Abstract
Background: The association between otitis media and vestibular symptoms has been hypothesized in the past. Thus, in this study, we aimed to critically analyze (based in a systematic review of the literature) whether patients who have otitis media are at greater risk of developing vestibular impairment or not.
Methods: We performed a systematic review of the literature and identified potentially relevant articles reporting vestibular symptoms and results of vestibular function tests in patients with otitis media through searches of the PubMED, Web of Science, Scopus, and Google Scholar databases. The quality of the final set of records was assessed using the “Newcaste–Ottawa Scale”.
Results: Of the 2334 records searched, 43 met our inclusion and exclusion criteria, and those included 2250 patients. The records comprised 20 longitudinal studies, 21 cross-sectional studies, and 2 case reports. Regarding the type of otitis media studied, 25 examined vestibular impairment in otitis media with effusion, 6 acute otitis media, and 12 chronic otitis media. Results of anamnesis, clinical exams, and several vestibular function tests are reported and critically discussed.
Conclusion: Most studies evaluating the association between otitis media and vestibular symptoms have potential methodological flaws. Clinical evidence suggests that patients with otitis media have increased chances for having vestibular symptoms, delayed acquisition of developmental milestones, and abnormalities in several vestibular function tests as compared with controls. Future studies with rigorous methodology aiming to assess the clinical significance (and prognostic factors) of the association between otitis media and vestibular impairment are warranted.
Several studies demonstrated long-term sequelae secondary to otitis media. However, the evidence supporting those assumptions are based in low-quality evidence. Thus, better structured studies are warranted to better understand the clinical relevance of such association.
Key message
Introduction
Otitis media is a disease with a high incidence, especially in young children. Worldwide, over 80% will be diagnosed at least once with acute otitis media before the age of 3 [Citation1], and 40% will have six or more recurrences before the age of 7 [Citation2]. Despite correct prevention and treatment, the incidence of complications of otitis media is still high, especially in underdeveloped countries. In the United States, $2.8 billion are spent every year in treating otitis media and complications [Citation3]. Several long-term sequelae such as sensorineural hearing loss, tinnitus, learning disabilities, and developmental delays have been extensively demonstrated [Citation4–6].
Recently, studies demonstrated the possibility of vestibular impairment as result of otitis media [Citation4,Citation7,Citation8]. It has been demonstrated that over 40% of patients who have chronic otitis media (COM) experience dizziness or vertigo through the course of disease [Citation7–9], and studies reported abnormal results in rotational chair, caloric, and vestibular-evoked myogenic potential (VEMP) tests [Citation7–10]. However, that the tests are negatively influenced by the presence of middle-ear pathologic lesions (tympanic membrane perforation, fibrosis, effusion, cholesteatoma) [Citation11] and/or conductive hearing loss [Citation8,Citation9,Citation12,Citation13] raises questions about whether findings actually demonstrate the presence of vestibular impairment or if they are the result of technical artifacts.
The objective of this study is to critically analyze (based in a systematic review of the literature) whether patients who have otitis media are at greater risk of developing peripheral vestibular impairment or not.
Material and methods
The protocol for this systematic review was designed in accordance with the PRISMA guidelines [Citation14]. We performed several literature searches in the PubMed, Scopus, and Web of Science databases from August 2017 to 11 November 2017, using relevant keywords and medical subject heading terms. Details of the database-specific search strategies are available in Supplementary Table S1 of the Supplementary material. We did not apply filters to the search. Results of all electronic searches were exported to Zotero v. 5.0.34.2 (Roy Rosenzweig Center for History and New Media, Fairfax, VA). Duplicates were excluded.
Eligibility criteria
We selected studies that reported clinical information and/or results of vestibular function tests in patients with otitis media. Considering the influence of the vestibular system in the development of co-ordination and fine and gross motor skills in children, we included studies reporting evaluation of those skills in patients with otitis media as well [Citation12,Citation13]. We excluded studies that (Citation1) did not have an available abstract; (Citation2) were review articles or letters to the editor; (Citation3) did not clearly define clinical evaluation methods and outcomes; (Citation4) included patients who had been subjected to ear surgery (except insertion of ventilation tubes); (Citation5) included patients with peri-lymphatic fistula; and (Citation6) histopathologic analyses of human temporal bones.
Study selection and data extraction
Two investigators independently performed the database searches and screened titles and abstracts for initial selection. We obtained and read full texts of potentially eligible articles. We assessed the quality of the articles using the guidelines of the “Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses” [Citation15,Citation16] ( and ; Supplementary Tables S2, S3, and S4). We followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement guidelines.
Table 1. Quality assessment of the records and potential biases detected in the longitudinal studies.
Table 2. Quality assessment of the records and potential biases detected in the cross-sectional studies.
From the selected articles, we extracted the following set of variables: (a) journal of publication and year, (b) country of origin, (c) setting, (d) design, (e) age group, (f) type of otitis media (effusion, acute, or chronic), (g) vestibular evaluation and testing, (h) intervention (if any), (i) results, and (j) conclusions. For longitudinal studies, the baseline data (“before” treatment or intervention) was collected and pooled with the data gathered from the cross-sectional studies. The results obtained after treatment or intervention were analyzed separately, and compared with the baseline results and/or with controls, when applicable. Data were organized in a workbook and built in the Microsoft Excel software v.15.40 (Microsoft Co., Redmond, WA).
Data analysis
Considering the heterogeneity of clinical presentations, age groups, and testing for peripheral vestibular symptoms, data pooling for meta-analysis was not possible. Thus, we performed a qualitative synthesis to evaluate the current evidence for vestibular impairment secondary to otitis media and the clinical value of clinical, laboratory, and vestibular function testing. Considering the variety of types of otitis media, we categorized the studies into three types (as per Bluestone et al.) [Citation17]: (a) OME: otitis media with effusion or non-suppurative COM , defined as the presence of middle-ear effusion for more than 3 months; (b) AOM: acute otitis media, defined as the presence of bulging of the tympanic membrane in association with other signs of acute infection (hyperemia, otalgia, otorrhea); and (c) COM: suppurative COM and COM with cholesteatoma, defined as the presence of a perforation of the tympanic membrane in association with chronic infection, with or without cholesteatoma. The gathered data were analyzed from November 2017 to February 2018.
Demography
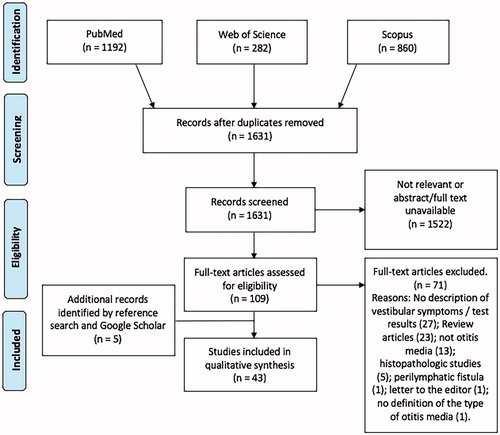
Our systematic searches identified 2334 articles; after application of the exclusion criteria, our final group of records comprised 43 articles (), all of which were published in peer-reviewed journals. Thirty-eight articles (88.3%) were written in English, and the remaining five (11.7%) in Japanese. The studies were based in 15 different countries; 2 were multi-centric (). Among records, 21 were cross-sectional studies, 20 were longitudinal studies, and 2 were case reports.
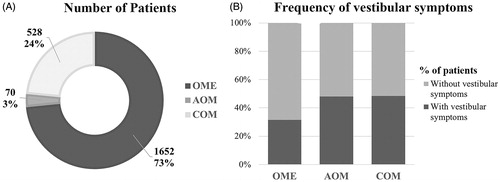
The selected records included 2250 patients with otitis media, who were evaluated for the presence/absence of peripheral vestibular dysfunction. Regarding age groups, 25 included patients younger than 18 years and 17 were in adults; the remaining study included subjects of all ages. The age of the patients of pediatric studies ranged from 7 months to 17 years, and age of adults ranged from 18 to 77 years. Twenty-five articles (58.1%) studied patients with OME (1652 patients), 6 (13.9%) AOM (70 patients), and 12 (28.0%) COM (528 patients) ().
Figure 3. Demographic information of the studies. A: Number of patients classified as OME, AOM, or COM among studies; B: Frequency of patients with each type of otitis media who had vestibular symptoms; dark gray area: percentage of patients who had vestibular symptoms; light gray area: percentage of patients who did not have vestibular symptoms.
Results
Quality assessment of the records
Regarding the quality assessment of records using the NOS, 26 were classified as “moderate”, 13 “low”, and 2 “very low” ( and ). Thirty-nine (95.1%) of the 41 longitudinal or cross-sectional studies were potentially affected by potential biases ( and ).
Evaluation of vestibular impairment secondary to otitis media
Patients with different types of otitis media (OME, AOM, COM) were evaluated by means of clinical evaluation, vestibular function tests, and questionnaires. The tests for evaluation of vestibular function included electronystagmography (ENG), caloric tests, VEMP, dynamic and static posturography, sway magnetometry, craniocorpography, and the subjective vertical visual (SVV). Eight studies used scales and clinical tests to evaluate coordination, balance and motor skills, which included the Peabody developmental motor scales (PDMS), Bruininks–Oseretsky test of motor proficiency (BOTMP), Stott test of motor impairment (STMI), and the motor accuracy test – revised (MAT-R; ).
Table 3. Demographic information and description of the vestibular testing performed in each clinical study, categorized accordingly to the type of otitis media.
Otitis media with effusion (OME)
Clinical information was available for 550 patients with OME and 176 healthy controls. The incidence of symptoms attributable to peripheral vestibular system dysfunction was higher in patients with OME (n = 173, 31.3%) than controls (n = 7, 3.97%) [Citation18–33].
The gross and fine motor skills of 343 children with OME were evaluated using scales and clinical tests: 327 (95.3%) were subjected to PDMS and/or BOTMP and 16 (4.7%) to STMI and MAT-R tests. No significant differences existed in the results of STMI and MAT-R tests of OME and control groups [Citation30]. Regarding the PDMS and BOTMP tests, all studies consistently claimed that children with OME performed significantly worse compared with controls [Citation12,Citation13,Citation20,Citation23,Citation27,Citation28]. However, the association between low scores in PDMS and BOTMP tests and presence of vestibular symptoms was inconsistent among studies. After placement of ventilation tubes or surgical drainage of the effusion, the PDMS and BOTMP results improved significantly in patients with OME compared with their pre-surgical results. Nevertheless, Hart et al. [Citation23] and Gawron et al. [Citation34] demonstrated that, regardless of post-surgical improvements, patients with OME were still performing worse than controls.
Of ENG tests in 334 children with OME [Citation13,Citation20,Citation21,Citation25,Citation26,Citation28,Citation35], abnormal findings were reported in 120 subjects (25.9%). Rates of abnormalities were significantly higher compared with controls in all studies except in the study of Engel-Yeger et al. (n = 20) [Citation13] ().
Table 4. ENG findings in patients with different types of otitis media.
VEMP tests were performed in 101 children with OME [Citation25,Citation28,Citation32]. VEMP tests using air-conducted stimuli did not elicit detectable responses in most patients with OME, and bone-conducted stimuli VEMP results varied greatly among studies (2–30% of absence of responses). Evaluation of the amplitude and latency of p13 and n26 waves (compared with controls) was inconsistent. Said et al. [Citation28] did not report significant differences in latencies or in amplitude; Kolkaila et al. [Citation25] determined delayed latencies but not amplitude differences; and Wang and Lee [Citation32] found delayed latencies and significantly smaller amplitudes. Regarding association of VEMP results with clinical symptoms, Kolkaila et al. [Citation25] (n = 30) found a significant positive association between dizziness complaints and latency of p13-n26 waves, findings that Said et al. [Citation28] (n = 50) contradicted. Wang and Lee [Citation32] reported that after surgical treatment (myringotomy or placement of ventilation tubes), significant decrease still existed in the amplitude of p13-n26 waves in OME patients compared with controls (n = 20).
Of the 211 patients subjected to dynamic or static posturography [Citation2,Citation24,Citation31,Citation34,Citation36–38], abnormalities reported in patients with OME compared with controls include the following: higher mean velocity [Citation2,Citation34], more falls during the exams (OME, 63%; controls, 8%) [Citation2], and increased sway amplitude during high-frequency stimuli [Citation24,Citation36]. In contrast, Ben-David et al. [Citation38] did not find differences in posturography results between the study and control groups. Most studies reported improvements in the posturography results after surgical treatment of the OME, which became comparable with the results of control groups. The only exception was Gawron et al. [Citation34] where despite improvements, OME subjects still performed worse than controls after 4 weeks of grommet insertion.
Acute otitis media (AOM)
The six studies involving patients with AOM included 70 subjects (age range 3.5–77) [Citation39–44]. Four studies [Citation39,Citation40,Citation43,Citation44] comprising 50 patients with AOM demonstrated that 24 (48%) complained of dizziness or vertigo. The vestibular symptoms disappeared after resolution of the acute infection (treatment with oral antibiotics and/or placement of ventilation tubes).
In 43 patients with AOM subjected to ENG and/or caloric tests, abnormalities were demonstrated on the ENG of 40 (93%), all of which suggest peripheral pathology (). Most patients with spontaneous nystagmus (92.8%) had irritative-type nystagmus beating toward the affected ear; only one patient had paretic-type nystagmus beating toward the contralateral ear. Caloric tests demonstrated decreased function in the affected ear in 8 of 26 subjects (30.7%).
Chronic otitis media (COM)
Studies involving patients with COM comprised 528 patients [Citation7–10,Citation45–52]. Description of clinical symptoms was available for 306 patients, 148 of whom (48.36%) complained of dizziness and/or vertigo [Citation7–10,Citation46,Citation47,Citation51]. However, only half of the clinical studies describe exclusion of patients who could have other causes for the vestibular symptoms (previous ear surgery, AOM, acute exacerbation of COM, labyrinthine fistula, systemic disease, head injury, use of ototoxic drugs, Meniere’s disease, vestibular neuritis, benign paroxysmal positional vertigo, diabetes, cardiovascular diseases, neurological diseases) [Citation7,Citation9,Citation10,Citation46]. Mostafa et al. [Citation7] demonstrated a trend for positive association between duration of COM and vestibular symptoms: the median in years of duration of disease in patients who had vestibular symptoms was higher as compared with patients without vestibular symptoms (6.0 and 2.0, respectively). Lee et al. [Citation8] performed the SVV in 25 patients, and demonstrated abnormal results in five (20%): two had the SVV tilting toward the affected ear and the other three to the contralateral non-diseased ear.
Of ENG tests performed in 175 patients, results demonstrated that 56% had spontaneous nystagmus () [Citation8,Citation47,Citation48]. Of the 198 patients subjected to caloric tests, 68 (34.3%) had abnormal results in the affected side [Citation7–9,Citation51,Citation52]. Clinically, there were no significant relations between the presence of vestibular symptoms and abnormal caloric responses [Citation7]. Rotatory chair tests of 60 patients with COM demonstrated a 70% incidence rate of phase defect, which had a reverse correlation with the frequency of chair rotation [Citation7]. The presence of abnormalities in the rotatory chair was positively associated with the presence of vertigo [Citation7].
A total of 277 patients (317 ears) with COM underwent VEMP tests [Citation7,Citation9,Citation10,Citation45,Citation46,Citation49,Citation50]. Of seven studies, six performed cervical VEMPs (cVEMP) (217 patients and 257 ears) and one tested both cVEMP and ocular (oVEMP) (85 patients, 117 ears). Chang et al. [Citation46] reported abnormalities in the oVEMP of 72 of 106 ears (68%). Among studies using cVEMPS, 160 of 317 ears (50.4%) had abnormal responses, defined as absence of response or delayed latency or decreased amplitude of waves p13-n26. Seo et al. [Citation10] reported that VEMP responses were more frequently absent in patients with COM who had disequilibrium (40%) than ones without disequilibrium (no absent responses). However, Mostafa et al. [Citation7] and Ho et al. [Citation9] demonstrated low correlation between VEMP abnormalities and clinical symptoms. Regarding posturography evaluation, Mostafa et al. [Citation7] did not find abnormal response patterns in their 60 subjects, but the authors did not include a control group in their study.
Discussion
Otitis media with effusion
The first description of possible connections between middle-ear effusion and disequilibrium was published in 1977 [Citation38]. Today, middle-ear effusion is acknowledged as one of the most common causes of dizziness in childhood [Citation12,Citation36,Citation37], and the real incidence may be higher than reported [Citation22,Citation34]. Children rarely complain objectively of balance-related issues. Instead, caregivers report symptoms such as clumsiness, frequent falls, or bumping into things [Citation2,Citation34,Citation36,Citation37]. The imprecise description of symptoms is reflected by the inconsistency between caregivers’ perception and the results of vestibular testing [Citation12,Citation33]. Thus, it seems plausible that clinical complaints become significant only when vestibular manifestations are severe, which is rarely seen in daily practice (unless when in association with acute infection) [Citation34,Citation37]. Nonetheless, it has been demonstrated that children with OME have more difficulties in maintaining balance in sensory-conflicting, dynamic situations, depending more heavily on visual cues to maintain balance [Citation36,Citation37]. Therefore, if treatment of the effusion is not timely, long-term vestibular sequela may occur [Citation34,Citation53].
Objective quantification of vestibular function is hard to achieve with tests available in daily practice. Results of caloric testing, ENG, and VEMPs are negatively influenced by the status of the middle-ear, and normative data in patients with OME are not available [Citation12,Citation13]. Further, co-operation of children for those tests is often limited [Citation13]. Thus, several authors sought alternative ways for quantifying vestibular impairment, including clinical tests, tests for analysis of motor skills, and posturography [Citation2,Citation36].
The low quality of the selected studies and the amount of potentially-biased studies are detrimental to an in-depth analysis of the results; however, the findings among patients with OME were very consistent. In general, all studies (except Ben-David et al. [Citation38]) demonstrated that children with OME had worse results in balance tests, delayed gross and fine motor skills, and several abnormalities in posturography results compared with healthy controls. Those abnormalities seemed to improve greatly after treatment of the middle-ear effusion [Citation27,Citation32]. Results of the tests, in association with the rapid improvements post-OME resolution, raised the hypothesis that vestibular symptoms secondary to OME are results of persistent pressure changes in the middle-ear, which encouraged some authors to suggest insertion of ventilation tubes in cases of moderate-to-severe vestibular symptoms or delayed motor development [Citation34,Citation37]. Nonetheless, the effect of learning curve between tests performed pre- and post-ventilation tubes must be considered as being partially responsible for the improvements observed in the second (post-surgical) test [Citation23].
Few available studies demonstrate vestibular sequelae secondary to OME. However, evidence suggests that there may be long-term vestibular impairment even after treatment of the effusion: Casselbrant et al. [Citation37] observed lower gain to rotational stimulus in children with past OME than controls. Aarhus et al. [Citation53] reported that adults who had OME or recurrent AOM during childhood had higher chances of having dizziness in adulthood as compared with people without a history of otitis media. And Gawron et al. [Citation34] found significantly lower scores in the results of balance tests in patients with OME even after insertion of ventilation tubes as compared with controls. Those results led to the hypothesis that chronic or repeated inflammation may cause permanent damage to the vestibular sensorial epithelium [Citation54]. However, it is unclear if those sequelae are caused by OME or if they occur only after acute infections and/or serous labyrinthitis episodes [Citation38]. Therefore, although the current evidence demonstrated the possibility of vestibular impairment secondary to OME, further well-designed studies in this regard may provide additional data to those pending questions.
Acute otitis media
Few studies evaluate long-term vestibular sequelae secondary to AOM. Several authors reported the presence of vestibular deficits after ear infection, which resolved completely after treatment [Citation41]. Although those results suggest no long-term vestibular sequela secondary to AOM, human temporal bones studies involving donors who had serous labyrinthitis secondary to AOM demonstrated significant, permanent vestibular hair cell loss in the maculae of otolithic organs [Citation55,Citation56]. Thus, further studies may shed light on whether AOM causes long-term vestibular sequels and which factors (severity, complications, single or repeated episodes) associate with the vestibular involvement.
Chronic otitis media
The prevalence of vestibular symptoms in patients who have COM is estimated at 40–60% [Citation7,Citation9]. Balance issues in patients with COM are frequently associated with peri-lymphatic fistulae or agudizations [Citation46]; when those are not present, symptoms are attributed to co-morbidities (such as diabetes and arterial hypertension) or presbyastasis, considering that COM has its peak incidence in older adults and the elderly [Citation57]. Symptoms of vestibular system dysfunction are not usually chief complaints of patients with COM [Citation46]: it is possible that the slow progression of the inflammatory damage to the sensorial and neural vestibular structures allow adequate central compensation for daily situations [Citation58]. However, patients may experience difficulties in maintaining postural stability and equilibrium in challenging situations, increasing the risk of disequilibrium and falls, especially in the elderly [Citation2,Citation7,Citation8].
Our literature search resulted in 12 records involving patients with COM. However, six of these records (50%) were analysis of the applicability and reliability of VEMPs in patients with COM; thus, only six studies (50%) were dedicated to specifically evaluate vestibular dysfunction secondary to COM. From those six, most are affected by potential biases ( and ): for example, only four studies clearly described their inclusion and exclusion criteria. Considering the large number of pathologic conditions that affect equilibrium and may occur in association with the COM [Citation59], future studies should be underpinned by strict exclusion criteria, minimizing methodologic flaws and increasing reliability in the results.
COM comprises a wide range of clinical symptoms and manifestations, including mild cases of chronic tympanic membrane perforation without chronic infection but also cases with extensive fibrosis, cholesteatoma, thick effusion, hyperplastic mucosa, and ossicular and bony destruction [Citation60]. Considering that the available vestibular tests in daily practice (ENG, calorics, VEMPs) are heavily influenced by the status of the middle-ear and conductive deficits, their results should be interpreted with caution [Citation8,Citation10]. The use of VEMPs using bone-conduction stimuli has been proposed as an alternative to the conventional air-conduction stimulus; however, such modality may stimulate the contralateral vestibule [Citation10], producing unreliable results when applied to clinical settings. Therefore, several tests which are not influenced by the middle-ear status have been proposed as alternatives, and those include clinical bedside tests (gait, static and dynamic postural tests, tests of the vestibule-ocular reflexes, cerebellar function tests, and neurologic evaluation), the video-head impulse test (vHIT), static and dynamic posturography, and the SVV test [Citation7,Citation8,Citation37]. To our knowledge, only one study performed any of those tests in patients with COM to evaluate vestibular function [Citation7].
In spite of the scarcity of well-designed studies [Citation10], there seem to be evidence of vestibular deficits secondary to COM. The clinical observation of high prevalence of disequilibrium and vertigo among studies involving patients with COM find support in histopathologic analyses of human temporal bones, which demonstrated changes to the vestibular sensorial epithelium of human temporal bones [Citation11,Citation60,Citation61]. In the clinical and histopathologic studies, there seemed to be a positive association between vestibular impairment and severity and duration of COM, potentially suggesting a cause–effect association [Citation7,Citation11]. Furthermore, there was a positive correlation between severity of sensorineural hearing loss and presence of vestibular symptoms, also suggesting combined cochlear-vestibular impairment secondary to COM [Citation7,Citation11]. Taken together, the evidence suggests that COM – solely – may lead to progressive and permanent vestibular damage [Citation7,Citation8,Citation11,Citation60,Citation61]. However, clinical implications for those findings are still somewhat obscure [Citation10]. Thus, to clarify the possible association between vestibular symptoms and COM, we propose further studies with well-defined methodology. It is imperative that these include (1) clear classification of the type of COM (non-suppurative, suppurative, with or without cholesteatoma); (2) otoscopic findings (size of perforation, presence of fibrosis, ossicular pathology, presence and characteristics of effusion, status of the middle-ear mucosa); (3) precise definition of exclusion criteria (fistulae, patients with a history of ear surgery, clinical otosclerosis, Meniere’s disease, uncontrolled arterial hypertension or dyslipidemia, head trauma, diabetes, malformations, syndromes); (4) results of hearing tests; and (5) standardized clinical examination, laboratorial evaluation, imaging (computerized tomography), and vestibular tests (bedside tests, vHIT, posturography, SVV).
Rafael_da_Costa_et_al._Supplemental_files.docx
Download MS Word (16.7 KB)Acknowledgements
The authors thank the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for the continued support; Thais Gomes Abrahao Elias for critically reviewing the manuscript; and Suzanne Schoenfelt for proofreading the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94.
- Casselbrant ML, Furman JM, Rubenstein E, et al. Effect of otitis media on the vestibular system in children. Ann Otol Rhinol Laryngol. 1995;104:620–624.
- Penido Nde O, Chandrasekhar SS, Borin A, et al. Complications of otitis media - a potentially lethal problem still present. Braz J Otorhinolaryngol. 2016;82:253–262.
- Paparella MM. Interactive inner-ear/middle-ear disease, including perilymphatic fistula. Acta Otolaryngol Suppl. 1991;485:36–45.
- Asbjornsen AE, Obrzut JE, Boliek CA, et al. Impaired auditory attention skills following middle-ear infections. Child Neuropsychol. 2005;11:121–133.
- Brooks DN. Otitis media and child development. Design factors in the identification and assessment of hearing loss. Ann Otol Rhinol Laryngol. 1979;88:29–47.
- Mostafa BE, Shafik AG, El Makhzangy AMN, et al. Evaluation of vestibular function in patients with chronic suppurative otitis media. ORL J Otorhinolaryngol Relat Spec. 2013;75:357–360.
- Lee JS, Lee SK, Shin IH, et al. Vestibular evoked myogenic potential according to middle ear condition in chronic otitis media with tympanic membrane perforation. Acta Otolaryngol. 2014;134:34–40.
- Ho K-Y, Chien C-Y, Tsai S-M, et al. Clinical significance of vestibular function with caloric and vestibular evoked myogenic potential testing for patients with simple chronic otitis media. J Int Adv Otol. 2012;8:447–452.
- Seo T, Miyamoto A, Saka N, et al. Vestibular evoked myogenic potential induced by bone-conducted stimuli in patients with conductive hearing loss. Acta Otolaryngol. 2008;128:639–643.
- Monsanto RDC, Schachern P, Paparella MM, et al. Progression of changes in the sensorial elements of the cochlear and peripheral vestibular systems: the otitis media continuum. Hear Res. 2017;351:2–10.
- Cohen H, Friedman EM, Lai D, et al. Balance in children with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 1997;42:10107–10115.
- Engel-Yeger B, Golz A, Parush S. Impact of middle ear effusion on balance performance in children. Disabil Rehabil. 2004;26:97–102.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Wells GA, Shea B, O’Connel D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Herzog R, Álvarez-Pasquin MJ, Díaz C, et al. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:19–154.
- Bluestone CD, Gates GA, Klein JO, et al. 1. Definitions, terminology, and classification of otitis media. Ann Otol Rhinol Laryngol. 2002;1;111:8–18.
- Editorial. Vertigo and glue ear in children. Clin Otolaryngol Allied Sci. 1978;3:198–200.
- Golz A, Angel-Yeger B, Parush S. Evaluation of balance disturbances in children with middle ear effusion. Int J Pediatr Otorhinolaryngol. 1998;43:21–26.
- Golz A, Westerman ST, Gilbert LM, et al. Effect of middle ear effusion on the vestibular labyrinth. J Laryngol Otol. 1991;105:987–989.
- Golz A, Netzer A, Angel-Yeger B, et al. Effects of middle ear effusion on the vestibular system in children. Otolaryngol Head Neck Surg. 1998;119:695–699.
- Grace ARH, Pfleiderer AG. Dysequilibrium and otitis media with effusion: what is the association? J Laryngol Otol. 1990;104:682–684.
- Hart MC, Nichols DS, Butler EM, et al. Childhood imbalance and chronic otitis media with effusion: effect of tympanostomy tube insertion on standardized tests of balance and locomotion. Laryngoscope. 1998;108:665–670.
- Jones NS, Prichard AJN, Radomskij P, et al. Imbalance and chronic secretory otitis media in children: effect of myringotomy and insertion of ventilation tubes on body sway. Ann Otol Rhinol Laryngol. 1990;99:477–481.
- Kolkaila EA, Emara AA, Gabr TA. Vestibular evaluation in children with otitis media with effusion. J Laryngol Otol. 2015;129:326–336.
- Koyuncu M, Saka MM, Tanyeri Y, et al. Effects of otitis media with effusion on the vestibular system in children. Otolaryngol Head Neck Surg. 1999;120:117–121.
- Orlin MN, Effgen SK, Handler SD. Effect of otitis media with effusion on gross motor ability in preschool-aged children: preliminary findings. Pediatrics. 1997;99:334–337.
- Said EA, Ahmed MK, Mohamed ES. Role of vestibular testing in deciding treatment strategies for children with otitis media with effusion. Egypt J Ear Nose Throat Allied Sci. 2015;16:151–159.
- Sommerfleck PA, Gonzalez Macchi ME, Weinschelbaum R, et al. Balance disorders in childhood: main etiologies according to age. Usefulness of the video head impulse test. Int J Pediatr Otorhinolaryngol. 2016;87:148–153.
- Von T, Deitz JC, McLaughlin J, et al. The effects of chronic otitis media on motor performance in 5- and 6-year-old children. Am J Occup Ther. 1988;42:421–426.
- Waldron MNH, Matthews JNS, Johnson IJM. The effect of otitis media with effusions on balance in children. Clin Otolaryngol Allied Sci. 2004;29:318–320.
- Wang M-C, Lee G-S. Vestibular evoked myogenic potentials in middle ear effusion. Acta Otolaryngol. 2007;127:700–704.
- Denning J, Mayberry W. Vestibular dysfunction in preschool children with a history of otitis media. Occup Ther J Res. 1987;7:335–348.
- Gawron W, Pospiech L, Orendorz-Fraczkowska K. An evaluation of postural stability and the effects of middle-ear drainage on vestibulo-spinal reflexes of children with chronic otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2004;68:1175–1179.
- Blayney AW, Colman BH. Dizziness in childhood. Clin Otolaryngol Allied Sci. 1984;9:77–85.
- Casselbrant ML, Redfern MS, Furman JM, et al. Visual-induced postural sway in children with and without otitis media. Ann Otol Rhinol Laryngol. 1998;107:401–405.
- Casselbrant ML, Furman JM, Mandel EM, et al. Past history of otitis media and balance in four-year-old children. Laryngoscope. 2000;110:773–778.
- Ben-David J, Podoshin L, Fradis M, et al. Is the vestibular system affected by middle ear effusion? Otolaryngol Head Neck Surg. 1993;109:421–426.
- Yamaguchi J, Yagi T, Baba S, et al. Inner ear damage in acute otitis media. Otol Jpn. 1992;2:259–263.
- Nishimura M, Doi K, Kubo T, et al. Inner ear damage in acute otitis media. Pract Otorhinolaryngol. 2000;93:455–459.
- Balatsouras DG, Kaberos A, Assimakopoulos D, et al. Etiology of vertigo in children. Int J Pediatr Otorhinolaryngol. 2007;71:487–494.
- Schaaf RC. The frequency of vestibular disorders in developmentally delayed preschoolers with otitis media. Am J Occup Ther. 1985;39:247–252.
- Eliashar R, Gross M, Saah D, et al. Vestibular involvement in myringitis bullosa. Acta Otolaryngol. 2004;124:249–252.
- Kim CH, Yang YS, Im D, et al. Nystagmus in patients with unilateral acute otitis media complicated by serous labyrinthitis. Acta Otolaryngol. 2016;136:559–563.
- Wang M-C, Liu C-Y, Yu EC-H, et al. Vestibular evoked myogenic potentials in chronic otitis media before and after surgery. Acta Otolaryngol. 2009;129:1206–1211.
- Chang C-W, Cheng P-W, Young Y-H. Inner ear deficits after chronic otitis media. Eur Arch Otorhinolaryngol. 2014;271:2165–2170.
- Kanaya T, Shirato M, Unno T. Equilibrium findings of chronic otitis media. Pract Otorhinolaryngol. 1982;75:2392–2398.
- Kanoh Y, Yagi T, Yoshimoto Y, et al. A study on preoperative electronystagmography in chronic otitis media. Equilibrium Res. 1980;39:43–48.
- Yang T-L, Young Y-H. Comparison of tone burst and tapping evocation of myogenic potentials in patients with chronic otitis media. Ear Hear. 2003;24:191–194.
- Zhou G, Poe D, Gopen Q. Clinical use of vestibular evoked myogenic potentials in the evaluation of patients with air-bone gaps. Otol Neurotol. 2012;33:1368–1374.
- Maw AR. Bobbing oscillopsia. Ann Otol Rhinol Laryngol. 1971;80:233–239.
- Siampara L, Mann SBS, Panda NK, Mehra YN. Audiovestibular profile in unilateral chronic suppurative otitis media. Indian J Otolaryngol Head Neck Surg. 1997;49:107–111.
- Aarhus L, Tambs K, Hoffman HJ, et al. Childhood otitis media is associated with dizziness in adulthood: the HUNT cohort study. Eur Arch Otorhinolaryngol. 2016;273:2047–2054.
- Joglekar S, Morita N, Cureoglu S, et al. Cochlear pathology in human temporal bones with otitis media. Acta Otolaryngol. 2010;130:472–476.
- Igarashi M, O-Uchi T, Isago H, et al. Utricular and saccular volumetry in human temporal bones. Acta Otolaryngol. 1983;95:75–80.
- Kaya S, Schachern PA, Tsuprun V, et al. Deterioration of vestibular cells in labyrinthitis. Ann Otol Rhinol Laryngol. 2017;126:89–95.
- Anmi K, Chiori K, Chikako T, et al. Statistical analysis of vertigo patients attending our clinic during the past 15 years. Pract Otorhinolaryngol. 1992;1992:7–13.
- Lacour M, Helmchen C, Vidal P-P. Vestibular compensation: the neuro-otologist’s best friend. J Neurol. 2016;263:54–64.
- Neuhauser HK, von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology. 2005;65:898–904.
- da Costa Monsanto R, Erdil M, Pauna HF, et al. Pathologic changes of the peripheral vestibular system secondary to chronic otitis media. Otolaryngol Head Neck Surg. 2016;155:494–500.
- Kodama A, Ishii T, Oka Y, et al. Histopathology of the inner ear in chronic otitis media. Equilibrium Res. 1988;47:94–100.