Abstract
Non-alcoholic fatty liver disease (NAFLD) is highly prevalent and causes an enormous burden to human health and health-care systems all over the world. A great proportion of this burden results from increased risk of cardiovascular diseases. Atrial fibrillation (AF) is the most common chronic heart arrhythmia globally and it increases the risk of embolic stroke and heart failure. Recent studies have explored the association between NAFLD and AF with somewhat conflicting results. However, ultrasound-verified prospective studies concur that NAFLD is associated with the incidence of AF. According to epidemiological evidence, the greater the prevalence of NALFD in a population, the stronger the association with AF incidence and prevalence. Specifically, diabetic individuals with NAFLD are at the greatest risk of AF. Additionally, the risk of AF may concentrate most in individuals with advanced NAFLD, particularly those with liver fibrosis. The possible mechanistic factors between NAFLD and AF, particularly obesity and systemic inflammation, are diverse and form a complex interplaying network. However, further studies are needed to elucidate whether NAFLD has a causative role in the development of AF. The purpose of this article is to review and discuss the epidemiologic evidence and possible mechanistic links between these two conditions.
Although epidemiologic studies have provided conflicting results on the association of NAFLD and AF, prospective studies with ultrasound-verified NAFLD concur that NAFLD is associated with about 2-fold greater incidence of AF among general population and about 6-fold greater incidence among subjects with type 2 diabetes.
The risk of AF among individuals with NAFLD is increased by other cardiovascular risk factors, especially type 2 diabetes and advanced age.
The possible mechanistic links between NALFD and AF are diverse, with obesity and systemic inflammation having a significant role, but further studies are needed until NAFLD can be established as a causal factor in the incidence of AF.
KEY MESSAGES
Introduction
Non-alcoholic fatty liver disease (NAFLD) parallels obesity and is defined as ectopic fat accumulation of at least 5% of liver weight without excess alcohol consumption or other secondary causes [Citation1]. Liver biopsy is the reference method to diagnose NAFLD, but nowadays, proton magnetic resonance spectroscopy and magnetic resonance imaging are also accepted methods for a definite diagnosis [Citation1]. Other imaging modalities, such as ultrasound and computed tomography, and different serum markers and scores are only referential but due to good availability they are widely used in the clinical practice [Citation1]. NAFLD is an umbrella term covering simple hepatosteatosis and non-alcoholic steatohepatitis (NASH), which, in turn, is divided into non-fibrotic NASH and fibrotic NASH, of which liver cirrhosis is the most progressive form [Citation1]. NAFLD may also lead to liver failure and hepatocellular carcinoma. The progression of NAFLD is slow and dependent on a complex interplay of metabolic, genetic, epigenetic as well as different environmental, hormonal and inflammatory factors [Citation2]. To date, the global prevalence of NAFLD is about 25% and the prevalence is still increasing [Citation3]. NALFD exposes to many extrahepatic diseases as well [Citation4]. For instance, the total risk of cardiovascular diseases (CVDs) is about doubled in the subjects with NAFLD as compared with those without NAFLD [Citation5].
Atrial fibrillation (AF) is characterized by irregular and rapid electronic and mechanic atrial activation, which leads to hemodynamic changes and a remodeling of the left atrial structure [Citation6]. It is the most common cardiac arrhythmia with a total prevalence of about 1–4% [Citation7]. The prevalence almost doubles with each decade of life, so that the estimated lifetime risk of having AF is about 25% [Citation7–9]. Male gender, obesity, smoking, hypertension, diabetes, coronary artery disease and heart failure are major risk factors of AF [Citation7]. Because AF implies an increased risk of embolic stroke, heart failure, myocardial infarction, dementia and chronic kidney disease, the human burden and health care costs related to AF are enormous [Citation7].
Recently published studies have explored the association between NAFLD and AF. The purpose of this article is to review this association, including the possible mechanistic links between these two conditions, and discuss avenues for future studies.
The epidemiology of NAFLD and AF
The literature review of the epidemiological studies of the association of NAFLD and AF was carried out using the Medline/PubMed database by combining the terms “NAFLD” and “atrial fibrillation” as well as “liver enzyme” and “atrial fibrillation”. Here, the epidemiological evidence from these searches is discussed.
Targher et al. were the first to show that ultrasound-based NAFLD is an independent risk factor of AF as they studied Italian subjects with type 2 diabetes (T2D) [Citation10]. Two years later, the association was shown in a Finnish community-based study cohort in which many of the study participants had hypertension [Citation11]. In the Finnish study, the odds for AF development in the follow-up after multiple adjustments were lower than in the study by Targher et al. (1.9 vs. 6.4). Another study on the same study cohort by Targher et al. showed that subjects with NAFLD had higher prevalence of AF as compared with the subjects without NAFLD in a cross-sectional setting [Citation12]. In contrast, two other cross-sectional studies did not detect an association between NAFLD/hepatosteatosis and AF. Markus et al. reported that ultrasound-verified hepatosteatosis (NAFLD was not defined separately) was not associated with a higher AF prevalence in a German community-based study cohort [Citation13]. Long et al. reported similar results on the association of computed tomography-verified NAFLD and AF in their study based on the Framingham cohort [Citation14]. It is noteworthy that no studies exist on the association of NAFLD and AF incidence with definitive liver biopsy-verified NAFLD.
The conflicting results may be explained by the differences between the study cohorts. The individuals in the German cohort and the Framingham cohort seemed to be younger and/or healthier than the individuals in the Finnish study cohort, let alone the individuals with T2D in the Italian cohort. This is reflected in the total AF prevalence and incidence in the study cohorts: 1.5% prevalence among the Germans, 3% incidence among the Framingham cohort (Americans), 10% incidence among the Finns, and 11% incidence and 12% prevalence among the Italians. In addition, because advancing age is an essential risk factor of AF development [Citation15], the age of the study cohort has a major effect on the AF prevalence. Thus, the differences in the outcomes may be explained by the subjects in the German study cohort being younger than those in the other cohorts. Moreover, in the German study by Markus et al., alcohol consumption or other secondary factors for hepatosteatosis were not specifically defined for all subjects with hepatosteatosis, while all the other studies studied only NAFLD, which may also affect the results.
NAFLD is the most common etiology of elevated liver enzymes [Citation1]. Thus, studies of the association between elevated liver enzymes and AF provide indirect evidence of the association between NAFLD and AF. Firstly, alanine aminotransferase (ALT) above the upper limit of normal has been reported to be prevalent in the subjects with AF [Citation16]. Elevated liver enzymes (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT)) have also been reported to predict AF development among middle-aged general population in two prospective studies [Citation13,Citation17]. However, one prospective study could only confirm this finding with GGT whereas ALT and AST had a U-shaped relationship with the risk of AF [Citation18] while another prospective study could not confirm the association between NAFLD and any liver enzyme [Citation19]. Convincingly, a recently published meta-analysis comprising nearly 240,000 subjects with NAFLD [Citation20], most of them from a poster presentation [Citation21] with more than 230,000 Korean study subjects with NAFLD diagnosed by Fatty Liver Index [Citation22], showed a clear linear association between NAFLD and the risk of AF [Citation20]. In total, NAFLD implied a 2.1-fold risk of later AF diagnosis [Citation20]. This report with large community-based study samples provides evidence that NAFLD is a predictor of AF. Moreover, the other two meta-analyses with smaller number of subjects support this view [Citation23,Citation24].
The epidemiological studies of associations between NAFLD (or hepatosteatosis) and AF are presented in (prospective studies) and (cross-sectional studies).
Table 1. The prospective studies of the association between NAFLD and AF.
Table 2. The cross-sectional studies of the association between NAFLD and AF.
According to the data from the studies above, the more progressive the NAFLD (NASH, fibrosis and liver cirrhosis), the greater the risk of AF may be. In line with this assumption of a linear association, prevalent AF has also been reported to be associated with liver stiffness in a small study of elderly Finnish subjects [Citation25]. The association was discovered by transient elastography [Citation26] in the total study population and separately in subjects with NAFLD assessed by NAFLD Fibrosis Score [Citation27]. Moreover, transient elastography values were higher in those participants with greater left atrial diameter, a collateral of AF [Citation25].
Noteworthy, there are some limitations in the epidemiological studies on NAFLD and AF. First, AF may be asymptomatic and paroxysmal, so that AF does not always lead to hospitalization or an exact diagnosis. Thus, AF is likely to be underdiagnosed in the clinical practice. Second, the study cohorts where NAFLD was detected by ultrasound may have been more thoroughly investigated in general as compared to studies where NAFLD was diagnosed by liver enzymes. This may lead to different results in the variables tested, such as AF prevalence rates. Third, even though NAFLD is the most common background for abnormal liver enzymes, there are numerous other reasons for abnormal liver enzymes. Additionally, a great proportion of subjects with NAFLD have liver enzymes within normal limits. Therefore, abnormal liver enzymes are more or less unspecific and insensitive surrogates for NAFLD.
In summary, the present data from epidemiological studies indicate that NAFLD is a risk factor of AF and that the more progressive the NAFLD, the stronger the association – a phenomenon also seen in the risk of other CVDs among NAFLD subjects [Citation28–30]. It can be concluded that advancing age and greater co-morbidity, in particular T2D, increase the risk of AF among the NAFLD subjects. This is not surprising because diabetes [Citation4] and advancing age, as a sign of a sum of cumulative metabolic exposures [Citation31], are both known to induce progression of NAFLD, i.e. the formation of fibrotic NASH, which, in turn, is associated with greater risk of CVDs as compared to non-fibrotic NASH [Citation28–30].
The possible mechanisms linking NAFLD to AF
NAFLD and AF share several risk factors and co-morbidities [Citation4,Citation15]. For instance, obesity is strongly linked to both NAFLD [Citation1] and AF [Citation32,Citation33]. However, there are also other possible mechanistic factors that can link NAFLD to AF.
Obesity, NAFLD and accumulation of ectopic fat in other anatomic sites such as the epicardium are parallel phenomena [Citation34]. Simultaneously, epicardial fat depots are closely linked to increased left ventricular mass and diastolic dysfunction through metabolic, endocrine and paracrine functions [Citation35,Citation36]. Additionally, epicardial fat is reported to be an independent predictor of AF in a population-based setting [Citation37,Citation38], and the association may be even greater than that of abdominal and overall adiposity [Citation37]. There are also reports of association of NAFLD with diastolic dysfunction [Citation36,Citation39–41], which is known to promote AF [Citation42,Citation43]. According to a report by Bonapace et al., NAFLD has an independent association with diastolic dysfunction in patients with T2D [Citation39]. However, adjustments were made for hypertension, triglycerides and HbA1C, but not for other components of metabolic syndrome (MetS), such as obesity or waist circumference, which are known to predict fat in the epicardium and other ectopic sites [Citation34,Citation44]. Likewise, although Fotbolcu et al. showed impaired diastolic function parameters in subjects with NAFLD as compared to those without NAFLD in a small cross-sectional study, they did not make any adjustments for clinically relevant confounding factors even though the subjects with NAFLD were far more obese and had more dyslipidemia and insulin resistance [Citation40]. In a Korean study, Jung et al. were able to show an independent association of NAFLD on diastolic dysfunction in the general population even after multiple adjustments including body mass index, hypertension, dyslipidemia, diabetes and insulin sensitivity [Citation41], and the Finnish study by Granér et al. reported that the hepatic triglyceride content correlated with the degree of diastolic dysfunction independently of epi- and pericardial triglycerides [Citation36]. Likewise, a very recently published prospective study showed that computed tomography-verified pericardial fat and hepatosteatosis predicted both left ventricular geometry and function [Citation45]. However, the effect of NAFLD on the increase of left ventricular mass in a long-term follow-up seems to be restricted only to subjects with MetS [Citation46]. Taken together, the current evidence, although scarce, shows that NAFLD may be independently associated with diastolic dysfunction. The association may be causal through systemic toxic effects such as adiponectin, inflammation and insulin, or the indirect effect of cardiometabolic diseases, such as hypertension and diabetes [Citation36,Citation45]. Thus, this link may provide one causal pathway from NAFLD to AF. However, further studies are needed to separate the effect of epicardial fat from liver fat on the development of AF.
NAFLD is reported to associate with heart rate recovery and other surrogates for autonomic nervous system dysfunction [Citation47,Citation48]. Longitudinal studies of the association are lacking and the causality between these two conditions remains unclear. Also epicardial fat correlates with autonomic dysfunction [Citation47], which complicates the assessment of hepatic fat accumulation as an independent risk factor for autonomic dysfunction. As autonomic dysfunction is a risk factor of AF [Citation49–51] it could hypothetically present a link between NAFLD and incident AF, but further studies are needed, taking into account confounding factors such as epicardial fat and MetS.
Structural and metabolic alterations in myocardial tissues and atrial conduction abnormalities, which are reported to have a central role in the development of AF [Citation52] but also to be a consequence of AF [Citation53], are reported to take place in NAFLD [Citation54,Citation55]. Atrial conductivity is impaired in subjects with epicardial fat as well [Citation52], and as there are no longitudinal studies on the association between NAFLD and cardiac electrophysiological properties, it is currently unknown whether NAFLD independently impairs atrial conductivity.
AF is reported to be a cause [Citation56,Citation57] and a consequence of systemic inflammation [Citation57–59]. Simultaneously, NAFLD associates with systemic inflammation [Citation60,Citation61], and systemic inflammation contributes to the progression of NAFLD [Citation2] and liver fibrosis [Citation62]. Additionally, NASH has been shown to induce the release of systemic inflammatory parameters, such as high sensitivity C-reactive protein, fibrinogen, plasminogen activator inhibitor-1, and reduced production of adiponectin, an adipokine that has anti-inflammatory effects, from adipose tissue [Citation63]. There are also other reports of the association of low levels of adiponectin, obesity and systemic inflammation [Citation64,Citation65]. Because a diminished adiponectin level is associated with NAFLD [Citation65] and may, although with conflicting reports, also be associated with AF [Citation64], hypoadiponectinemia may partly explain why obesity and systemic inflammation seem to have a central role in the association between NAFLD and AF.
There is a network of cross-links and interplay between the above-mentioned associations. For instance, obesity is associated with autonomic dysfunction [Citation66,Citation67], reported to induce diastolic dysfunction [Citation68], to be a cause and a consequence of systemic inflammation [Citation69–71] and to impair atrial conductive properties [Citation72,Citation73]. Moreover, systemic inflammation is also known to repress atrial conductivity [Citation74] and to associate with autonomic dysfunction [Citation75], which deepens the impairment of atrial conductive properties [Citation51].
As stated above, NAFLD has several potential causal mechanisms that may provoke AF. To date, we are unable to differentiate the effects of distinct ectopic fat depots, especially epicardial and liver fat, on the development of AF. In addition, it is interesting that NAFLD seems not to predict AF if MetS is taken into consideration as a confounding factor [Citation76]. This may not be surprising as subjects with NAFLD but without MetS do not to have an increased long-term overall CVD risk as compared to healthy controls [Citation46,Citation77]. This may indicate that MetS is the real etiology behind the increased risk of AF in subjects with NAFLD as the majority of them have MetS [Citation78]. Thus, perhaps NAFLD has no independent role in the pathophysiology of AF if MetS is not present. Therefore, further studies with biopsy, magnetic resonance imaging, or magnetic resonance-proton density fat fraction proven NAFLD, long-term follow-up and exclusion of relevant confounding factors, such as T2D, MetS and epicardial fat, are required before the independent role of NAFLD in the development of AF can be established [Citation79]. However, based on the current literature, the possible mechanisms linking NAFLD to AF are presented in .
Figure 1. The possible mechanisms linking NAFLD to AF. NAFLD associates with diastolic dysfunction, impaired atrial conductivity, obesity and autonomic dysfunction, and is reported to be a cause and a consequence of systemic inflammation. All these conditions are risk factors of AF. The risk factors also form a complex interplay with each other. As confounding factors such as MetS, T2D and epicardial fat accumulation are prevalent in patients with NAFLD, further investigation is still needed to establish the independent causative role of NAFLD in the development of AF. The associations are illustrated with dashed lines and causalities with arrows. AF: atrial fibrillation; MetS: metabolic syndrome; NAFLD: non-alcoholic fatty liver disease; T2D: type 2 diabetes.
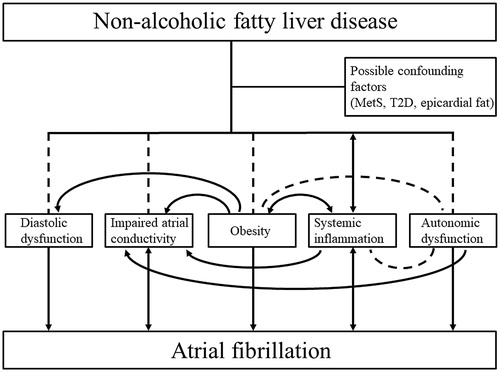
Whether AF can induce the progression of NAFLD is not known. However, it is possible that AF may provoke the progression of pre-existing NAFLD via systemic inflammation. The dynamic progression of NAFLD is silent and takes years or decades [Citation1], making it difficult to study rigorously.
NAFLD and other cardiac arrhythmias
Along with AF, NAFLD has also been reported to associate with other cardiac arrhythmias and cardiac conducting abnormalities [Citation80]. For instance, NAFLD associates with QTc prolongation in subjects with T2D [Citation81] and in general population [Citation82]. Correspondingly, NAFLD associates with non-fatal ventricular arrhythmias [Citation83]. Moreover, NAFLD associates with heart blocks (several atrioventricular conduction impairments combined to one variable) and the more progressive NAFLD is, the greater the association seems to be [Citation84]. As these studies are cross-sectional, the causality between NAFLD and the above-mentioned conditions is yet to be shown.
Future perspectives
Histology and magnetic resonance imaging are the golden standards to define NAFLD [Citation1]. However, studies of the association between NAFLD and AF using these methods to detect NAFLD do not exist. Because liver biopsy is an invasive procedure and thus cannot be performed without a suspicion of liver morbidity, the study populations where histological evaluation has been performed are not representative of the general NAFLD population. Nevertheless, studies with biopsy-proven NAFLD could give us information of how different histologic features of NAFLD associate with AF. Moreover, long-term follow-up studies on NAFLD proven by magnetic resonance imaging or magnetic resonance-proton density fat fraction and with exclusion of MetS and/or epicardial fat would be needed to establish the individual role of NAFLD in the development of AF.
Whether a reduction of liver fat or improvement of liver histology reduces the future risk of AF has not been studied. However, it is interesting that a meta-analysis of pioglitazone use and the risk of AF showed that pioglitazone may confer some protection against AF in patients with T2D [Citation85]. Because pioglitazone has been reported to diminish liver fat and/or improve liver histology among subjects with NAFLD [Citation86–88] and to increase the level of circulating adiponectin [Citation89], the possible protection against AF by pioglitazone may come out via beneficial effects on liver histology and adiponectin level.
Further studies would also be needed to elucidate whether diminished adiponectin level has an active role in the pathogenesis of NAFLD and AF. This could provide new therapeutic targets on NAFLD and AF progression [Citation89].
Conclusions
There is growing epidemiologic evidence of the association of NAFLD and the incidence of AF. Ultrasound-verified prospective studies consistently demonstrate that NAFLD is associated with the incidence of AF. The more morbid or older the population, the stronger the association seems to be, possibly due to higher prevalence of progressive NAFLD stages such as fibrosis and NASH. As confounding factors such as MetS, T2D and epicardial fat accumulation are prevalent in patients with NAFLD, further investigation is still needed to establish the independent causative role of NAFLD in the development of AF.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140.
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab Clin Exp. 2016;65:1038–1048.
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64.
- Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, et al. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: a systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11 Suppl 1:S209–S216.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–e88.
- Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654.
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046.
- Targher G, Valbusa F, Bonapace S, et al. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One.2013;8:e57183.
- Karajamaki AJ, Patsi OP, Savolainen M, et al. Non-alcoholic fatty liver disease as a predictor of atrial fibrillation in middle-aged population (OPERA Study). PLoS One. 2015;10:e0142937.
- Targher G, Mantovani A, Pichiri I, et al. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci. 2013;125:301–309.
- Markus MR, Meffert PJ, Baumeister SE, et al. Association between hepatic steatosis and serum liver enzyme levels with atrial fibrillation in the general population: The Study of Health in Pomerania (SHIP). Atherosclerosis. 2016;245:123–131.
- Long MT, Yin X, Larson MG, et al. Relations of liver fat with prevalent and incident atrial fibrillation in the Framingham Heart Study. J Am Heart Assoc. 2017;6:e005227.
- Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468.
- Makar GA, Weiner MG, Kimmel SE, et al. Incidence and prevalence of abnormal liver associated enzymes in patients with atrial fibrillation in a routine clinical care population. Pharmacoepidem Drug Safe. 2008;17:43–51.
- Sinner MF, Wang N, Fox CS, et al. Relation of circulating liver transaminase concentrations to risk of new-onset atrial fibrillation. Am J Cardiol. 2013;111:219–224.
- Alonso A, Misialek JR, Amiin MA, et al. Circulating levels of liver enzymes and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities cohort. Heart. 2014;100:1511–1516.
- Schutte R, Whincup PH, Papacosta O, et al. Liver enzymes are not directly involved in atrial fibrillation: a prospective cohort study. Eur J Clin Invest. 2017;47:583–590.
- Wijarnpreecha K, Boonpheng B, Thongprayoon C, et al. The association between non-alcoholic fatty liver disease and atrial fibrillation: a meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:525–532.
- You SC, Yang P, Kim T, et al. Non-alcoholic fatty liver disease is independently associated with new onset atrial fibrillation: a nationwide cohort study in Korea. J Am Coll Cardiol. 2016;67:854.
- Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
- Minhas AM, Usman MS, Khan MS, et al. Link between non-alcoholic fatty liver disease and atrial fibrillation: a systematic review and meta-analysis. Cureus. 2017; 69:e1142.
- Zhou Y, Lai C, Peng C, et al. Nonalcoholic fatty liver disease as a predictor of atrial fibrillation: a systematic review and meta-analysis. Adv Interv Cardiol. 2017;13:250–257.
- Karajamaki AJ, Kettunen O, Lepojarvi S, et al. Presence of atrial fibrillation is associated with liver stiffness in an elderly Finnish population. PLoS One. 2017;12:e0173855.
- Mikolasevic I, Orlic L, Franjic N, et al. Transient elastography (FibroScan((R))) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease – Where do we stand? World J Gastroenterol. 2016;22:7236–7251.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854.
- Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554.
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e10.
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565.
- Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17:774.
- Tsang TS, Barnes ME, Miyasaka Y, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233.
- Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477.
- Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest. 2015;125:1790–1792.
- Matloch Z, Kotulak T, Haluzik M. The role of epicardial adipose tissue in heart disease. Physiol Res. 2016;65:23–32.
- Graner M, Nyman K, Siren R, et al. Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circ Cardiovasc Imaging. 2014;8:e001979.
- Wong CX, Sun MT, Odutayo A, et al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e004378.
- Bos D, Vernooij MW, Shahzad R, et al. Epicardial fat volume and the risk of atrial fibrillation in the general population free of cardiovascular disease. JACC Cardiovasc Imaging. 2017;10:1405–1407.
- Bonapace S, Perseghin G, Molon G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–395.
- Fotbolcu H, Yakar T, Duman D, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457–463.
- Jung JY, Park SK, Ryoo JH, et al. Effect of non-alcoholic fatty liver disease on left ventricular diastolic function and geometry in the Korean general population. Hepatol Res. 2017;47:522–532.
- Nagarakanti R, Ezekowitz M. Diastolic dysfunction and atrial fibrillation. J Interv Card Electrophysiol. 2008;22:111–118.
- Uetake S, Maruyama M, Yamamoto T, et al. Left ventricular stiffness estimated by diastolic wall strain is associated with paroxysmal atrial fibrillation in structurally normal hearts. Clin Cardiol. 2016;39:728–732.
- Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12:31–42.
- Shah RV, Anderson A, Ding J, et al. Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2017;10:1016–1027.
- Käräjämäki AJ, Bloigu R, Kauma H, et al. Non-alcoholic fatty liver disease with and without metabolic syndrome: different long-term outcomes. Metabolism. 2017;66:55–63.
- Cho KI, Jo EA, Cho SH, et al. The influence of epicardial fat and nonalcoholic fatty liver disease on heart rate recovery in metabolic syndrome. Metab Syndr Relat Disord. 2017;15:226–232.
- Liu YC, Hung CS, Wu YW, et al. Influence of non-alcoholic fatty liver disease on autonomic changes evaluated by the time domain, frequency domain, and symbolic dynamics of heart rate variability. PLoS One. 2013;8:e61803.
- Park HW, Shen MJ, Lin SF, et al. Neural mechanisms of atrial fibrillation. Curr Opin Cardiol. 2012;27:24–28.
- Perkiomaki J, Ukkola O, Kiviniemi A, et al. Heart rate variability findings as a predictor of atrial fibrillation in middle-aged population. J Cardiovasc Electrophysiol. 2014;25:719–724.
- Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021.
- Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res. 2014;102:205–213.
- Eijsbouts SC, Majidi M, van Zandvoort M, et al. Effects of acute atrial dilation on heterogeneity in conduction in the isolated rabbit heart. J Cardiovasc Electrophysiol. 2003;14:269–278.
- Ballestri S, Lonardo A, Bonapace S, et al. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724–1745.
- Ozveren O, Izgi C, Eroglu E, et al. Doppler tissue evaluation of atrial conduction properties in patients with non-alcoholic fatty-liver disease. Ultrason Imaging. 2016;38:225–235.
- Wijesurendra RS, Casadei B. Atrial fibrillation: effects beyond the atrium? Cardiovasc Res. 2015;105:238–247.
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270.
- Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502.
- Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010.
- Ndumele CE, Nasir K, Conceicao RD, et al. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–1932.
- Foroughi M, Maghsoudi Z, Khayyatzadeh S, et al. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. 2016;5:28–9175.
- Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20:2515–2532.
- Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring). 2008; Jun16:1394–1399.
- Ding YH, Ma Y, Qian LY, et al. Linking atrial fibrillation with non-alcoholic fatty liver disease: potential common therapeutic targets. Oncotarget. 2017;8:60673–60683.
- Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079.
- Indumathy J, Pal GK, Pal P, et al. Association of sympathovagal imbalance with obesity indices, and abnormal metabolic biomarkers and cardiovascular parameters. Obes Res Clin Pract. 2015;9:55–66.
- Sztajzel J, Golay A, Makoundou V, et al. Impact of body fat mass extent on cardiac autonomic alterations in women. Eur J Clin Invest. 2009;39:649–656.
- Lavie CJ, Alpert MA, Arena R, et al. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102.
- Bleau C, Karelis AD, St-Pierre DH, et al. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev. 2015;31:545–561.
- Exley MA, Hand L, O'Shea D, et al. Interplay between the immune system and adipose tissue in obesity. J Endocrinol. 2014;223:R41–R48.
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S.
- Mahajan R, Lau DH, Brooks AG, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66:1–11.
- Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100.
- Tselentakis EV, Woodford E, Chandy J, et al. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J Surg Res. 2006;135:68–75.
- Luttmann-Gibson H, Suh HH, Coull BA, et al. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med. 2010;67:625–630.
- Käräjämäki AJ. Non-alcoholic fatty liver disease (NAFLD) - perspectives to etiology, complications and lipid metabolism [dissertation]. Acta Universitatis Ouluensis. D, Medica: University of Oulu; 2018.
- Younossi ZM, Otgonsuren M, Venkatesan C, et al. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism. 2013;62:352–360.
- Yki JH. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910.
- Mantovani A, Nascimbeni F. Is it time to include non-alcoholic fatty liver disease in the current risk scores for atrial fibrillation? Dig Liver Dis. 2018;50:626–628.
- Mantovani A. Nonalcoholic fatty liver disease (NAFLD) and risk of cardiac arrhythmias: a new aspect of the liver-heart axis. J Clin Transl Hepatol. 2017;5:134–141.
- Targher G, Valbusa F, Bonapace S, et al. Association of nonalcoholic fatty liver disease with QTc interval in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2014;9:e88371–e88669.
- Hung CS, Tseng PH, Tu CH, et al. Nonalcoholic fatty liver disease is associated with QT prolongation in the general population. J Am Heart Assoc. 2015;4:e001820.
- Mantovani A, Rigamonti A, Bonapace S, et al. Nonalcoholic fatty liver disease is associated with ventricular arrhythmias in patients with type 2 diabetes referred for clinically indicated 24-hour Holter monitoring. Diabetes Care. 2016;39:1416–1423.
- Mantovani A, Rigolon R, Pichiri I, et al. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PLoS One. 2017;12:e0185459.
- Zhang Z, Zhang X, Korantzopoulos P, et al. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord. 2017;17:96.
- Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307.
- Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685.
- Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184.
- Polyzos SA, Mantzoros CS. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: a systematic review. Metabolism. 2016;65:1297–1306.
