221 women (OR 3.12 in women vs. OR 1.17 in men). In the DIACORE SDB substudy, association of severe SDB and DKD was similar in men and women.
Several different pathophysiological mechanisms have been discussed to explain the relation between DKD and SDB. SDB might contribute to the development of DKD mediated by SDB-related chronic intermittent hypoxia, oxidative stress, inflammation and endothelial dysfunction [Citation27]. In patients with DM2, SDB is associated with increased oxidative stress as well as with impaired microvascular and endothelial regulation [Citation28]. SDB-related reactive oxygen species and systemic inflammation contribute to atherosclerosis [Citation27] and may also contribute to chronic kidney disease progression [Citation29]. In addition, sleep fragmentation results in higher sympathetic activity and activation of the renin-angiotensin-aldosterone system [Citation27,Citation29]. SDB is an established treatable cause of arterial hypertension [Citation27,Citation30]. This can lead to renal fibrosis [Citation31], causing proteinuria and decreased eGFR [Citation27]. Patients with untreated SDB have a lower renal plasma flow and a higher filtration fraction in comparison to SDB-treated patients [Citation32].
On the other hand, DKD might cause SDB through a variety of mechanisms [Citation33]: chronic kidney disease, which is one component of DKD, that might be associated with excessive fluid volume with a potential shift during recumbency towards the neck area causing pharyngeal narrowing [Citation34,Citation35]. Hanly and Pierratos [Citation36] demonstrated that nocturnal hemodialysis improves SDB associated with chronic renal failure. Other possible pathomechanisms could be alterations of chemoreflex responsiveness and accumulation of uremic toxins [Citation37].
The strengths of our study are the large sample size and high-resolution phenotyping of a study sample representative from general DM2 patients without selection for extreme obesity; thus, enabling us to investigate the effects specific to sex. There are limitations that warrant discussion: first, we cannot conclude a causal relationship of the underlying mechanism; specifically, it is unclear whether SDB causes DKD or vice versa, or whether there is an association without a causal relationship. Patients with manifest DKD or even sub-clinical manifestations of decreased eGFR or UACR may suffer from fluid overload contributing to the development of SDB [Citation34,Citation35]. Longitudinal data would be required to establish SDB as a risk factor for incident DKD or to document SDB as a monitor of disease. Second, though the use of portable respiratory devices instead of inpatient overnight polysomnography is well established and validated for assessment of SDB [Citation17–19], we cannot distinguish between obstructive and central sleep apnea due to simplified monitoring. Third, the degree of albuminuria was determined from the albumin-creatinine-ratio in random spot urine in place of three measurements. Still, this practice was shown to be accurate in predicting nephropathy and appropriate for epidemiological studies [Citation38].
In summary, findings from this large sample of DM2 patients demonstrate that more severe SDB is significantly associated with lower eGFR and increased albuminuria, independent of known modulators of DKD. Further research on SDB in patients with DM2 including longitudinal analyses and interventional studies to improve the understanding of the underlying pathophysiology of DKD and the role of SDB as a potential treatment target is warranted.
Acknowledgements
We thank all participating patients of the DIACORE study. We thank the physicians and health insurance companies supporting the DIACORE study: Axel Andreae, Gerhard Haas, Sabine Haas, Jochen Manz, Johann Nusser, Günther Kreisel, Gerhard Bawidamann, Frederik Mader, Susanne Kißkalt, Johann Hartl, Thomas Segiet, Christiane Gleixner, Christian Scholz, Monika Schober (Chief of Supply Management, Allgemeine Ortskrankenkasse Bayern), Cornelia Heinrich (Communication Manager, Allgemeine Ortskrankenkasse Bayern), Thomas Bohnhoff (Disease Management, Techniker Krankenkasse), Thomas Heilmann (Head of Disease Management, Techniker Krankenkasse), Stefan Stern (Consulting Physician, Allgemeine Ortskrankenkasse Bayern), Andreas Utz (Head of Department, Allgemeine Ortskrankenkasse Bayern), Georg Zellner (Chief of Supply Management, Deutsche Angestellten Krankenkasse), Werner Ettl (Barmer-GEK), Thomas Buck (Barmer-GEK), Rainer Bleek (IKK classic) and Ulrich Blaudzun (IKK classic). We further thank the study nurses for their excellent work in performing the study visits: Simone Neumeier, Sarah Hufnagel, Isabell Haller, Petra Jackermeier, Sabrina Obermüller, Christiane Ried, Ulrike Hanauer, Bärbel Sendtner, Natalia Riewe-Kerow. Konstantin Dumann and Britta Hörmann (PhD-Students) are thanked for their exceptional work in performing the study visits.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Quiroga B, Arroyo D, de Arriba G. Present and future in the treatment of diabetic kidney disease. J Diabetes Res. 2015;2015:801348.
- Ghaderian SB, Hayati F, Shayanpour S, et al. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev. 2015;4:28–33.
- Slabaugh SL, Curtis BH, Clore G, et al. Factors associated with increased healthcare costs in Medicare Advantage patients with type 2 diabetes enrolled in a large representative health insurance plan in the US. J Med Econ. 2015;18:106–112.
- Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63:A7.
- United States Renal Data System. 2014 Annual Data Report: epidemiology of kidney disease in the United States. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014.
- Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314.
- Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904.
- Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–1679.
- Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610.
- Becker HF. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2002;107:68–73.
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014.
- Laaban J-P, Daenen S, Leger D, et al. Prevalence and predictive factors of sleep apnoea syndrome in type 2 diabetic patients. Diabetes Metab. 2009;35:372–377.
- Kraus MA, Hamburger RJ. Sleep apnea in renal failure. Adv Perit Dial. 1997;13:88–92.
- Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care. 2013;36:3718–3725.
- Furukawa S, Saito I, Yamamoto S, et al. Nocturnal intermittent hypoxia as an associated risk factor for microalbuminuria in Japanese patients with type 2 diabetes mellitus. Eur J Endocrinol. 2013;169:239–246.
- Dörhöfer L, Lammert A, Krane V, et al. Study design of DIACORE (DIAbetes COhoRtE) – a cohort study of patients with diabetes mellitus type 2. BMC Med Genet. 2013;14:25.
- Chen H, Lowe AA, Bai Y, et al. Evaluation of a portable recording device (ApneaLink) for case selection of obstructive sleep apnea. Sleep Breath. 2009;13:213–219.
- Erman MK, Stewart D, Einhorn D, et al. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–392.
- Arzt M, Woehrle H, Oldenburg O, et al. Prevalence and predictors of sleep-disordered breathing in patients with stable chronic heart failure: the SchlaHF Registry. JACC Heart Fail. 2016;4:116–125.
- Stuck A. The new “International Classification of Sleep Disorders”. Somnologie. 2015;19:126–132.
- Weinreich G, Armitstead J, Topfer V, et al. Validation of ApneaLink as screening device for Cheyne-Stokes respiration. Sleep. 2009;32:553–557.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Leong WB, Nolen M, Thomas GN, et al. The impact of hypoxemia on nephropathy in extremely obese patients with type 2 diabetes mellitus. J Clin Sleep Med. 2014;10:773–778.
- Agrawal V, Vanhecke TE, Rai B, et al. Albuminuria and renal function in obese adults evaluated for obstructive sleep apnea. Nephron Clin Pract. 2009;113:7.
- Schober A-K, Neurath MF, Harsch IA. Prevalence of sleep apnoea in diabetic patients. Clin Respir J. 2011;5:165–172.
- Levy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015.
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia-revisited-the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45.
- Adeseun GA, Rosas SE. The impact of obstructive sleep apnea on chronic kidney disease. Curr Hypertens Rep. 2010;12:378–383.
- Hu X, Fan J, Chen S, et al. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens. 2015;17:215–222.
- Wu H, Zhou S, Kong L, et al. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol Lett. 2015;232:340–348.
- Kinebuchi S-i, Kazama JJ, Satoh M, et al. Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci. 2004;107:317–322.
- Abuyassin B, Sharma K, Ayas NT, et al. Obstructive sleep apnea and kidney disease: a potential bidirectional relationship. J Clin Sleep Med. 2015;11:915–924.
- Fritz A. Sleep apnea syndrome in chronic renal failure. Somnologie. 2014;18:127–131.
- Roumelioti M-E, Brown LK, Unruh ML. The relationship between volume overload in end-stage renal disease and obstructive sleep apnea. Semin Dial. 2015;28:508–513.
- Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–107.
- Langevin B, Fouque D, Leger P, et al. Sleep apnea syndrome and end-stage renal disease. Cure after renal transplantation. Chest. 1993;103:1330–1335.
- Pugliese G, Solini A, Fondelli C, et al. Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency And Cardiovascular Events (RIACE) study. Nephrol Dial Transplant. 2011;26:3950–3954.
- Buyukaydin B, Akkoyunlu ME, Kazancioglu R, et al. The effect of sleep apnea syndrome on the development of diabetic nephropathy in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;98:140–143.