Abstract
Background: Atrial fibrillation (AF)-European guidelines suggest the use of biomarkers to stratify patients for stroke and bleeding risks. We investigated if a multibiomarker strategy improved the predictive performance of CHA2DS2-VASc and HAS-BLED in anticoagulated AF patients.
Methods: We included consecutive patients stabilized for six months on vitamin K antagonists (INRs 2.0–3.0). High sensitivity troponin T, NT-proBNP, interleukin-6, von Willebrand factor concentrations and glomerular filtration rate (eGFR; using MDRD-4 formula) were quantified at baseline. Time in therapeutic range (TTR) was recorded at six months after inclusion. Patients were follow-up during a median of 2375 (IQR 1564–2887) days and all adverse events were recorded.
Results: In 1361 patients, adding four blood biomarkers, TTR and MDRD-eGFR, the predictive value of CHA2DS2-VASc increased significantly by c-index (0.63 vs. 0.65; p = .030) and IDI (0.85%; p < .001), but not by NRI (−2.82%; p < .001). The predictive value of HAS-BLED increased up to 1.34% by IDI (p < .001). Nevertheless, the overall predictive value remains modest (c-indexes approximately 0.65) and decision curve analyses found lower net benefit compared with the originals scores.
Conclusions: Addition of biomarkers enhanced the predictive value of CHA2DS2-VASc and HAS-BLED, although the overall improvement was modest and the added predictive advantage over original scores was marginal.
Recent atrial fibrillation (AF)-European guidelines for the first time suggest the use of biomarkers to stratify patients for stroke and bleeding risks, but their usefulness in real world for risk stratification is still questionable.
In this cohort study involving 1361 AF patients optimally anticoagulated with vitamin K antagonists, adding high sensitivity troponin T, N-terminal pro-B-type natriuretic peptide, interleukin 6, von Willebrand factor, glomerular filtration rate (by the MDRD-4 formula) and time in therapeutic range, increased the predictive value of CHA2DS2-VASc for cardiovascular events, but not the predictive value of HAS-BLED for major bleeding. Reclassification analyses did not show improvement adding multiple biomarkers.
Despite the improvement observed, the added predictive advantage is marginal and the clinical usefulness and net benefit over current clinical scores is lower.
Key Messages
Introduction
Atrial fibrillation (AF) is associated with high morbidity and mortality, with an increased risk of stroke and thromboembolism [Citation1]. Oral anticoagulation (OAC) is highly effective reducing the risk of stroke/systemic embolism (by 64%) and all-cause mortality (by 26%), compared to placebo/control [Citation2].
To aid decision-making for thromboprophylaxis, several clinical risk stratification schemes have been developed. Currently, the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled] vascular disease, age 65–74 years and sex category [female]) emphasizes a risk factor-based approach [Citation3], and has been proposed by guidelines to aid decision-making for OAC [Citation4,Citation5]. Currently used clinical risk scores have only modest capability in predicting those at “high risk” for thromboembolic events [Citation6]. Hence, there has been interest into the incorporation of biomarkers to improve the prediction power of these clinical risk scores, thus refining risk stratification [Citation7–9]. Indeed, we have previously demonstrated how different (single) biomarkers could predict adverse events and may provide complementary prognostic information to an established clinical risk score [Citation9].
Although guidelines recommend the use of the CHA2DS2-VASc score [Citation4,Citation5], its value in predicting thromboembolism after initiating anticoagulation is controversial. Importantly, adverse thromboembolic events in anticoagulated AF patients still remain high [Citation5].
Apart from the reduction in thromboembolism, OAC increases the risk of bleeding. The HAS-BLED score incorporates the more common bleeding risk factors in AF patients, and particularly draws attention to the reversible bleeding risk factors (e.g. uncontrolled hypertension (H), labile INRs (L), concomitant use of NSAIDs, bleeding predisposition or excess alcohol (D), etc.) to be addressed by the responsible clinician during the follow-up [Citation10]. A “high risk” HAS-BLED score (i.e. ≥3) is not a reason to withhold OAC, but such patients should be “flagged-up” for more careful review and follow-up [Citation11]. Nonetheless, stroke and bleeding risks closely track each other [Citation12].
Although current guidelines encourage the use of clinical scores for stroke and bleeding risk prediction, their predictive value is modest. Prior studies examining the impact of biomarkers in enhancing stroke and bleeding risk prediction have been performed in highly selected anticoagulated trial cohorts. Based on these studies, the recently published European guidelines on the management of AF recommended that the use of (multiple) biomarkers for assessing thrombotic and haemorrhagic risk may be considered (Grade IIb, level of evidence B) [Citation5].
Enhancement of stroke and bleeding risk prediction using multiple biomarkers requires additional studies in “real world” cohorts. In the present study, we tested a multi-biomarker strategy, exploring different facets of underlying pathophysiological mechanisms related to AF (stroke and systemic embolism), bleeding and all cause death in a cohort of anticoagulated AF patients.
Methods
Subjects, clinical data collection and follow-up
From 1 May 2007 to 1 December 2007, consecutive patients with permanent or paroxysmal AF who were taking vitamin K antagonist (VKA) were recruited from our outpatient anticoagulation clinic in the Universitary Hospital Morales Meseguer (Murcia, south-east of Spain). In order to homogenize the study sample, all patients had good anticoagulation control and consistently achieved an INR between 2.0 and 3.0 during at least the previous six months of clinic visits (thus, time in therapeutic range [TTR] of 100%). All were anticoagulated with acenocoumarol. Apart from baseline homogeneity, the six preceding months of good anticoagulation control would ensure that the impact of the biomarkers could not be related to poor anticoagulation control, enabling us to investigate the “real” effect of biomarkers.
Patients with prosthetic heart valves, rheumatic AF, acute coronary syndrome, stroke (ischaemic or embolic), potentially unstable chest pain or any haemodynamic instability, as well as patients who had hospital admission or surgical intervention in the preceding six months were excluded from the study. On entering the study, a complete medical history was recorded for each patient.
The CHA2DS2-VASc stroke risk score was recorded at baseline. Similarly, the HAS-BLED bleeding risk score was calculated as a measure of baseline bleeding risk, as the result of adding 1 point to hypertension, abnormal renal and liver function (1 point each), stroke, bleeding history or predisposition, labile INR, old age (≥65 years) and use of drugs and alcohol concomitantly (1 point for each). The TTR at 6 months after entry was calculated using the method of Rosendaal [Citation13].
The median follow-up was 2375 (IQR 1566-2887) days and the information was obtained from visits to the anticoagulation clinic, the hospital electronic medical records system or, when unavailable, by telephone interview. The last follow-up visit was carried out on 26 January 2016 and no patient was lost.
The primary endpoints were adverse cardiovascular events (the composite of stroke/systemic embolism, acute coronary syndrome, acute heart failure and cardiovascular death), major bleeding events and all-cause deaths. Stroke was also analysed separately as primary endpoint. For composite of cardiovascular events, cardiovascular death was defined as a death caused by sudden death, progressive congestive heart failure, fatal MI or procedure-related. Major bleeding events were assessed by the 2005 International Society on Thrombosis and Haemostasis criteria [Citation14]. The definitions were as follows: fatal or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome or bleeding causing a fall in haemoglobin level of ≥20 g/L (1.24 mmol/L) or leading to transfusion of ≥2 units of whole blood or red cells. All adverse events were identified, confirmed and recorded by the investigators.
The study protocol was approved by the Ethics Committee from University Hospital Morales Meseguer and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patients gave informed consent to participation in the study.
Renal function, liver function and alcohol use
At study entry, serum creatinine levels were recorded and estimated glomerular filtration rate (eGFR) was calculated using the simplified Modification of Diet in Renal Disease (MDRD)-4 equation: eGFR (ml/min/1.73 m2) = 186 × [serum creatinine (mg/dl)]−1.154× (age)−0.203× (0.742 if female) × (1.21 if black) [Citation15,Citation16]. Thus, renal impairment was defined as a glomerular filtration rate below 60 ml/min/1.73 m2. Liver function was defined as abnormal when the presence of cirrhosis or bilirubin more than double over the normal range with AST/ALT/AP more than triple over the normal range was documented in the hospital electronic medical records or detected in the daily practice blood samples. Alcohol use was defined as excessive if eight or more alcohol drinks (units) were ingested a week.
Blood samples and laboratory analysis
Blood samples were drawn at baseline atraumatically and without stasis into syringes preloaded with trisodium citrate (0.011 M). Platelet-poor plasma fractions were obtained by centrifugation at 4 °C for 20 min at 2200 × g. Aliquots were stored at −80 °C to allow batch analysis.
Von Willebrand factor (vWF) levels were assessed in an automated coagulometer ACL top 3G, Hemosil von Willebrand factor, (IL instruments, Milan, Italy). The inter- and intra- assay variation coefficient was 1.4% and the lower limit of detection was 2.2 UI/dl. High sensitivity troponin T (hsTnT), high sensitivity interleukin-6 (hsIL6) and N-terminal fragment B-type natriuretic peptide (NT-proBNP) levels were assessed by electrochemiluminescence in an automated analyser (Cobas e 601, Roche Diagnostica, Mannheim, Germany). The intra-assay coefficient of variation was 5.6% and the lower limits of detection of these assays were 3.0 pg/ml for hsTnT, 1.5 pg/ml for hsIL6 and 5.0 pg/ml for NT-proBNP.
Statistical analysis
Continuous variables are presented as a mean ± SD or median (interquartile range, IQR), as appropriate and categorical variables as a percentage. Area under the curve (AUC, a measure of the c-index) analyses were generated to test the predictive discrimination of each biomarker to identify association with adverse events during follow-up. The value with the best sensitivity and specificity for each adverse event was chosen as cut-off point and if the AUC did not result in a significant point, we use the 4th quartile value.
To relate the entire panel of biomarkers to the incidence of each clinical event, we used a multivariable Cox regression model adjusted for CHA2DS2-VASc or HAS-BLED scores. We included in multivariate analysis those biomarkers that showed a p value <.150 in the univariate analysis.
We also estimated the AUC for multivariable models incorporating biomarkers plus CHA2DS2-VASc or HAS-BLED scores and compared them with the original models including only the CHA2DS2-VASc or HAS-BLED scores by the method of DeLong [Citation17]. Net reclassification improvement (NRI) and integrated discriminatory improvement (IDI) were performed according to the methods described by Pencina et al [Citation18]. NRI and IDI were designed to assess the risk refinement (enhanced risk differentiation) provided by one or more new markers. IDI represents the average improvement of a new model in relation to the prediction of true events, discounting any worsening by the prediction of false events. NRI has two components, subjects without events and subjects with events. If subjects without events are correctly reclassified into lower-risk category, NRI demonstrate positive reclassification. If subjects with events are correctly reclassified into higher risk category, NRI also demonstrates positive re-classification. Conversely, if subjects with events are incorrectly reclassified into lower risk category, NRI demonstrate negative re-classification; likewise, if subjects without events are incorrectly reclassified into higher-risk category, the NRI demonstrate negative re-classification.
Goodness-of-fit of the new models were evaluated using the Hosmer–Lemeshow test.
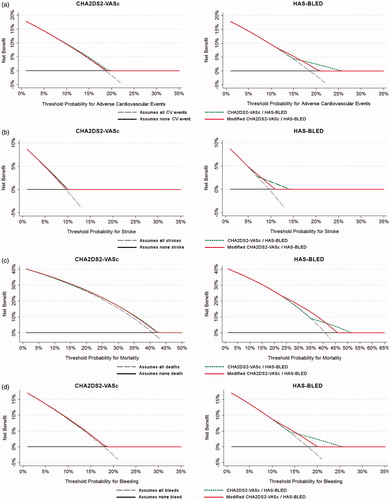
Clinical usefulness and net benefit of the modified CHA2DS2-VASc and HAS-BLED scores were estimated according with decision curve analyses (DCAs) [Citation19,Citation20], in order to identify patients who will have any of the adverse events evaluated, based on the predictions of the modified risks scores when are compared with the originals. The DCA shows the clinical usefulness of each new model based on a continuum of potential thresholds for adverse events (x axis) and the net benefit of using the model to stratify patients at risk (y axis) relative to assuming that no patient will have an adverse event. The basic interpretation of DCA is that the strategy with the highest net benefit at a particular threshold probability has the highest clinical value. In this study, the prediction models are represented by dashed green lines (original scores) and red lines (modified scores). Those models that are the farthest away from the slanted dashed grey line (i.e. assume all adverse events) and the horizontal black line (i.e. assume none adverse event) demonstrate the higher net clinical benefit.
A p value <.05 was accepted as statistically significant. Statistical analyses were performed using SPSS 21.0 (SPSS, Inc., Chicago, IL), MedCalc v. 16.4.3 (MedCalc Software bvba, Ostend, Belgium) and STATA v. 12.0 (Stata Corp., College Station, TX) for Windows.
Results
We studied 1361 patients (49% male; median age 76 years [IQR 71-81]) whose clinical characteristics are summarised in . This accounted for approximately 14% of the total number of patients anticoagulated in our unit, and the 33% of all patients with AF from the clinic. The median CHA2DS2-VASc score was 4 (3–5) and 94% had a CHA2DS2-VASc score ≥2. Median follow-up was 2375 (1566-2887) days, and during this period, 274 patients (3.10%/year) had an adverse cardiovascular event, 130 (1.47%/year) patients had an ischaemic stroke, 250 (2.83%/year) suffered from a major haemorrhagic episode and 551 (6.23%/year) died.
Table 1. Patients characteristics.
Cox regression analyses for the composite of adverse cardiovascular events, major bleeding and death are summarized in Supplementary Tables I, II, III and IV. For stroke, only vWF remained significant (Supplementary Table II); while for mortality, all biomarkers were significant after adjusting for CHA2DS2-VASc score (Supplementary Table III). After adjusting for HAS-BLED score, only vWF and MDRD-4 remained significantly associated with bleeding (Supplementary Table IV).
shows the predictive value of clinical risk scores for all endpoints and the incremental predictive value after adding the analysed biomarkers. Prediction of the composite cardiovascular events showed an improvement in c-indexes after adding all described biomarkers, for both CHA2DS2-VASc and HAS-BLED scores (0.63 vs. 0.65 and 0.60 vs. 0.64, respectively, both p < .05). The prediction of mortality was improved after adding the four biomarker panel, eGFR MDRD-4 and TTR values respectively to CHA2DS2-VASc and HAS-BLED.
Table 2. Additive value of adding von Willebrand factor, high sensitivity troponin T, N-terminal fragment B-type natriuretic peptide, high sensitivity interleukin-6, time in therapeutic range and modification of diet in renal disease equation for prognosis in AF (n = 1361).
The IDI demonstrated a modest gain in sensitivity (less than 1.5%) of the new CHA2DS2-VASc model (i.e. with biomarkers added) for predicting all events (p < .05). For predicting mortality, the improvement was higher (3.62%, p < .001). For HAS-BLED, there was an increment in predictive value for all events, including bleeding (1.34%) and mortality (5.0%) (both p < .001).
Using the NRI for the composite of adverse cardiovascular events and stroke, higher proportion of patients were incorrectly reclassified into risk categories by the new CHA2DS2-VASc model (i.e. with biomarkers added). Thus, there was a significant negative reclassification (−2.82% and −2.56%, respectively, both p < .05) with the modified CHA2DS2-VASc compared with the original CHA2DS2-VASc. Despite the small improvement of sensitivity, there was also a non-significant negative reclassification for all events with the new HAS-BLED model (i.e. with biomarkers added) when compared with the original clinical score (). Given the p values in the Hosmer–Lemeshow test, the new predictive models were properly calibrated (Supplementary Table V).
DCA graphically demonstrates a lower net benefit and clinical usefulness of the modified CHA2DS2-VASc and HAS-BLED scores (i.e. with biomarkers added; red lines) compared with the original CHA2DS2-VASc and HAS-BLED clinical scores (dashed green lines), since none of the modified models are farthest away from the slanted dashed grey line and the horizontal black line in comparison with the original models ().
Figure 1. Decision curve analyses for the original and modified CHA2DS2-VASc and HAS-BLED risk scores (adding von Willebrand factor, high sensitivity troponin, N-terminal fragment B-type natriuretic peptide, high sensitivity interleukin-6, time in therapeutic range and Modification of Diet in Renal Disease equation for the estimation of glomerular filtration rate). (a) Composite of adverse cardiovascular events. (b) Stroke. (c) All-cause mortality. (d) Major bleeding.
Discussion
In this study, we show how a multi-biomarker strategy enhances the predictive value of CHA2DS2-VASc and HAS-BLED scores in stable AF patients well controlled by VKA treatment, although overall predictive value remained modest (c-indexes approximately 0.65) and the added predictive advantage over clinical risk scores was marginal. Indeed, our DCA found a lower net benefit of the modified scores (i.e. with biomarkers added) compared with the original CHA2DS2-VASc and HAS-BLED clinical scores.
The use of biomarkers for improving prognostics tools in different cardiovascular diseases has gained much interest in the last decade. In the biomarker substudies to recent large phase-III stroke prevention trials in AF, several biomarkers (e.g. troponins or NT-proBNP) were significantly predictive for adverse events [Citation21–23]. Nonetheless, the study of prothrombotic biomarkers in AF patients, especially in patients taking oral anticoagulation, have reported conflicting results [Citation24].
Currently used clinical risk scores have modest predictive capability for thromboembolic events and mortality [Citation25]. In the present “real world” study, we have clearly shown how different biomarkers, when assessed individually can significantly improve the predictive value of the guideline-recommended clinical risk scores, CHA2DS2-VASc and HAS-BLED, over a long follow-up period, similar to the reported biomarker substudies of the randomized trials.
In clinical trials, patients are often carefully selected, whereas AF patients in “real life” clinical practice tend to be older, with associated comorbidities and polypharmacy [Citation26]. These patient-centred factors that may make accurate estimation of stroke and bleeding risk more difficult. While we have previously shown the prognostic value of each biomarkers separately, we have now investigated in a large “real world” cohort of anticoagulated AF patients that vWF (an established biomarker of endothelial damage/dysfunction) was an independent predictor for thrombotic, bleeding events and death; also, both hsTnT and hsIL6 levels provided complementary prognostic information to the clinical risk scores for the prediction of long-term cardiovascular events and death; and NT-proBNP also improved the prediction of stroke/systemic embolism and mortality. All these biomarkers have been assessed individually, so the implementation of a multimarker strategy would clearly improve risk stratification.
What about the long term follow-up?
In the present study, we have reanalysed the role of vWF in prognosis, after more than six years of follow-up. Indeed, vWF is a simple prognostic biomarker in anticoagulated AF patients and, while the addition of vWF levels to the CHA2DS2-VASc and the HAS-BLED scores statistically improved prediction for some endpoints, the absolute changes and clinical value or impact on decision-making was minimal [Citation27]. Indeed, vWF was the only biomarker independently associated with all adverse events, whereas the rest of biomarkers were only predictive for death, with the exception of hsTnT, which was also predictive for the composite cardiovascular events endpoint. Indeed, many biomarkers can be simultaneously predictive of thromboembolism, bleeding, heart failure, myocardial infarction and death, which may cause some confusion to clinicians over which endpoint to focus on, when deciding on interventions such as OAC. This is unfortunately also true for several risk factors in the atrial fibrillation risk scores, and not only a concern for biomarkers. Thus, for example, age, hypertension or previous stroke are both, risk factors for stroke and bleeding [Citation3,Citation10].
The recent published European guidelines for the management of AF [Citation5] suggested that (multiple) biomarkers may be considered for the prediction of stroke and bleeding adverse events in anticoagulated AF patients, based on data collected from highly selected clinical trial patients. However, this approach has never been previously validated in “real world” patients. Also, the possibility of risk stratification in “relatively low risk” patients (CHA2DS2-VASc: 0-1) has not been evaluated.
While it is clear from our study is that biomarkers, single or multiple, may be helpful to refine assessment of AF risk, at least statistically, but it remains uncertain if these biomarkers bring new, different pathophysiological factors into risk prediction in AF patients or whether they simply are a measure of disease severity (or “disease burden”). AF clinical risk factors are indicators of a biological process that relates to AF, while measured “biomarkers” (whether blood tests, urine markers, imaging, etc.) are related to an AF-causing process (or consequence), but do not necessarily contribute by themselves to the biology of AF [Citation28]. Indeed, AF may well be a surrogate marker of vascular damage and subsequently atherothrombosis. Of note, we have observed in a “real world” cohort of anticoagulated outpatients with AF, a high incidence of cardiovascular events and mortality [Citation25].
Adding multiple biomarkers may help improve prediction of “high risk” patients, since, for example, von Willebrand factor, IL-6, troponin T and NT-proBNP could show increased levels. All these biomarkers could also be elevated in haemodynamically unstable AF patients. However, the approach we present in this study included patients haemodynamically stable, with the absence of adverse events in the previous six months, and with excellent anticoagulation control (TTR 100%). Although many AF patients would not necessarily be under the same conditions, this approach gives us the opportunity of assessing the real impact of common biomarkers on prognosis, excluding major confounding from biomarker changes in relation to recent adverse events or poor anticoagulation control. Thus, future studies investigating improvements in risk prediction using multiple biomarkers including both stable and non-stable patients may enhance the generalizability, but the cost-effectiveness of routinely checking biomarkers in AF patients is limited and questionable for everyday practice, adding substantial complexity, expense and lack of practicality for everyday decision-making [Citation29].
Limitations
Some selection bias cannot be excluded as our patients were clinically stable at entry, and thus unstable AF patients who are more prone to have adverse events were excluded. Similarly, we selected “anticoagulation experienced” patients with good anticoagulation control during the six preceding months before study entry (i.e. INR 2.0–3.0 during the previous six months), to ensure that the impact of the biomarkers could not be related to suboptimal anticoagulation control or recent adverse events. As we emphasized, the inclusion of such “non-stable” patients could potentially lead to even greater biases and confounding, and misleading results when interpreting the impact of biomarkers on risk scores, which was our principal study objective.
Nevertheless, the long follow-up period and the standard care received makes our “real world” population different from selected clinical trial cohorts where biomarkers have been investigated. Unfortunately, we do not have data on TTR during follow-up, which could have some influence on prognosis [Citation30]. We also selected biomarkers on the basis of our previous studies, and we acknowledge that numerous other biomarkers that were not tested, which might have (or have not) provided additional information. Cardiovascular mortality was only recorded if we were sure of cause of death, and this could be lead to an underestimation of this adverse event. Of note, our study was performed in an outpatient anticoagulation clinic, where all patients were derived from other clinicians to start or manage anticoagulation. Therefore, we do not have data on non-OAC patients, nor their CHA2DS2-VASc score. Also, most of our patients fulfilled criteria for OAC (i.e. CHA2DS2-VASc ≥2) and only 6% of our cohort had CHA2DS2-VASc 0 or 1. Thus, we did not perform a particular analysis on low stroke risk patients. Finally, our study was performed with patients taking VKA and not direct oral anticoagulants, thus our results cannot be translated so such a population.
In conclusion, addition of multiple biomarkers significantly enhanced the predictive value of CHA2DS2-VASc and HAS-BLED scores in well anticoagulated and stable AF patients. However, the overall predictive value remained modest and the added predictive advantage over current clinical scores was marginal. This marginal improvement has to be balanced against the loss of simplicity and practicality, for “quick” everyday clinical use given that decision-making would require availability of laboratory results.
Supplemental_Material_revised_R2.docx
Download MS Word (29.2 KB)Disclosure statement
VR has received funding for consultancy and lecturing from Bristol-Myers-Squibb, Bayer and Boehringer-Ingelheim. FM has received funding for research, consultancy and lecturing from Abbott, Boston Scientifics, Bayer, Astra Zeneca, Daiichi-Sankyo, BMS/Pfizer and Boehringer-Ingelheim. GYHL has received funding for consultancy from Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife and Daiichi-Sankyo; and for lecturing from Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche and Daiichi-Sankyo. No fees are received personally.
None declared in relation to this manuscript for other authors.
Additional information
Funding
References
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272.
- Camm AJ, Lip GY, De Caterina R, et al. Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962.
- Lip GY. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J. 2013;34:1041–1049.
- Hijazi Z, Oldgren J, Siegbahn A, et al. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–1480.
- Kornej J, Apostolakis S, Bollmann A, et al. The emerging role of biomarkers in atrial fibrillation. Can J Cardiol. 2013;29:1181–1193.
- Vilchez JA, Roldan V, Hernandez-Romero D, et al. Biomarkers in atrial fibrillation: an overview. Int J Clin Pract. 2014;68:434–443.
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100.
- Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a 'real world' nationwide cohort study. Thromb Haemost. 2011;106:739–749.
- Gallego P, Roldan V, Torregrosa JM, et al. Relation of the HAS-BLED bleeding risk score to major bleeding, cardiovascular events, and mortality in anticoagulated patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:312–318.
- Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239.
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694.
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254.
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845.
- Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172.
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574.
- Vickers AJ, Cronin AM, Elkin EB, et al. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53.
- Hijazi Z, Wallentin L, Siegbahn A, et al. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol. 2013;61:2274–2284.
- Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation. 2012;125:1605–1616.
- Ruff CT, Giugliano RP, Braunwald E, et al. Cardiovascular biomarker score and clinical outcomes in patients with atrial fibrillation: a subanalysis of the ENGAGE AF-TIMI 48 randomized clinical trial. JAMA Cardiol. 2016;1:999–1006.
- Danese E, Montagnana M, Cervellin G, et al. Hypercoagulability, D-dimer and atrial fibrillation: an overview of biological and clinical evidence. Ann Med. 2014;46:364–371.
- Jover E, Roldan V, Gallego P, et al. Predictive value of the CHA2DS2-VASc score in atrial fibrillation patients at high risk for stroke despite oral anticoagulation. Rev Esp Cardiol (Engl Ed). 2012;65:627–633.
- Freedman B, Lip GY. “Unreal world; or real world; data in oral anticoagulant treatment of atrial fibrillation”. Thromb Haemost. 2016;116:587–589.
- García-Fernández A, Roldán V, Rivera-Caravaca JM, et al. Does von Willebrand factor improve the predictive ability of current risk stratification scores in patients with atrial fibrillation? Sci Rep. 2017;7:41565.
- Kirchhof P, Lip GY, Van Gelder IC, et al. Comprehensive risk reduction in patients with atrial fibrillation: Emerging diagnostic and therapeutic options. Executive summary of the report from the 3rd AFNET/EHRA consensus conference. Thromb Haemost. 2011;106:1012–1019.
- Szymanski FM, Lip GY, Filipiak KJ, et al. Stroke risk factors beyond the CHA(2)DS(2)-VASc Score: can we improve our identification of “High Stroke Risk” Patients With Atrial Fibrillation? Am J Cardiol. 2015;116:1781–1788.
- Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:84–91.
