Abstract
Objectives: The biological mechanism by which smoking reduces the risk of pre-eclampsia (PE) is unresolved. We studied serum levels of soluble fms-like tyrosine kinase 1 (sFlt-1), placental growth factor (PlGF) and their ratio, in addition to soluble endoglin (sEng) in early and late pregnancy to ascertain whether these factors are altered in women who smoke.
Subjects and methods: First trimester serum samples were available from 217 women who later developed PE and 238 women who did not develop PE. Second/third trimester serum samples were available from 174 PE and 54 non-PE women.
Results: PE women who smoked during pregnancy had elevated first trimester concentrations of serum PlGF [geometric mean (95% CI): 39.8 (32.6–48.5) pg/ml, p = .001] and reduced sEng concentration [5.0 (4.6–5.6) ng/ml, p = .047] compared to PE non-smokers [30.0 (28.1–32.1) pg/ml and 6.1 (5.9–6.4) ng/ml, respectively]. Non-smoking women in the PE group had the highest sFlt-1/PlGF ratio in early and late pregnancy.
Conclusions: The protective effect of smoking in reducing the risk of PE may be due to the early pregnancy change towards pro-angiogenic marker profile. Also, in late pregnancy, smoking exerted effect in sFlt-1/PlGF ratio in PE pregnancies, and may complicate its use as a prognostic and diagnostic marker.
Smoking appears to have angiogenic effects in early pregnancy with reduced sEng concentrations and elevated PlGF concentrations in both normal and PE pregnancies.
Throughout pregnancy, smoking exerted effect in PlGF concentration and sFlt-1/PlGF ratio in PE pregnancies, and thus may complicate its use as a prognostic and diagnostic marker.
Key messages
Introduction
Pre-eclampsia (PE) is a complex pregnancy disorder defined by new-onset hypertension and proteinuria after 20 weeks of gestation, or new-onset PE-associated signs in the absence of proteinuria [Citation1]. The pathogenesis of PE remains poorly understood due to its heterogeneous, multi-systemic nature. The conventional risk factors for the development of PE include nulliparity, multifoetal gestations, previous history of PE, chronic hypertension, obesity, diabetes mellitus, vascular and connective tissue disorders, age >35 years at first pregnancy and African-American ethnicity [Citation2]. An obstetrical paradox is that maternal smoking appears to be protective for the development of PE. There is a robust body of literature that has consistently demonstrated a reduced risk of PE in women who smoke when they are pregnant [Citation3–5]. However, the biological mechanism by which smoking during pregnancy reduces the risk of PE is still unresolved.
In the last decade, research attention has increasingly focused on imbalances in maternal pro-angiogenic placental growth factor (PlGF) and anti-angiogenic soluble fms-like tyrosine kinase 1 (sFlt-1) in the pathogenesis of PE [Citation6–10]. Elevated levels of s-Flt1 and reduced levels of PlGF have been observed in women with PE, as well as in pregnant women prior to development of PE [Citation6]. The high sFlt-1/PlGF ratio has been demonstrated to already exist before PE is established, and thus might serve as a diagnostic tool to predict future PE [Citation10]. Different cut-offs for the sFlt1/PlGF ratio have also been proposed to allow for the assessment of PE [Citation10–13]. The soluble form of endoglin (sEng) has also been shown to have anti-angiogenic activity and increased levels have been reported in PE women two to three months before the onset of the disease [Citation7].
Different concentrations of angiogenic markers have been reported in smoking and non-smoking PE women. Smoking appears to cause a pro-angiogenic state, particularly by lowering maternal sFlt-1 concentrations [Citation14–16]. The effect of smoking on sEng concentrations is less well studied.
In this observational study, we have compared the first and second/third trimester serum concentrations of sFlt-1, PlGF and their ratio, in addition to sEng, in women who had smoked before or during pregnancy to those who had never smoked. Since altered levels of these markers precede the clinical onset of the disease, the PE and non-PE women were investigated separately. We also tested within these subgroups recently recommended rule-out cut-off values.
Methods
Study design and aim
Data for the present study come from the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC), a cross-sectional case-control multicentre study with a nationwide clinical and DNA database on PE and non-PE women, including their partners and infants. The cohort was established in order to identify genetic risk factors for PE. Details of the study design, methods and procedures have been described elsewhere [Citation17]. Herein, we investigated whether maternal serum concentrations of sFlt-1, PlGF, sEng and sFlt-1/PlGF ratio available from FINNPEC participants associate with maternal smoking status and number of cigarettes smoked.
Study subjects
Originally in the FINNPEC Study, 1450 women with PE and 1065 non-PE women were recruited at the five Finnish university hospitals. In this study, we focused on a subset of women from whom first and second/third trimester serum samples were available. All participants provided written informed consent, and the FINNPEC study protocol was approved by the coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa.
Inclusion criteria
Nulliparous or multiparous women with a singleton pregnancy were eligible for the study. PE was defined as hypertension and proteinuria occurring after 20 weeks of gestation. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, and proteinuria as the urinary excretion of ≥0.3 g protein in a 24-hour specimen, or 0.3 g/l, or two ≥1+ dipstick readings in a random urine sample with no evidence of a urinary tract infection. Women who suffered from proteinuria without hypertension (n = 5) were included in the non-PE group. Furthermore, women who suffered from gestational hypertension but did not meet the full criteria for PE were included in the non-PE group. Each diagnosis was ascertained from hospital records and independently confirmed by a research nurse and a study physician.
Exclusion criteria
Exclusion criteria were multiple pregnancy, maternal age less than 18 years and an inability to provide informed consent based on information being offered in Finnish or Swedish.
Background and obstetric data
Extensive information on family history, medical history, obstetric history, pregnancy complications, pregnancy outcome, proteinuria, blood pressure, laboratory measurements, delivery and newborn was obtained from the hospital records and maternity cards (women in Finland are given a personal maternity card at their first visit to a maternity centre, and all visits are recorded on these cards). Data on pre-pregnancy weight, height, and diastolic and systolic blood pressure at first antenatal visit were obtained from maternity cards. Data on smoking status and the number of cigarettes smoked were collected from the maternity cards and complemented from the background information questionnaires if needed. The woman was considered to be a pre-pregnancy smoker if she smoked within 6 months of conception. Women were further divided into subgroups based on their smoking status (current smokers/pre-pregnancy smokers/non-smokers).
Serum samples and angiogenic markers
First and second/third trimester serum samples were collected from women receiving care from the Hospital District of Helsinki and Uusimaa. In Finland, a routine first-trimester screening test for foetal chromosome abnormalities is performed at health centres and the samples are routinely stored. These screening samples (range 9–15 weeks of gestation) of the FINNPEC mothers were retrospectively tracked and analysed. Serum samples from the second/third trimesters (range 20–42 weeks of gestation) were collected at hospitals for study protocol.
Maternal serum sFlt-1 and PlGF concentrations were measured using sFlt-1 and PlGF electro-chemiluminescence immunoassays (ECLIA; Roche Diagnostics GmbH, Mannheim, Germany) on a cobas e601 analyser (Hitachi High Technology Co., Tokyo, Japan). Serum concentrations of sEng (CD105) were measured using the human Quantikine Endoglin ELISA kit (R&D Systems, Abingdon, UK) according to the manufacturer’s instructions. The intra-assay and inter-assay coefficients of variation for PlGF were <0.8% and <2.3% in the concentration range 96–1020 pg/ml, for sFlt-1 <1.4% and <1.7% in the range 97–5390 pg/ml.
Recently recommended rule-out cut-off values of 33 (from 20 weeks to delivery), rule-in cut-offs of 85 (until 33 weeks 6 days) and 110 (from 34 weeks to delivery) were tested for the Elecsys immunoassay sFlt-1/PlGF ratio [Citation18]. Furthermore, we tested a cut-off for the sFlt-1/PlGF ratio that was presented very recently in the PROGNOSIS study [Citation10]. Zeisler et al. [Citation10] derived a single cut-off value independent of the weeks of gestation: values below 38 were considered negative and were used to rule-out PE within 1 week after assessment of the ratio.
Statistical analysis
Statistical tests were performed with IBM SPSS Statistics version 20 (Armonk, NY). The normality of variable distributions was verified with the Kolmogorov–Smirnov test. Logarithmic transformation was used when appropriate. Each biomarker was ln-transformed to correct for right-skewness, and estimated means were back-transformed as geometric means and 95% confidence intervals for purposes of presentation. For the continuous variables, comparisons between groups were analysed with general linear model univariate ANOVA at baseline and with linear mixed models during the pregnancy. Selected co-variables [parity, maternal age, body mass index (BMI), gestational weeks at sampling] were included in the models as covariates. Normality was assessed by plotting the residuals.
For the categorical variables, the comparisons were performed with the Fisher’s exact test. With skewed distributions, comparisons between continuous variables were performed by the Mann–Whitney U-test.
Results
The maternal and perinatal characteristics of PE and non-PE women according to smoking status are presented in . Smokers were younger in both PE and non-PE groups and, in the PE group, smokers had higher BMI compared to non-smokers. There were no differences in any other characteristics between smokers and non-smokers including birthweight, gestational weeks and percentage of small for gestational age (SGA) foetuses (birthweights below –2.0 SD units, according to Finnish standards).
Table 1. Maternal and perinatal characteristics according to smoking status in FINNPEC.
Angiogenic markers at baseline
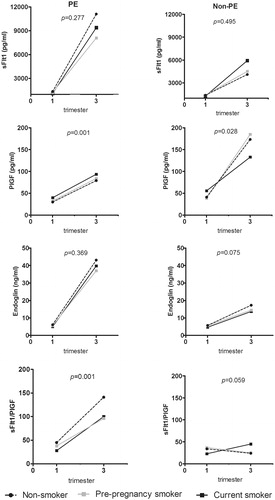
First trimester concentrations of angiogenic markers in PE and non-PE women according to smoking status are presented in . There were no differences in sFlt-1 concentrations in early pregnancy between current, pre-pregnancy and non-smokers in PE or in non-PE group. Current smokers had elevated first trimester concentrations of PlGF compared to non-smokers in both PE and control groups. Furthermore, current smokers had reduced first trimester levels of sEng and sFlt-1/PlGF ratio compared to non-smokers in both PE and non-PE groups ().
Table 2. First trimester concentrations of angiogenic markers according to smoking status, geometric mean (95% CI).
Angiogenic markers during pregnancy
Concentrations of angiogenic markers and sFlt-1/PlGF ratio during pregnancy in PE and non-PE women according to the smoking categories are presented in . Smoking status was not associated with sFlt-1 and sEng concentrations among PE or non-PE women. In the PE group, current smokers had highest PlGF concentrations in early and late pregnancy. Whereas in the non-PE group, current smokers had lowest concentrations in late pregnancy. Non-smoking women in the PE group had the highest sFlt-1/PlGF ratio in early and late pregnancy. Smoking status did not affect the ratio in the non-PE group.
Cut-offs
The proportion of PE women whose sFlt-1/PlGF ratio was below the cut-off of 33 (rule-out according to the NICE guideline [Citation18]), and below the cut-off 38 was higher in the group of smoking PE women (). There were no differences in the percentages of smoking and non-smoking women exceeding the cut-offs of 85 (rule-in between 20 and 33 weeks six days according to the NICE guideline [Citation18]) or 110 (rule-in between 34 weeks 0 days and delivery according to the NICE guideline [Citation18]) when the sampling time was taken into account. In the non-PE women, there were women who exceeded both NICE rule-in cut-offs (85 and 110) (), but there were no differences in the number of women below or above the cut-offs between smokers and non-smokers in the non-PE group.
Table 3. Proportion of women below/above different cut-off values for soluble fms-like tyrosine kinase 1 (sFlt-1)/placental growth factor (PlGF) ratios.
Angiogenic markers and number of cigarettes smoked before pregnancy
The first trimester concentrations of sFlt-1, PlGF, sEng and the sFlt-1/PlGF ratio in pre-eclamptic and non-PE groups according to the number of cigarettes smoked daily before pregnancy are presented in Supplementary Figure 1. An association was found between the number of cigarettes smoked and concentrations of PlGF in pre-eclamptic women. Those PE women who smoked 16 or more cigarettes per day had higher concentrations of PlGF than non-smoker PE women (Supplementary Figure 1(B)). Furthermore, an association was found between the number of cigarettes smoked and the sFlt-1/PlGF ratio in both PE and non-PE women (Supplementary Figure 1(C)). In PE women, the more cigarettes smoked, the lower the sFlt1-1/PlGF ratio. Whereas, in non-PE women, the opposite association was observed. There was no association between sFlt-1 concentration and the number of cigarettes smoked (Supplementary Figure 1(A)), but among PE women, those who smoked ≥16 cigarettes per day had lower concentrations of sEng than non-smokers (Supplementary Figure 1(D)).
Angiogenic markers and number of cigarettes smoked during pregnancy
The first trimester concentrations of sFlt-1, PlGF, sEng and the sFlt1/PlGF ratio in pre-eclamptic and non-PE groups according to the number of cigarettes smoked during pregnancy are presented in Supplementary Figure 2. An association was found between the number of cigarettes smoked and concentrations of PlGF in both PE and non-PE women (Supplementary Figure 2(B)). Those non-PE women who smoked six or more cigarettes per day had higher concentrations of PlGF than non-smoker PE women (Supplementary Figure 2(B)) and those PE women who smoked 0–5 cigarettes per day had higher concentrations of PlGF than non-smoker PE women (Supplementary Figure 1(B)). In addition, an association was found between the number of cigarettes smoked and the sFlt-1/PlGF ratio in both PE and non-PE women (Supplementary Figure 2C): non-smokers had higher ratios than those PE women who smoked daily six or more cigarettes and those non-PE women who smoked 0–5 cigarettes.
Discussion
Serum concentrations of sFlt-1, sEng and sFlt-1/PlGF ratio levels are usually found to be higher and PlGF concentrations lower and, thus, anti-angiogenic in PE [Citation6,Citation10,Citation19]. An obstetrical paradox has been that maternal smoking appears to be protective for the development of PE. The biological mechanism behind this is largely unknown but it may relate to inhibitory effects of smoking by-products such as nicotine and carbon monoxide (CO) on the endothelial dysfunction [Citation20]. In this study, we compared the first and second/third trimester serum concentrations of sFlt-1, PlGF and their ratio in addition to sEng in PE and non-PE women according to their smoking status in the FINNPEC cohort. The overall interpretation of our results is that smoking appeared to have angiogenic effects in early pregnancy with reduced sEng and elevated PlGF concentrations. In the longitudinal analyses, current smokers in the PE group had elevated PlGF concentrations throughout pregnancy. Furthermore, they had decreased sFlt-1/PlGF pregnancy. These findings support the hypothesis that smoking may decrease the risk of PE by altering the angiogenic markers during pregnancy.
There is a robust body of literature that consistently has demonstrated a reduced risk of PE in women who smoke when they are pregnant [Citation3–5]. Accordingly in the whole FINNPEC study population, controls reported to smoke more before and during pregnancy than PE women [Citation17]. Although smoking appears to protect from PE, it should be noted that it is also associated with many adverse pregnancy outcomes including preterm labour, placental abruption and foetal growth restriction/low birth weight [Citation21,Citation22]. However, within this study, we were not able to detect association with smoking and gestational weeks at delivery or relative birth weight.
Previously, angiogenic markers have been shown to be expressed differentially in smoking and non-smoking women with PE. Lower sFlt-1 and higher PlGF concentrations have been reported in PE women who smoked compared to PE women who did not [Citation15,Citation16,Citation23–25]. In line with these findings, we showed that the effect of smoking in reducing the risk of PE in early pregnancy may be due to the increase in PlGF and the decrease in sEng concentration. As a novel observation, we also showed associations between number of cigarettes and these markers. Wikström et al. [Citation5] have previously found a dose-dependent risk for PE development. For pregnant women who smoked 1–9 cigarettes per day, the risk was reduced by 34% while those who smoked 10 or more cigarettes daily, reduced the risk by 50%. Interestingly, the risk reduction was observed only when the mother smoked throughout the whole pregnancy.
Data regarding sEng levels and smoking in PE are scarce. To our knowledge, this is the first study to show lower sEng concentrations at first trimester in PE mothers who smoke. Previously, Levine et al. [Citation7] have suggested that sEng concentrations may be lower among healthy pregnant smokers which was observed also in this study. In addition, Jeyabalan et al. [Citation23] have shown that sEng concentration was lower in smokers with multifoetal gestations and diabetes but not in PE.
Although there are no formal guidelines regarding the use of the sFlt-1/PlGF ratio, consensus statements have been developed by international experts on the clinical use of the Elecsys immunoassay sFlt-1/PlGF ratio [Citation18]. We retrospectively investigated the feasibility of these relatively recently established sFlt-1/PlGF cut-offs according to smoking status. The proportion of PE women whose ratio was below the cut-offs of 33 or 38 was higher in the group of PE women who smoked, when the sampling time was taken into account. This may suggest that the different sFlt-1/PlGF ratio cut-offs are needed for smokers. However, larger studies are needed to confirm these observations.
Strengths and limitations
Our study has several strengths. The diagnostic criteria for PE were well defined. Furthermore, detailed clinical information allowed us to accurately define the phenotypes. Detailed phenotyping enabled us to include various adjustments for maternal characteristics. Clinical covariates affecting angiogenic levels (parity, age, BMI, gestational weeks at sampling) were taken into account when aiming to define the role of smoking in PE. Furthermore, data on smoking were collected from two distinct sources which could reduce the possible bias in maternal report of smoking.
Our study also has certain limitations. The inter-individual variations in serum concentrations were relatively large and the sample size was small, especially when further dividing the study population into subcategories. Particularly, the number of current smokers was very low in the non-PE group and the inter-individual variations in serum concentrations were large. In addition, there was a very small number of samples available from the second trimester. We also lack biochemical confirmation of smoking status (e.g. cotinine concentrations). It has been postulated that products other than the nicotine in cigarettes might protect against PE [Citation5]. Wikström et al. [Citation5] assessed the effect of snuff use during pregnancy and it was not observed to reduce the risk for PE. This suggests that exposure to the combustible products of cigarette smoke, such as CO may offer the protective effect for PE. Accordingly, Cudmore et al. [Citation26] have reported that CO and CO-releasing molecules lower sFlt1 and sEng production in endothelial cells and placental organ cultures, thus potentially providing a molecular explanation for the lower circulating levels of sFlt1 and sEng noted in smokers during pregnancy. This study, however, did not address the biological mechanisms by which smoking may affect angiogenic factors in PE women, and further studies are warranted to define the pathways.
Conclusions
We provide further evidence that the protective effect of smoking in reducing the risk of PE may be due to changes in angiogenic marker profiles. Smoking appeared to have pro-angiogenic effects in early pregnancy with reduced sEng and elevated PlGF concentrations in both normal and PE pregnancies. During pregnancy, smoking exerted an angiogenic effect in sFlt-1/PlGF ratio in PE pregnancies, and thus may complicate its use as a prognostic and diagnostic marker.
Supplementary_figure_2_revision_190417.tif
Download TIFF Image (2.5 MB)Supplementary_figure_1_revision_190417.tif
Download TIFF Image (2.8 MB)Acknowledgements
We are indebted to all the FINNPEC study participants. We appreciate the contribution of the present or former members of the FINNPEC Study Group: Tia Aalto-Viljakainen, Jenni Heikkinen-Eloranta, Reija Hietala, Miira Klemetti, Susanna Sainio and Terhi Saisto, Eeva Ekholm and Kaarin Mäkikallio-Anttila (Turku University Central Hospital), Marja Vääräsmäki (Oulu University Hospital), Leena Georgiadis and Leea Keski-Nisula (Kuopio University Hospital), Jukka Uotila (Tampere University Hospital), Sanna Heino, Tea Kaartokallio, Inkeri Lokki and Marja Vilkki (University of Helsinki). The expert technical assistance of Eija Kortelainen, Susanna Mehtälä, Hanna Nurmi, Aija Lähdesmäki, Satu Leminen and Christina Salmén is gratefully acknowledged.
The FINNPEC core investigator group
Hannele Laivuori (PI)
Medical and Clinical Genetics, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
Obstetrics and Gynecology, University of Helsinki and Helsinki University Hospital, Helsinki Finland
Institute for Molecular Medicine Finland/HiLIFE, University of Helsinki, Helsinki, Finland
Seppo Heinonen
Obstetrics and Gynecology, University of Helsinki and Helsinki University Hospital, Helsinki Finland
Eero Kajantie
Chronic Disease Prevention Unit, National Institute for Health and Welfare, Helsinki, Finland
Children’s Hospital, Helsinki University Hospital and University of Helsinki, Helsinki, Finland
PEDEGO Research Unit, MRC Oulu, Oulu University Hospital and University of Oulu, Oulu, Finland
Juha Kere
Department of Biosciences and Nutrition, and Science for Life Laboratory, Karolinska Institutet, Stockholm, Sweden
Molecular Neurology Research Program, University of Helsinki, Helsinki, Finland
Folkhälsan Institute of Genetics, Helsinki, Finland
Katja Kivinen
Division of Cardiovascular Medicine, University of Cambridge, Cambridge, UK
Anneli Pouta
PEDEGO Research Unit, MRC Oulu, Oulu University Hospital and University of Oulu, Oulu, Finland
Department of Government Services, National Institute for Health and Welfare, Helsinki, Finland
Disclosure statement
The authors state that there are no conflicts of interest.
Additional information
Funding
References
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131.
- Eiland E, Nzerue C, Faulkner M. Preeclampsia 2012. J Pregnancy. 2012;2012:586578.
- Wei J, Liu CX, Gong TT, et al. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget. 2015;6:43667–43678.
- England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci. 2007;12:2471–2483.
- Wikström AK, Stephansson O, Cnattingius S. Tobacco use during pregnancy and preeclampsia risk: effects of cigarette smoking and snuff. Hypertension. 2010;55:1254–1259.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683.
- Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005.
- Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31:33–46.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1–58.e8.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352.
- Rana S, Karumanchi SA, Levine RJ, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–142.
- Stepan H, Hund M, Gencay M, et al. A comparison of the diagnostic utility of the sFlt-1/PlGF ratio versus PlGF alone for the detection of preeclampsia/HELLP syndrome. Hypertens Pregnancy. 2016;35:295–305.
- Powers RW, Roberts JM, Cooper KM, et al. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. Am J Obstet Gynecol. 2005;193:185–191.
- Jeyabalan A, Powers RW, Durica AR, et al. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am J Hypertens. 2008;21:943–947.
- Mijal RS, Holzman CB, Rana S, et al. Midpregnancy levels of angiogenic markers in relation to maternal characteristics. Am J Obstet Gynecol. 2011;204:244.e1–212.
- Jääskeläinen T, Heinonen S, Kajantie E, et al. Cohort profile: the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC). BMJ Open. 2016;6:e013148.
- National Institute for Health and Care Excellence (NICE). PlGF-based testing to help diagnose suspected pre-eclampsia (Triage PlGF test, Elecsys immunoassay sFlt-1/PlGF ratio, DELFIA Xpress PlGF 1-2-3 test, and BRAHMS sFlt-1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio): NICE diagnostics guidance [DG23]; 2016.
- Widmer M, Villar J, Benigni A, et al. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007;109:168–180.
- Karumanchi A, Levine RJ. How does smoking reduce the risk of preeclampsia? Hypertension. 2010;55:1100–1101.
- Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182:465–472.
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tobacco Res. 2004;6(Suppl.2):S125–S140.
- Jeyabalan A, Powers RW, Clifton RG, et al. Effect of smoking on circulating angiogenic factors in high risk pregnancies. PLoS One. 2010;5:e13270.
- Llurba E, Sánchez O, Domínguez C, et al. Smoking during pregnancy: changes in mid-gestation angiogenic factors in women at risk of developing preeclampsia according to uterine artery Doppler findings. Hypertens Pregnancy. 2013;32:50–59.
- Kahn SR, Almeida ND, McNamara H, et al. Smoking in preeclamptic women is associated with higher birthweight for gestational age and lower soluble fms-like tyrosine kinase-1 levels: a nested case control study. BMC Pregnancy Childbirth. 2011;11:91.
- Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797.