Abstract
Abnormalities in body composition can occur at any body weight. Low muscle mass is a predictor of poor morbidity and mortality and occurs in several populations. This narrative review provides an overview of the importance of low muscle mass on health outcomes for patients in inpatient, outpatient and long-term care clinical settings. A one-year glimpse at publications that showcases the rapidly growing research of body composition in clinical settings is included. Low muscle mass is associated with outcomes such as higher surgical and post-operative complications, longer length of hospital stay, lower physical function, poorer quality of life and shorter survival. As such, the potential clinical benefits of preventing and reversing this condition are likely to impact patient outcomes and resource utilization/health care costs. Clinically viable tools to measure body composition are needed for routine screening and intervention. Future research studies should elucidate the effectiveness of multimodal interventions to counteract low muscle mass for optimal patient outcomes across the healthcare continuum.
Low muscle mass is associated with several negative outcomes across the healthcare continuum.
Techniques to identify and counteract low muscle mass in clinical settings are needed.
Key messages
Introduction
Measures of body mass such as weight and body mass index (BMI) have long been regarded as practical and sensitive for the prediction of health risks and outcomes. Although of value, these measurements do not depict an individual’s variability in body composition (i.e. lean versus adipose tissue). Body composition can be variable among individuals of the same body size, confounding the association between body weight and health, . Abnormalities in body composition such as low muscle mass are powerful predictors of morbidity and mortality, particularly in clinical settings where the disease or illness itself can lead to this condition [Citation1,Citation2]. In this narrative review, we provide an overview of the importance of low muscle mass on health outcomes for patients in inpatient, outpatient and long-term care clinical settings, as well as a past year review of the literature.
Low muscle mass: a new face of an old problem
From a theoretical standpoint, skeletal muscle is a primary driver of the relationship between body composition and clinical outcomes, as it is involved in mobility, strength and balance. However, there are several methods to measure body composition and as a result, there are many constructs and terms that ultimately reflect this compartment in the research hereby discussed (). Therefore, for clarity, we will refer to “muscle mass” when providing an overview and discussion of the topic and will use the terminology described in when referring to specific research findings. The latter will reflect the compartment being assessed by the body composition technique used in the study.
Table 1. Commonly used terminology in body composition research.
Several cutpoints have been used to define “low” muscle mass. We present the most commonly used ones in , although this list is not exhaustive. The term “sarcopenia” has often been used to describe low muscle mass in clinical settings. However, the term is now widely accepted to also include measures of strength, muscular performance, or physical performance [Citation1,Citation2] and usually used in context of ageing. Notably, cutoffs for low muscle mass are mostly derived from relatively healthy cohorts and hence are of limited applicability to different patient populations and clinical settings. However, they provide a benchmark for quantifying degrees of muscle depletion as it correlates to changes in health status. The absence of standardized diagnostic criteria for low muscle mass precludes a more comprehensive characterization of the prevalence and significance of this condition among different cohorts of patients, an issue discussed later in this review.
Table 2. Common cut-points to define low muscle mass.
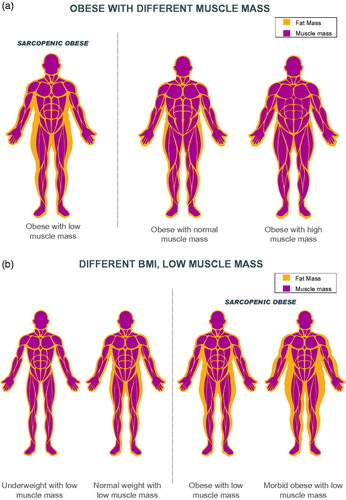
Low muscle mass is not restricted to individuals with a small frame, . The concurrent appearance of low muscle mass with high adiposity (also termed sarcopenic obesity) is the new face of an old problem, common in people who face chronic diseases. This condition has a compounding impact on health and outcomes. To date, medical practice relies on measures of body size such as BMI; however, BMI does not necessarily relate to muscle mass, a limitation that may impact the ability of healthcare professionals to carry out targeted interventions directed at addressing low muscle mass rather than interventions focused on addressing adiposity. The prevalence of low muscle mass relates to age, sex, diet, physical activity, disease state and hormonal regulation and other metabolic abnormalities in both obese and non-obese states.
Although most studies to date have only quantified muscle mass, the “quality” of the muscle is also a determinant of outcomes. This feature is assessed by the amount of fat infiltration in skeletal muscle (myosteatosis) using computerized tomography (CT) or magnetic resonance imaging. CT images provide measures of macroscopic intermuscular fat and muscle attenuation, the latter of which is inversely associated with muscle fat content [Citation3] The lower the muscle attenuation or radiodensity, the higher the amount of fat infiltration into muscle. This feature is an emerging prognostic indicator although primarily studied under the auspices of cancer, where imaging techniques are available for its assessment.
Selected techniques available for the estimation of the muscle mass compartment have been described elsewhere [Citation4,Citation5]. The reader is referred to previous articles for a comprehensive discussion of the benefits and limitations of available body composition techniques [Citation6].
Muscle loss across the continuum of care: an under-recognized phenomenon
Each sub-section of this article begins with an overview of the literature (expert opinion) followed by a summary of a year of recent publications. Our approach to focus on 12 months of publications was to highlight how the field has evolved into the current state of knowledge and how this has shaped current understanding of the role of low muscle mass in health care.
We included only studies using body composition techniques and not those using anthropometry (through equations or direct measures). As the purpose of this manuscript was to provide an overview of the most recent literature rather than a systematic review, criteria for selection of the publications were more lenient. Observational studies, longitudinal studies, and clinical trials were included. No exclusion criteria were set other than mentioned above. The search for January 2016–January 2017 publications was conducted exclusively on PubMed yielding 2350 articles. Search terms included those related to muscle mass and clinical outcomes, Supplementary Material.
One hundred and forty-three relevant articles in a single year were selected and are included in our discussion below, highlighting emerging research on the impact of muscle mass in various cohorts (). Articles were organized by clinical care settings: inpatient (surgery, cardiovascular disease, renal disease, chronic obstructive pulmonary disease [COPD], critical illness, “other”), outpatient (cancer, liver conditions, and primary care/population) and long-term care. Not all publications provided sufficient details regarding the study cohort; as such, samples of individuals undergoing surgery or any other procedure that typically requires at least one inpatient day are discussed in the context of inpatient (vs outpatient) settings. As discussed above, the terminology herein will refer to specific compartments being measured/estimated when discussing specific study findings (per ), except when discussing the assimilated data in general terms/collection of evidence.
Table 3. Summary of studies in a single year assessing the effect of muscle mass on clinical outcomes.
Inpatient settings
Low muscle mass in hospitalized patients is a prevalent phenomenon that worsens with increased time spent admitted to a hospital [Citation7]. This is led by a variety of reasons ranging from hyper-catabolism, inadequate food intake and/or immobility, depending on the condition being investigated. The ability to conduct in-depth body composition assessments in inpatient settings has contributed to a large number of investigations, including recent ones. We identified 79 articles that examined the relationship between muscle mass and outcomes published within the timeline described above. These included the following settings and conditions: surgical (n = 49), heart conditions (n = 10), renal disease (n = 5), COPD (n = 2), critical illness (n = 6) and other conditions not otherwise specified (n = 7) among hospitalized patients.
Surgery
Approximately 9.7 million inpatient surgical procedures occur in the United States each year [Citation8]. Identifying potential modifiable risk factors for complications and mortality is essential for optimal post-surgical care, such as immune status, nutritional status and muscle mass. Surgery invokes a catabolic environment and alterations in nutritional status possibly driven by nausea, vomiting, decreased appetite, etc. [Citation9], creating an environment conducive to muscle loss.
Low muscle mass is an economic burden to the health care system, as this condition is associated with an average increase of over $14,000 in total hospital costs per patient undergoing major abdominal surgery compared to patients with normal muscle amounts [Citation10]. Post-surgical complications such as sepsis, infection, prolonged ventilation and pneumonia are reported to occur at a higher rate and survival is shorter in individuals with low muscle mass undergoing different types of surgery [Citation11]. In patients with colorectal cancer and documented low muscle mass, hospital length of stay is longer, the infection risk is greater and more inpatient rehabilitation care is required than in patients with normal muscle mass [Citation12]. Furthermore, having low muscle is an independent predictor of post-operative infections and rehabilitation care among hospitalized older adults (≥65 years old) [Citation12]. Low muscle mass is also associated with a higher incidence of surgical complications and shorter survival in numerous different cancer types [Citation13]. In response to the significant burden of this condition, some programs have implemented preoperative lifestyle interventions that incorporate exercise, nutrition interventions, stress reduction, smoking cessation and spirometer exercises [Citation14]. The effectiveness of each intervention is still unknown but has been embraced by patients awaiting surgery [Citation14].
A year at a glance
Fourty-nine studies reported the impact of muscle mass on outcomes in surgical patients; most (n = 44, 89.8%) used CT images, four used bioelectrical impedance analysis (BIA, reporting fat-free mass or estimated skeletal muscle) and one used dual-energy X-ray absorptiometry (DXA, reporting fat-free mass). The majority of these publications (n = 26, 53%) were conducted in individuals with cancer. The remaining studies included diverse patient populations comprising of individuals undergoing surgery for liver or lung transplant, upper abdominal surgery, spine surgery, proctocolectomy, hip arthroplasty, intrahepatic portosystemic shunt placement, pancreaticoduodenectomy, pancreatic resection, aneurysm or general gastrointestinal resection. Of these, 28 studies investigated the impact of muscle mass on survival and most (n = 22; 78.6%) reported a positive association between survival and muscle mass [Citation15–36]. One publication reported that low muscle mass was not associated with survival in multivariate analysis (only in univariate analysis) [Citation37], and another found that low muscle mass was prognostic of survival only in the presence of obesity [Citation38], suggesting that disease factors and/or excess adiposity might work synergistically with muscle to predict outcomes. Four other publications reported no association between the amount of muscle mass and survival in individuals undergoing nephrectomy for kidney cancer [Citation39], pancreatoduodenectomy [Citation40], esophagectomy [Citation41] or left ventricular assist device implantation [Citation42]. The lack of an association could be partially explained by the patients included, who may have experienced body composition changes after neoadjuvant therapy [Citation41] or who had advanced cancer [Citation39]. One of the studies that found no association between muscle mass and overall survival also reported that higher muscle attenuation (i.e. better muscle quality) was associated with longer survival [Citation40].
Post-operative complications were additional common outcomes found to be associated with low muscle mass or attenuation in 18 studies [Citation28,Citation32,Citation34,Citation36,Citation38–40,Citation43–53], except for one [Citation43], possibly due to the high rates of complication in the entire sample. Low muscle attenuation [Citation24] and low muscle mass [Citation47,Citation54,Citation55] were associated with longer length of hospital stay and hospital readmissions [Citation34,Citation44,Citation48,Citation56,Citation57]. Other outcomes that were reported to be associated with low muscle mass included general morbidity [Citation24,Citation34,Citation41], surgical complications [Citation58], reduced physical function [Citation57,Citation59], lower quality of life [Citation59], higher inflammatory response after surgery [Citation60], discharge destination to a nursing or rehabilitation facility [Citation34,Citation56] and higher hospitalization costs [Citation10,Citation61,Citation62]. One study reported a null association between muscle mass and quality of life post-discharge [Citation63]; although the population was large (n = 215), and diagnoses within the sample were diverse (cancer and non-cancer patients), which could have concealed significant sub-group associations.
In sum, individuals undergoing surgery have diverse clinical backgrounds. Nevertheless, low muscle mass and attenuation have been associated with shorter survival and worse post-operative complications, among other negative outcomes, in most studies.
Cardiovascular disease/conditions
Cardiovascular disease is a leading cause of death worldwide. Heart failure, in particular, is highly prevalent, affecting approximately one in five adults during their lifetime [Citation64] and might trigger substantial loss of muscle mass.
Obesity is a risk factor for heart disease, and many patients, therefore, have obesity at disease presentation. However, body weight may be artificially elevated due to edema and can conceal underlying low muscle mass. This influences body composition measurement since hydration may affect some measures of muscle mass. Nevertheless, the presence of low muscle mass is estimated to occur in almost 20% of patients with stable heart failure [Citation65]. It is associated with a decline in several functional parameters such as strength (handgrip and quadriceps), total peak VO2, walking distance (6-meter walk tests) [Citation65], abnormal cardiac parameters and cardiac perfusion [Citation66].
A year at a glance
Ten articles investigated the association between low muscle mass with clinical outcomes in heart diseases/conditions in acute care settings. All studies except one (DXA) reported muscle mass using CT images. The patient populations of interest included individuals undergoing procedures such as transcatheter aortic valve implantation, aortic aneurysm repair or left ventricular assist device implantation. Higher muscle mass was associated with improved overall survival in most [Citation30–33,Citation50,Citation67], but not all studies [Citation42]. Though low muscle mass was not associated with overall survival in one publication, patients with lower muscle had higher rates of inpatient death or prolonged length of hospital stay [Citation42]. Compared to low muscle, having a higher muscle mass was also positively associated with better physical function [Citation49, Citation68,Citation69], New York Heart Association score [Citation33], fewer post-operative complications [Citation32,Citation50], shorter length of stay [Citation42,Citation49,Citation69] and less ventilator support [Citation32]. Among these discussed, low muscle mass was a negative prognostic factor in cardiovascular disease.
Renal disease
In individuals with chronic kidney disease (CKD), systemic inflammation, transient catabolic comorbidities, nutrient losses during dialysis, endocrine abnormalities (such as resistance to insulin, growth hormone and insulin-like growth factor), hyperglycemia, hyperparathyroidism and loss of blood during hemodialysis are prevalent [Citation70]. Additionally, reduced protein diets of 0.6–0.8 g/kg/day may be recommended to patients, not on dialysis. These factors contribute to muscle wasting, which is usually reported under the auspices of protein-energy wasting [Citation70]. In individuals undergoing dialysis, old age, comorbidities, subjective global assessment score > 1, inactivity, low albumin and inflammation (C-reactive protein) were associated with low handgrip strength but not with low muscle mass measured by DXA [Citation71]. In the same study, body composition alone was not associated with poorer survival; however, low strength alone (hazard ratio [HR] 1.98, 95% confidence interval [CI]: 1.01–3.87, p = .04) or in combination with low muscle mass (HR: 1.93, 95% CI: 1.01–3.71, p = .04) was more strongly associated with higher mortality [Citation71]. These findings suggest that strength and muscle mass – while highly related – are two entities differently affecting outcomes in this population.
A year at a glance
A variety of body composition techniques were used in patients with CKD including CT images, DXA, BIA, bioelectrical impedance spectroscopy and magnetic resonance imaging. In these studies, higher muscle mass was associated with better physical function [Citation72,Citation73]; both the amount of muscle mass [Citation74] and muscle attenuation [Citation75] were indicative of better survival, although another study reported no associations [Citation76]. In the latter publication [Citation76], all patients had sarcopenic obesity and were actively undergoing maintenance hemodialysis, and no standard cut points of low muscle mass could predict survival. Overall, these studies suggest that muscle mass is associated with poorer outcomes in renal disease patients, but the effect of obesity and other confounders such stage of the disease might mitigate these associations.
COPD
Weight loss is common in individuals with COPD, instigated by the difficulty of eating [Citation77] and higher energy expenditure [Citation78,Citation79]. Fat-free mass represents a large portion of this weight loss, as 15–40% of COPD patients are reported to have low fat-free mass [Citation80,Citation81]. Patients with COPD are also three times more likely to have low fat-free mass with obesity (sarcopenic obesity), poor physical performance (i.e. lower 6-minute walk test distance) and higher systemic inflammation [Citation82].
A year at a glance
Recent clinical evidence demonstrates that individuals with COPD with higher muscle mass (measured by BIA or DXA) experienced better outcomes (e.g. improved forced expiratory volume and COPD assessment test score and reduced dyspnea) [Citation83] and lower occurrence of osteopenia/osteoporosis than COPD patients with low muscle mass [Citation84]. Importantly, the prevalence of low muscle mass was higher in men with COPD compared to matched males with normal lung function. There was a relationship between muscle mass and airflow limitation [Citation84], which suggests that disease severity may induce or exacerbate muscle loss in individuals with COPD. The studies in the past year show that muscle mass is a predictor of important disease-specific outcomes.
Critical illness
Almost 6 million individuals are admitted to intensive care units (ICU) in the United States each year. The most frequent reasons for admission include respiratory system issues with ventilator support required, acute myocardial infarction, intracranial haemorrhage or cerebral infarction, percutaneous cardiovascular procedure with stent and sepsis [Citation85].
The stress response to trauma induces a negative protein balance and resistance to anabolic signals; this reaction, along with physical inactivity [immobility] ultimately leads to proteolysis and loss of muscle mass [Citation86]. Up to 63% of individuals admitted to the ICU on ventilator support have low muscle mass [Citation87], and this percentage is higher in patients ≥ 65 years old [Citation88]. Muscle wasting is likely to worsen during the hospital stay due to considerable systemic inflammation, pre-existing comorbidities, multi-organ dysfunction and prolonged bed rest [Citation89]. Numerous studies have shown that low muscle mass is associated with lower ventilator-free and ICU days [Citation88] and shorter survival [Citation87,Citation88,Citation90].
A year at a glance
In six studies evaluating the critical illness population, muscle mass was usually measured via CT images; one study used BIA-derived phase angle, which is an indicator of cell membrane function and nutritional status [Citation91]. Higher muscle mass or muscle attenuation was unanimously associated with better survival [Citation91–96]. More specifically, greater muscle attenuation was associated with lower 6-month mortality (HR per 10 Hounsfield units: 0.640; 95% CI:0.55–0.72, p < .001) in mechanically ventilated critically ill patients, after adjustment for the Acute Physiological, Age and Chronic Health Evaluation (APACHE) II score, BMI and muscle mass [Citation94]. Phase angle was predictive of 28-day mortality (OR: 0.63, 95% CI: 0.78–0.96, p = .008) in individuals in the ICU [Citation91]. Trauma patients (n = 23,622) in the lowest quartile of muscle mass had over nine times greater odds of death compared to those in the highest quartile (OR: 9.15, 95% CI not reported, p < .001) [Citation93]. In terms of outcomes other than survival, two studies reported an inverse association between length of stay and muscle mass [Citation92] and density [Citation94]. In emergency surgery, higher muscle mass was predicative of lower surgical morbidity, but not survival in multivariate analysis [Citation92]; however, this study used only psoas muscle, which may not be representative of whole-body skeletal muscle [Citation97]. Overall, both the amount of muscle mass and attenuation are usually indicative of survival and other clinical outcomes.
Other conditions
Individuals with aspiration pneumonia, abdominal wall hernias and pancreatic disease are also inpatients, and at risk for muscle loss (measured by BIA in four studies and CT images in two studies). In general, higher muscle mass was associated with better outcomes such as physical function [Citation98], disease-specific outcomes (pancreatic exocrine insufficiency) [Citation99], less dysphagia [Citation100,Citation101], shorter length of stay [Citation102] and survival [Citation103,Citation104].
Outpatient settings
Cancer
Individuals with cancer make up a large proportion of patients receiving care in outpatient settings. The tumour-bearing state often induces metabolic alterations such as anorexia, hypoanabolism or hypercatabolism [Citation105,Citation106], which might elicit dramatic changes in body composition.
As obesity is a risk factor for certain types of cancer, many newly diagnosed patients have obesity, and up to 15% of patients with obesity have low muscle mass [Citation107]. Low muscle is associated with worse prognosis including poorer quality of life and function [Citation108], severe treatment toxicity, more postoperative infections and complications, incidence of hospitalization, longer length of hospital stay and shorter survival [Citation108–110].
CT images are regularly obtained for diagnostic and follow-up purposes and are an opportunistic method to accurately assess muscle mass, usually using the 3rd lumbar vertebra as a landmark [Citation111]. The availability of these images allows for a large number of studies exploring the association between muscle mass and patient outcomes. Additionally, the assessment of muscle attenuation is also possible; CT-assessed low muscle attenuation is of emerging importance as it may relate to even higher indication of poor patient prognosis, as discussed elsewhere [Citation110,Citation112,Citation113].
A year at a glance
Twenty-five of the studies in outpatient populations were in oncology; all of them included measurement of muscle mass using CT images. Many of these publications found that higher amounts of muscle mass were associated with improved survival [Citation114–127]. Others reported a statistically significant association between survival and intramuscular adipose tissue [Citation125,Citation126,Citation128–131]. Few studies found a significant association only in sub-groups of patients, such as those with a high neutrophil/lymphocyte ratio in males with small cell lung cancer [Citation132], disease with lymph node involvement in individuals with esophageal squamous cell carcinoma [Citation58] or pancreatic cancer patients with a BMI ≥ 22 kg/m2 [Citation133], suggesting that disease status and body weight might influence the impact of muscle mass on survival. One study reported only a trend towards significance for the association between muscle mass and survival [Citation134], and another found that muscle mass was predictive of survival only in univariate analysis [Citation97]. In the outpatient oncology setting, only one investigation reported no association between body composition and survival in advanced non-small cell lung cancer [Citation135].
Treatment toxicity [Citation135,Citation136], poorer radiographic and objective response to therapy [Citation117], fewer treatment cycles completed [Citation134], and shorter time to tumour progression [Citation135] were also associated with low muscle mass. Some studies reported no association of muscle mass or attenuation and the following outcomes: time to disease progression [Citation135], toxicity to chemotherapy [Citation135] and objective response to therapy [Citation117]. However, these studies were either carried out in advanced cancer patients with almost 10% weight loss in six months [Citation135] or were in a sample that reported high muscle density was associated with radiographic complete response, but not objective response rates [Citation117]. Therefore, body composition is still a useful tool for predicting outcomes in outpatient cancer settings.
Cancer research in one year shows that while low muscle mass might predict lower survival in most studies, factors such as inflammation, disease stage, and BMI might mitigate this relationship. Body composition might also relate to other prognostic outcomes.
Liver diseases
Liver conditions largely comprise of cirrhosis and liver disease. Cirrhosis is a highly catabolic condition due to insufficient liver glycogen storage, metabolic patterns are similar to that observed in healthy individuals after 2–3 days of starvation [Citation137]. Of particular concern is high use of amino acids as fuel, leading to the breakdown of muscle tissue.
Up to 43% of patients with cirrhosis have low muscle mass, 20% have low muscle concurrent with obesity (sarcopenic obesity), and 52% have low muscle attenuation [Citation138]. Importantly, patients with any body composition abnormality have shorter survival compared to patients with normal body composition [Citation138].
A year at a glance
Five studies investigated the impact of muscle mass on outcomes in liver conditions, using muscle mass from CT images (n = 3), estimated muscle mass from BIA data, (n = 1), or both (n = 1). In these individuals, low muscle mass was associated with worse albumin levels [Citation139], Child-Pugh scores [Citation139], physical function [Citation140] (correlation only) and shorter survival [Citation138,Citation141,Citation142]. Body composition in these studies was, therefore, a useful tool for prognostication.
Primary care and population
Body composition abnormalities such as low muscle mass can occur across age, sex and BMI groups [Citation143]. Hence, it is plausible that a seemingly healthy individual might have low muscle mass. Research on the implications of low muscle mass is limited in younger adults. Instead, community-dwelling older adults are the most common population studied in primary care and population-level investigations. As such, low muscle mass has been associated with increased risk of falls, osteoporosis, hospital re-admission and difficulties in activities of daily living [Citation1]. At the same time, fat mass increases until the sixth decade of life [Citation143], increasing the risk of these individuals to develop sarcopenic obesity.
Among older adults, men often have higher rates of low muscle mass than women [Citation144], due in part to the higher amounts of muscle men have earlier in life. However, the mortality risk is higher in women with low muscle [Citation144]. This is likely because women have higher fat mass and lower muscle mass and strength than men, increasing their risk of developing functional decline.
Physical function is also critical in older adults, as measures such as grip strength and gait speed are inversely associated with disability and mortality [Citation145,Citation146]. In fact, some suggest that these measures are a different construct than muscle mass [Citation147], and including measures of physical function might be more predictive of negative outcomes than measures/estimations of muscle mass alone [Citation146,Citation148]. The term sarcopenia is used in this setting when both low mass and function/performance are taken into account [Citation1,Citation2].
A year at a glance
Twenty articles investigated low muscle mass in primary care, mainly in healthy community-dwelling older adults. Muscle mass was measured using BIA (n = 9), DXA (n = 7), CT images (n = 3) or magnetic resonance imaging (n = 1). Higher muscle mass and not having sarcopenia was positively associated with serum albumin levels [Citation149] better physical function [Citation150–159], higher quality of life [Citation157,Citation159] and longer survival [Citation154,Citation160–162]. Higher muscle mass was also negatively associated with cardiovascular disease risk factors [Citation163], idiopathic pulmonary fibrosis [Citation154], low bone mineral density [Citation164], bone fractures [Citation165], hospitalization [Citation161], poor pulmonary function [Citation166]; sarcopenia was associated with poorer disease-specific outcomes such as ankylosing spondylitis [Citation164] and poor renal function [Citation167]. Better muscle attenuation was associated with improved balance [Citation150], quality of life [Citation159] and survival [Citation162]. Notably, one study [Citation168] found no association between estimated muscle mass (from BIA) and quality of life in older adults; only physical activity, handgrip strength and balance were associated with overall quality of life. In older individuals, strength declines more rapidly than muscle mass and factors such as muscle attenuation, metabolism, aerobic capacity, insulin resistance, fibrosis and neural activation might be more relevant for physical function and impaired mobility (and thus the quality of life) than muscle mass alone [Citation169–171].
The impact of muscle mass and sarcopenia on patient/healthcare outcomes has also been investigated on a population level (mainly community-dwelling middle-age or older adults) in fourteen studies, which primarily utilized DXA alone (n = 10) or in combination with air displacement plethysmography (n = 1), followed by BIA (n = 2) and CT images (n = 1). Survival was longer in those with more muscle mass [Citation172–177]. Sarcopenia was associated with lower bone mineral density [Citation178] and osteoporosis risk [Citation179], and low muscle mass was associated with poor pulmonary function [Citation180] and physical function [Citation179,Citation181], and higher fracture risk [Citation182,Citation183], frailty [Citation181], and morbidity [Citation184]. One study reported that slow walking speed, but not low muscle mass was associated with increased risk of hospitalization or higher likelihood of short-term nursing facility stay in women aged ≥65 years [Citation185].
The outcomes assessed in this group of individuals are diverse. Although low muscle mass is predictive of many of these outcomes in primary care and population level settings, the interplay between other ageing-related factors (e.g. hormonal changes, muscle energetics, neural connectivity, muscle blood flow, etc.) is also thought to contribute to mobility-disability in this population.
Long-term care settings
The pathophysiology of low muscle mass in long-term care residents is multi-factorial and consists of morphological changes (e.g. type II muscle fiber loss), hormonal changes (e.g. lower testosterone, estrogen and growth hormone), higher inflammation/oxidative stress and lifestyle influences (low physical activity and energy intake) [Citation186]. Comorbidities such as heart disease, dementia and Parkinson’s disease are common and contribute to further nutritional decline [Citation187].
Low muscle mass in long-term care is most often investigated as a construct of sarcopenia (i.e. low mass and function). Sarcopenia in individuals in long-term care is more prevalent than in community-dwelling adults [Citation188]. In individuals age 70 years and older, over one-third have sarcopenia and this condition is associated with a higher risk of death compared to residents without sarcopenia after adjusting for age, sex, physical impairment, BMI and a number of comorbidities (HR 2.34, 95% CI 1.04–5.24) [Citation189].
A year at a glance
Dual X-ray absorptiometry (n = 5) and BIA (n = 2) were the only measurement methods used in seven studies in the long-term care setting. Cognitive function was the most frequently reported outcome, and most did not find any association between sarcopenia and this parameter [Citation190–191]. Activities of daily living were found to be associated with muscle mass and sarcopenia in two studies [Citation192,Citation193], but not another [Citation191]. Mixed findings were also apparent when the relationship between muscle mass [Citation193] or sarcopenia [Citation191] and nutritional status as measured by the Mini Nutritional Assessment was investigated (positive relationship [Citation193]; null [Citation191]). Individuals with lower muscle mass had worse Alzheimer’s severity [Citation194] and individuals with sarcopenia had poorer survival [Citation195]. A year of research had no conclusive evidence for a diverse array of outcomes.
Treating low muscle mass: Addressing an unmet medical need
The above discussion, complimented by a one-year glimpse at publications, showcases the rapidly growing research of body composition in clinical settings. As we discuss, low muscle mass is prevalent across the continuum of care and is a predictor of poor outcomes. Therefore, the potential clinical benefits of preventing and reversing low muscle mass in patients are likely to impact not only patient outcomes but also resource utilization/health care costs [Citation61,Citation196–198].
Some clinicians may question the anabolic potential of patients who suffer from acute or chronic conditions associated with muscle loss. However, evidence suggests that interventions can help to maintain or rebuild muscle in these patients [Citation199]. For instance, cancer patients were found to have a normal anabolic response to nutrition interventions [Citation200], not differently than healthy persons [Citation201], even after surgery [Citation202]. Also, in patients with COPD, the anabolic response to nutrition is not different from that of healthy individuals [Citation203].
New perspectives
The availability of body composition techniques is still limited in clinical settings. Nonetheless, as the value of assessing muscle mass and mitigating muscle loss emerges, so will the recognition of its use for adequate patient screening and monitoring, and we anticipate a surge of technological developments and financial investments. The recent establishment of ICD-10-CM code for sarcopenia by the Centers for Disease Control and Prevention highlights the momentum for such change in perspective and practice. With the establishment of the ICD-10 code in the US, muscle loss as sarcopenia is now recognized as a condition that can be documented and reported within the healthcare setting and for data collection. The code, M62.84, is now available for use by the medical community effective 1 October 2016. The new code designation for this condition has the potential to affect research and development of treatments. It calls into action a need to establish clear clinical guidelines for diagnosis, early intervention and treatment of muscle loss. It will potentially enable development and reimbursement of diagnostic tools to measure muscle mass. It also opens new avenues for development of novel therapeutics targeting muscle loss that could receive FDA approval. Having an ICD-10 code will also enable data capture from electronic medical records, death certificates and other system data sources, which will help the understanding of health economics associated with this condition, also helping researchers to access this data more easily. Overall, the ICD-10 code gives validity to the importance of muscle mass in driving long-term health benefits across the continuum of care.
Where do we go from here?
With this increasing evidence on the importance of maintaining muscle mass in various clinical settings, it is critical to develop effective health screening tools to identify people at risk of losing muscle. Tools such as the SARC-F (strength, assistance in walking, rise from a chair, climb stairs, falls) questionnaire [Citation204] are quick and valid [Citation205] in older individuals, but the psychometric properties of such instruments in clinical populations has not been determined. In addition, there is a need for clinically viable tools to measure muscle mass (and attenuation) at the bedside and develop early intervention strategies to mitigate muscle loss. Currently, body composition measurement tools (e.g. DXA, CT) although available to specific clinical settings are not widely available to the general population. Hand held tools such as ultrasound and BIA show promise [Citation206] and are in various stages of validation in different clinical settings. Measurements of muscle function such as grip strength are very good predictors of mobility limitations in older adults, as previously discussed [Citation207]. Therefore, regulatory agencies have suggested improvement in both muscle mass and physical function or survival as endpoints, although these outcomes are ambiguous [Citation208,Citation209].
For such tools to become routine in clinical practice, healthcare professionals need to be educated on the importance of muscle loss, and how to incorporate routine screening and intervention for these conditions into their clinical practice. For example, screening for muscle loss could be included as part of the Welcome to Medicare and annual Medicare wellness preventive exams in the United States.
In order to provide a foundation for clinical measurement of body composition, a consensus definition of low muscle is needed for various clinical populations. The use of CT images has been recently popularized due to its widespread availability. Total muscle cross-sectional area at the 3rd lumbar vertebrae is highly correlated with whole-body muscle mass [Citation210] and is often used to identify low muscle. The use of a single muscle group (e.g. psoas) is a new trend in body composition assessment. However, this methodology is poorly correlated with total skeletal area and may impede correct interpretation of prognosis [Citation97]. Furthermore, this particular muscle might atrophy due to comorbidities such as low back pain or hip osteoarthritis independent of surrounding musculature [Citation211,Citation212]. Some research suggests using linear measures of two muscle groups (psoas and paraspinal muscles) in combination with age and sex can accurately identify individuals with low muscle mass [Citation213], although alternative measurements of total muscle cross-sectional area have been strongly discouraged [Citation214].
Although CT images using total muscle cross-sectional area at the third lumbar vertebra offer an accurate assessment of whole body skeletal muscle mass, certain conditions (i.e. critical care settings) limit weight-bearing activity and are associated with disproportional muscle atrophy in the lower limbs [Citation215]. Therefore, the accuracy of CT images for whole-body muscle may be compromised, although little is known on this topic. Furthermore, CT imaging may not be sensitive enough to detect body composition changes in short time periods [Citation216]. Ultrasound is emerging as the method of choice for such settings where selected muscle groups can be assessed individually [Citation217], although protocols for accurate assessment are still underway.
There is a considerable need to counteract the loss of muscle mass, strength and physical function. Possible dietary interventions might include protein/amino acid formulas, creatine, β-hydroxy-β-methylbuthyrate and micronutrients, among others. Exercise typically consists of strength or aerobic activity in a supervised or home-based intervention. A recent systematic review concluded that exercise improves muscle mass, strength and physical performance in healthy adults 60 years and older with additional benefit of nutrition in a small proportion of studies [Citation218]. The purported limited effectiveness of nutrition could be due to the great heterogeneity in the type and duration of dietary supplement protocols. Future well-designed clinical trials that combine some form of nutrition and exercise such as the Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy (MENAC) [Citation219] and Nutrition and Exercise in Critical Illness (NEXIS) [Citation220] will elucidate the potential feasibility and efficacy of methods to counteract muscle loss.
Supplemental Material
Download MS Word (13.6 KB)Disclosure statement
Carolyn Alish and Suzette Pereira are employees of Abbott. Carla Prado has received speaker honorarium from Abbott. Nicolaas E. Deutz has received research funding and speaker honorarium from Abbott. Bret H. Goodpaster has received research funding from Abbott. Steven B. Heymsfield serves on Medical Advisory Board for Tanita Corporation.
Additional information
Funding
References
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423.
- Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558.
- Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210:489–497.
- Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom. 2015;18:467–471.
- Heymsfield SB, Gonzalez MC, Lu J, et al. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74:355–366.
- Prado CM, Pinto CM, Gonzalez MC, et al. Techniques for Assessment of Body Composition in Health. Scientific American Nutrition. Hamilton, Ontario, Canada: Decker Intellectual Properties; 2017.
- Koukourikos K, Tsaloglidou A, Kourkouta L. Muscle atrophy in intensive care unit patients. Acta Inform Med. 2014;22:406–410.
- Agency for Healthcare Research and Quality. Inpatient vs. Outpatient Surgeries in U.S. Hospitals Rockville, MD, USAMarch 2015. [cited 2018 Aug 24]. Available from: www.hcup-us.ahrq.gov/reports/infographics/inpt_outpt.jsp.
- Bisgaard T, Kehlet H. Early oral feeding after elective abdominal surgery—what are the issues? Nutrition. 2002;18:944–948.
- Gani F, Buettner S, Margonis GA, et al. Sarcopenia predicts costs among patients undergoing major abdominal operations. Surgery. 2016;160:1162–1171.
- Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261.
- Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936.
- Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and somplications in surgical sncology: A review of the current literature. J Surg Oncol. 2015;112:503–509.
- Friedman J, Lussiez A, Sullivan J, et al. Implications of sarcopenia in major surgery. Nutr Clin Pract. 2015;30:175–179.
- Hale AL, Twomey K, Ewing JA, et al. Impact of sarcopenia on long-term mortality following endovascular aneurysm repair. Vasc Med. 2016;21:217–222.
- Higashi T, Hayashi H, Taki K, et al. Sarcopenia, but not visceral fat amount, is a risk factor of postoperative complications after major hepatectomy. Int J Clin Oncol. 2016;21:310–319.
- Lee S, Paik HC, Haam SJ, et al. Sarcopenia of thoracic muscle mass is not a risk factor for survival in lung transplant recipients. J Thorac Dis. 2016;8:2011–2017.
- Fukushima H, Nakanishi Y, Kataoka M, et al. Postoperative changes in skeletal muscle mass predict survival of patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy. Clin Genitourin Cancer. 2017;15:e229–e238.
- Hervochon R, Bobbio A, Guinet C, et al. Body mass index and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg. 2017;103:287–295.
- Hirasawa Y, Nakashima J, Yunaiyama D, et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. 2016;23:1048–1054.
- Huang DD, Chen XX, Chen XY, et al. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol. 2016;142:2347–2356.
- Ishihara H, Kondo T, Omae K, et al. Sarcopenia predicts survival outcomes among patients with urothelial carcinoma of the upper urinary tract undergoing radical nephroureterectomy: a retrospective multi-institution study. Int J Clin Oncol. 2017;22:136–144.
- Itoh S, Yoshizumi T, Kimura K, et al. Effect of Sarcopenic Obesity on Outcomes of Living-Donor Liver Transplantation for Hepatocellular Carcinoma. Anticancer Res. 2016;36:3029– 3034.
- Malietzis G, Currie AC, Athanasiou T, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103:572–580.
- Okumura S, Kaido T, Hamaguchi Y, et al. Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24:1037–1045.
- Paireder M, Asari R, Kristo I, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43:478–484.
- Psutka SP, Boorjian SA, Moynagh MR, et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol. 2016;195:270–276.
- Suzuki Y, Okamoto T, Fujishita T, et al. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92–97.
- van Dijk DP, Bakens MJ, Coolsen MM, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:317–326.
- Drudi LM, Phung K, Ades M, et al. Psoas muscle area predicts all-cause mortality after endovascular and open aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2016;52:764–769.
- Mok M, Allende R, Leipsic J, et al. Prognostic value of fat mass and skeletal muscle mass determined by computed tomography in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2016;117:828–833.
- Paknikar R, Friedman J, Cron D, et al. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151:745–750.
- Saji M, Lim DS, Ragosta M, et al. Usefulness of psoas muscle area to predict mortality in patients undergoing transcatheter aortic valve replacement. Am J Cardiol. 2016;118:251–257.
- Bokshan SL, Han AL, DePasse JM, et al. Effect of sarcopenia on postoperative morbidity and mortality after thoracolumbar spine surgery. Orthopedics. 2016;39:e1159–e1e64.
- Hamaguchi Y, Kaido T, Okumura S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101:565.
- Izumi T, Watanabe J, Tohyama T, et al. Impact of psoas muscle index on short-term outcome after living donor liver transplantation. Turk J Gastroenterol. 2016;27:382–388.
- Rutten IJ, Ubachs J, Kruitwagen RF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43:717–724.
- Boer BC, de Graaff F, Brusse-Keizer M, et al. Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis. 2016;31:1117–1124.
- Peyton CC, Heavner MG, Rague JT, et al. Does sarcopenia impact complications and overall survival in patients undergoing radical nephrectomy for stage III and IV kidney cancer? J Endourol. 2016;30:229–236.
- Van Rijssen LB, van Huijgevoort NC, Coelen RJ, et al. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann Surg Oncol. 2017;24:272–280.
- Grotenhuis BA, Shapiro J, van Adrichem S, et al. Sarcopenia/muscle mass is not a prognostic factor for short- and long-term outcome after esophagectomy for cancer. World J Surg. 2016;40:2698–2704.
- Heberton GA, Nassif M, Bierhals A, et al. Usefulness of psoas muscle area determined by computed tomography to predict mortality or prolonged length of hospital stay in patients undergoing left ventricular assist device implantation. Am J Cardiol. 2016;118:1363–1367.
- Chemama S, Bayar MA, Lanoy E, et al. Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol. 2016;23:3891–3898.
- Lou N, Chi CH, Chen XD, et al. Sarcopenia in overweight and obese patients is a predictive factor for postoperative complication in gastric cancer: a prospective study. Eur J Surg Oncol. 2017;43:188–195.
- Makiura D, Ono R, Inoue J, et al. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: a retrospective cohort study. J Geriatr Oncol. 2016;7:430–436.
- Nishigori T, Okabe H, Tanaka E, et al. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol. 2016;113:678–684.
- Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol. 2016;23:556–564.
- Lindqvist C, Majeed A, Wahlin S. Body composition assessed by dual-energy X-ray absorptiometry predicts early infectious complications after liver transplantation. J Hum Nutr Diet. 2017;30:284–291.
- Dahya V, Xiao J, Prado CM, et al. Computed tomography-derived skeletal muscle index: A novel predictor of frailty and hospital length of stay after transcatheter aortic valve replacement. Am Heart J. 2016;182:21–27.
- Garg L, Agrawal S, Pew T, et al. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol. 2017;119:457–460.
- Fujikawa H, Araki T, Okita Y, et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg Today. 2017;47:92–98.
- Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15:934–936.
- Nishida Y, Kato Y, Kudo M, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. 2016;20:1586–1594.
- Kalafateli M, Mantzoukis K, Choi Yau Y, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the model for end-stage liver disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121.
- Tsaousi G, Kokkota S, Papakostas P, et al. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur J Cancer Care. 2017;26:e12491.
- Onesti JK, Wright GP, Kenning SE, et al. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatol. 2016;16:284–289.
- Weig T, Milger K, Langhans B, et al. Core muscle size predicts postoperative outcome in lung transplant candidates. Ann Thorac Surg. 2016;101:1318–1325.
- Harada K, Ida S, Baba Y, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29:627–633.
- Jo S, Park SB, Kim MJ, et al. Comparison of balance, proprioception and skeletal muscle mass in total hip replacement patients with and without fracture: a pilot study. Ann Rehabil Med. 2016;40:1064–1070.
- Reisinger KW, Derikx JP, van Vugt JL, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2016;35:924–927.
- Sousa AS, Guerra RS, Fonseca I, et al. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr. 2016;70:1046–1051.
- van Vugt JLA, Buettner S, Levolger S, et al. Low skeletal muscle mass is associated with increased hospital expenditure in patients undergoing cancer surgery of the alimentary tract. PLoS One. 2017;12:e0186547.
- Aahlin EK, Trano G, Johns N, et al. Health-related quality of life, cachexia and overall survival after major upper abdominal surgery: a prospective cohort study. Scand J Surg. 2017;106:40–46.
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41.
- Fulster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34:512–519.
- Uematsu M, Akashi YJ, Ashikaga K, et al. Association between heart rate at rest and myocardial perfusion in patients with acute myocardial infarction undergoing cardiac rehabilitation - a pilot study. Aoms. 2012;4:622–630.
- Matsubara Y, Matsumoto T, Inoue K, et al. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J Vasc Surg. 2017;65:1390–1397.
- Bekfani T, Pellicori P, Morris DA, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41–46.
- Zuckerman J, Ades M, Mullie L, et al. Psoas muscle area and length of stay in older adults undergoing cardiac operations. Ann Thorac Surg. 2017;103:1498–1504.
- Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398.
- Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9:1720–1728.
- Hsiao SM, Tsai YC, Chen HM, et al. Association of fluid status and body composition with physical function in patients with chronic kidney disease. PLoS One. 2016;11:e0165400.
- Segura-Orti E, Gordon PL, Doyle JW, et al. Correlates of physical functioning and performance across the spectrum of kidney function. Clin Nurs Res. 2018;27:579–596.
- Barros A, Costa BE, Mottin CC, et al. Depression, quality of life, and body composition in patients with end-stage renal disease: a cohort study. Rev Bras Psiquiatr. 2016;38:301–306.
- Locke JE, Carr JJ, Nair S, et al. Abdominal lean muscle is associated with lower mortality among kidney waitlist candidates. Clin Transplant. 2017;31:e12911.
- Malhotra R, Deger SM, Salat H, et al. Sarcopenic obesity definitions by body composition and mortality in the hemodialysis patients. J Ren Nutr. 2017;27:84–90.
- Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J. 2014;44:1504–1520.
- Slinde F, EllegÅRd L, GrÖNberg AM, et al. Total energy expenditure in underweight patients with severe chronic obstructive pulmonary disease living at home. Clin Nutr. 2003;22:159–165.
- Baarends EM, Schols AM, Westerterp KR, et al. Total daily energy expenditure relative to resting energy expenditure in clinically stable patients with COPD. Thorax. 1997;52:780–785.
- Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83.
- Schols AM, Soeters PB, Dingemans AM, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–1156.
- Joppa P, Tkacova R, Franssen FM, et al. Sarcopenic obesity, functional outcomes, and systemic inflammation in patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2016;17:712–718.
- Pothirat C, Chaiwong W, Phetsuk N, et al. The relationship between body composition and clinical parameters in chronic obstructive pulmonary disease. J Med Assoc Thai. 2016;99:386–393.
- Hwang JA, Kim YS, Leem AY, et al . Clinical implications of sarcopenia on decreased bone density in men with COPD. Chest. 2017;151:1018–1027.
- Society of Critical Care Medicine. Critical Care Patients. Mount Prospect, IL; 2016 [cited 2018 Aug 24].
- Preiser J-C, van Zanten ARH, Berger MM, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care. 2015;19:35.
- Weijs PJ, Looijaard WG, Dekker IM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18:R12.
- Moisey LL, Mourtzakis M, Cotton BA, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206.
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). Jpen J Parenter Enteral Nutr. 2016;40:159–211.
- Akahoshi T, Yasuda M, Momii K, et al. Sarcopenia is a predictive factor for prolonged intensive care unit stays in high-energy blunt trauma patients. Acute Med Surg. 2016;3:326–331.
- Thibault R, Makhlouf AM, Mulliez A, et al. Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: the international prospective observational study phase angle project. Intensive Care Med. 2016;42:1445–1453.
- Dirks RC, Edwards BL, Tong E, et al. Sarcopenia in emergency abdominal surgery. J Surg Res. 2017;207:13–21.
- Leeper CM, Lin E, Hoffman M, et al. Computed tomography abbreviated assessment of sarcopenia following trauma: the CAAST measurement predicts 6-month mortality in older adult trauma patients. J Trauma Acute Care Surg. 2016;80:805–811.
- Looijaard WG, Dekker IM, Stapel SN, et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20:386.
- Shibahashi K, Sugiyama K, Kashiura M, et al. Decreasing skeletal muscle as a risk factor for mortality in elderly patients with sepsis: a retrospective cohort study. J Intensive Care. 2017;5:8
- Wallace JD, Calvo RY, Lewis PR, et al. Sarcopenia as a predictor of mortality in elderly blunt trauma patients: Comparing the masseter to the psoas using computed tomography. J Trauma Acute Care Surg. 2017;82:65–72.
- Rutten IJG, Ubachs J, Kruitwagen RFPM, et al. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle. 2017;8:630–638.
- Sousa AS, Guerra RS, Fonseca I, et al. Sarcopenia and length of hospital stay. Eur J Clin Nutr. 2016;70:595–601.
- Shintakuya R, Uemura K, Murakami Y, et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology. 2017;17:70–75.
- Maeda K, Akagi J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int. 2016;16:515–521.
- Maeda K, Takaki M, Akagi J. Decreased skeletal muscle mass and risk factors of sarcopenic dysphagia: A prospective observational cohort study. J Gerontol A Biol Sci Med Sci. 2016;72:1290–1294.
- Rinaldi JM, Geletzke AK, Phillips BE, et al. Sarcopenia and sarcopenic obesity in patients with complex abdominal wall hernias. Am J Surg. 2016;212:903–911.
- Maeda K, Akagi J. Muscle mass loss is a potential predictor of 90-day mortality in older adults with aspiration pneumonia. J Am Geriatr Soc. 2017;65:e18–e22.
- Perez-Zepeda MU, Sgaravatti A, Dent E. Sarcopenia and post-hospital outcomes in older adults: a longitudinal study. Arch Gerontol Geriatr. 2017;69:105–109.
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99.
- Purcell SA, Elliott SA, Baracos VE, et al. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr. 2016;70:1230–1238.
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635.
- Wallengren O, Lundholm K, Bosaeus I. Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer. 2013;21:1569–1577.
- Prado CMM, Mourtzakis M, Baracos V, et al. Overweight and obese patients with solid tumors may have sarcopenia, poor prognosis and early features of cachexia. Int J Body Compos Res. 2010;8:7–15.
- Prado CM, Cushen SJ, Orsso CE, et al. Sarcopenia and cachexia in the era of obesity: clinical and nutritional Impact. Proc Nutr Soc. 2016;75:188–198.
- Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–2338.
- Xiao J, Caan BJ, Weltzien E, et al. Associations of pre‐existing co‐morbidities with skeletal muscle mass and radiodensity in patients with non‐metastatic colorectal cancer. J Cachexia Sarcopenia Muscle. 2018;9:654–663.
- Daly L, Prado CM, Ryan A. A window beneath the skin: how computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc Nutr Soc. 2018;77:135–151.
- Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). 2016;95:e3164.
- Begini P, Gigante E, Antonelli G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. 2017;16:107–114.
- Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1339–1344.
- Chu MP, Lieffers J, Ghosh S, et al. Skeletal muscle density is an independent predictor of diffuse large B-cell lymphoma outcomes treated with rituximab-based chemoimmunotherapy. J Cachexia Sarcopenia Muscle. 2017;8:298–304.
- Daly LE, Power DG, O'Reilly A, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116:310–317.
- Fukushima H, Nakanishi Y, Kataoka M, et al. Prognostic significance of sarcopenia in patients with metastatic renal cell carcinoma. J Urol. 2016;195:26–32.
- Giap F, Lau SKM, Gannavarapu BS, et al. Impact of cachexia at diagnosis on radiotherapy utilization and survival in non-small cell lung cancer. Jco. 2016;34:133.
- Hiraoka A, Hirooka M, Koizumi Y, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2017;47:558–565.
- Ishihara H, Kondo T, Omae K, et al. Sarcopenia and the modified glasgow prognostic score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first-line sunitinib treatment. Targ Oncol. 2016;11:605–617.
- Kamachi S, Mizuta T, Otsuka T, et al. Sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment. Hepatol Res. 2016;46:201–208.
- Liu J, Motoyama S, Sato Y, et al. Decreased skeletal muscle mass after neoadjuvant therapy correlates with poor prognosis in patients with esophageal cancer. Ar. 2016;36:6677–6685.
- Malietzis G, Johns N, Al-Hassi HO, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263:320–325.
- Rollins KE, Tewari N, Ackner A, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35:1103–1109.
- Taguchi S, Akamatsu N, Nakagawa T, et al. Sarcopenia evaluated using the skeletal muscle index is a significant prognostic factor for metastatic urothelial carcinoma. Clin Genitourin Cancer. 2016;14:237–243.
- Kumar A, Moynagh MR, Multinu F, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142:311–316.
- Rier HN, Jager A, Sleijfer S, et al. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017;31:9–15.
- Sjoblom B, Gronberg BH, Wentzel-Larsen T, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016;35:1386–1393.
- Veld J, Vossen JA, De Amorim Bernstein K, et al. Adipose tissue and muscle attenuation as novel biomarkers predicting mortality in patients with extremity sarcomas. Eur Radiol. 2016;26:4649–4655.
- Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer. 2016;24:2075–2084.
- Ninomiya G, Fujii T, Yamada S, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg. 2017;39:45–51.
- Xiao DY, Luo S, O'Brian K, et al. Impact of sarcopenia on treatment tolerance in United States veterans with diffuse large B-cell lymphoma treated with CHOP-based chemotherapy. Am J Hematol. 2016;91:1002–1007.
- Srdic D, Plestina S, Sverko-Peternac A, et al. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support Care Cancer. 2016;24:4495–4502.
- Shachar SS, Deal AM, Weinberg M, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017;23:658–665.
- Owen OE, Trapp VE, Reichard GA, Jr, et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72:1821–1832.
- Montano-Loza AJ, Angulo P, Meza-Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126–135.
- Hara N, Iwasa M, Sugimoto R, et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med. 2016;55:863–870.
- Itoh S, Shirabe K, Yoshizumi T, et al. Skeletal muscle mass assessed by computed tomography correlates to muscle strength and physical performance at a liver-related hospital experience. Hepatol Res. 2016;46:292–297.
- Hanai T, Shiraki M, Ohnishi S, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res. 2016;46:743–751.
- Nishikawa H, Enomoto H, Ishii A, et al. Prognostic significance of low skeletal muscle mass compared with protein-energy malnutrition in liver cirrhosis. Hepatol Res. 2017;47:1042–1052.
- Prado CM, Siervo M, Mire E, et al. A population-based approach to define body-composition phenotypes. Am J Clin Nutr. 2014;99:1369–1377.
- Batsis JA, Mackenzie TA, Barre LK, et al. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007.
- Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560.
- Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality: results from the InCHIANTI study. J Gerontol A-Biol. 2009;64A:377–384.
- Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40.
- Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65.
- Bouchi R, Fukuda T, Takeuchi T, et al. Sarcopenia is associated with incident albuminuria in patients with type 2 diabetes: a retrospective observational study. J Diabetes Investig. 2017;8:783–787.
- Anderson DE, Quinn E, Parker E, et al. Associations of computed tomography-based trunk muscle size and density with balance and falls in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:811–816.
- Bai HJ, Sun JQ, Chen M, et al. Age-related decline in skeletal muscle mass and function among elderly men and women in Shanghai, China: a cross sectional study. Asia Pac J Clin Nutr. 2016;25:326–332.
- Chang JS, Kim TH, Kim H, et al. Qualitative muscle mass index as a predictor of skeletal muscle function deficit in Asian older adults. Geriatr Gerontol Int. 2017;17:99–107.
- Han DS, Chang KV, Li CM, et al. Skeletal muscle mass adjusted by height correlated better with muscular functions than that adjusted by body weight in defining sarcopenia. Sci Rep. 2016;6:19457.
- Nishiyama O, Yamazaki R, Sano H, et al. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology. 2017;22: 480–485.
- Orsatti FL, Nunes PR, Souza AP, et al. Predicting functional capacity from measures of muscle mass in postmenopausal women. Pm R. 2017;9:596–602.
- Schweitzer L, Geisler C, Johannsen M, et al. Associations between body composition, physical capabilities and pulmonary function in healthy older adults. Eur J Clin Nutr. 2017;71:389–394.
- Silva Neto LS, Karnikowski MG, Osorio NB, et al. Association between sarcopenia and quality of life in quilombola elderly in Brazil. Int J Gen Med. 2016;9:89–97.
- Tramontano A, Veronese N, Sergi G, et al. Prevalence of sarcopenia and associated factors in the healthy older adults of the Peruvian Andes. Arch Gerontol Geriatr. 2017;68:49–54.
- Trombetti A, Reid KF, Hars M, et al. Age-associated declines in muscle mass, strength, power, and physical performance: impact on fear of falling and quality of life. Osteoporos Int. 2016;27:463–471.
- Graf CE, Herrmann FR, Spoerri A, et al. Impact of body composition changes on risk of all-cause mortality in older adults. Clin Nutr. 2016;35:1499–1505.
- Wu TY, Liaw CK, Chen FC, et al. Sarcopenia screened with SARC-F questionnaire is associated with quality of life and 4-year mortality. J Am Med Dir Assoc. 2016;17:1129–1135.
- Zhao Q, Zmuda JM, Kuipers AL, et al. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing. 2016;45:529–534.
- Rodriguez AJ, Scott D, Khan B, et al. Low relative lean mass is associated with increased likelihood of abdominal aortic calcification in community-dwelling older Australians. Calcif Tissue Int. 2016;99:340–349.
- El Maghraoui A, Ebo'o FB, Sadni S, et al. Is there a relation between pre-sarcopenia, sarcopenia, cachexia and osteoporosis in patients with ankylosing spondylitis? BMC Musculoskelet Disord. 2016;17:268.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424–3431.
- Inomoto A, Fukuda R, Deguchi Phn J, et al. The association between the body composition and lifestyle affecting pulmonary function in Japanese workers. J Phys Ther Sci. 2016;28:2883–2889.
- Yang R, Zhang Y, Shen X, et al. Sarcopenia associated with renal function in the patients with type 2 diabetes. Diabetes Res Clin Pract. 2016;118:121–129.
- Haider S, Luger E, Kapan A, et al. Associations between daily physical activity, handgrip strength, muscle mass, physical performance and quality of life in prefrail and frail community-dwelling older adults. Qual Life Res. 2016;25:3129–3138.
- Visser M, Deeg DJ, Lips P, et al. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386.
- McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 2014;3:9.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064.
- Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7:290–298.
- Chuang SY, Hsu YY, Chen RC, et al. Abdominal obesity and low skeletal muscle mass jointly predict total mortality and cardiovascular mortality in an elderly Asian population. J Gerontol A Biol Sci Med Sci. 2016;71:1049–1055.
- Pasco JA, Mohebbi M, Holloway KL, et al. Musculoskeletal decline and mortality: prospective data from the Geelong Osteoporosis Study. J Cachexia Sarcopenia Muscle. 2017;8:482–489.
- Reinders I, Murphy RA, Brouwer IA, et al. Muscle quality and myosteatosis: novel associations with mortality risk: the age, gene/environment susceptibility (AGES)-reykjavik study. Am J Epidemiol. 2016;183:53–60.
- Spahillari A, Mukamal KJ, DeFilippi C, et al. The association of lean and fat mass with all-cause mortality in older adults: The Cardiovascular Health Study. Nutr Metab Cardiovasc Dis. 2016;26:1039–1047.
- Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol. 2016;117:1355–1360.
- Scott D, Seibel M, Cumming R, et al. Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: the concord health and ageing in men project. J Bone Miner Res. 2017;32:575–583.
- He H, Liu Y, Tian Q, et al. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27:473–482.
- Oliveira PD, Wehrmeister FC, Perez-Padilla R, et al. Relationship between body composition and pulmonary function in early adult life: a cross-sectional analysis nested in two birth cohort studies. PLoS One. 2016;11:e0163428.
- Hirani V, Naganathan V, Blyth F, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: the concord health and ageing in men project. Age Ageing. 2017;46:413–420.
- Hars M, Biver E, Chevalley T, et al. Low lean mass predicts incident fractures independently from frax: a prospective cohort study of recent retirees. J Bone Miner Res. 2016;31:2048–2056.
- Sornay-Rendu E, Duboeuf F, Boutroy S, et al. Muscle mass is associated with incident fracture in postmenopausal women: The OFELY study. Bone. 2017;94:108–113.
- An KO, Kim J. Association of sarcopenia and obesity with multimorbidity in korean adults: a nationwide cross-sectional study. J Am Med Dir Assoc. 2016;17:960 e1–967.
- Cawthon PM, Lui LY, McCulloch CE, et al. Sarcopenia and health care utilization in older women. J Gerontol A Biol Sci Med Sci. 2017;72:95–101.
- Bauer JM, Kaiser MJ, Sieber CC. Sarcopenia in nursing home residents. J Am Med Dir Assoc. 2008;9:545–551.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748–759.
- Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13:121–126.
- Huang CY, Hwang AC, Liu LK, et al. Association of dynapenia, sarcopenia, and cognitive impairment among community-dwelling older Taiwanese. Rejuvenation Res. 2016;19:71–78.
- Moon JH, Moon JH, Kim KM, et al. Sarcopenia as a predictor of future cognitive impairment in older adults. J Nutr Health Aging. 2016;20:496–502.
- Yamanouchi A, Yoshimura Y, Matsumoto Y, et al. Severely decreased muscle mass among older patients hospitalized in a long-term care ward in Japan. J Nutr Sci Vitaminol (Tokyo). 2016;62:229–234.
- Sugimoto T, Ono R, Murata S, et al. Sarcopenia is associated with impairment of activities of daily living in Japanese patients with early-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2017;31:256–258.
- Tufan A, Bahat G, Ozkaya H, et al. Low skeletal muscle mass index is associated with function and nutritional status in residents in a Turkish nursing home. Aging Male. 2016;19:182–186.
- Takagi D, Hirano H, Watanabe Y, et al. Relationship between skeletal muscle mass and swallowing function in patients with Alzheimer’s disease. Geriatr Gerontol Int. 2016;17:402–409.
- Yalcin A, Aras S, Atmis V, et al. Sarcopenia and mortality in older people living in a nursing home in Turkey. Geriatr Gerontol Int. 2017;17:1118–1124.
- Janssen I, Shepard DS, Katzmarzyk PT, et al. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85.
- Du K, Goates S, Arensberg MB, et al. Impact of sarcopenia on falls and hospitalization in community dwelling adults. J Frailty Aging. 2017;6(S1):165.
- Goates S, Du K, Arensberg M, et al. Cost burden of sarcopenia associated hospitalization in adults by age and race/ethnicity. ISPOR 22nd Annual International Meeting. Boston, MA; 2017.
- Yoshimura Y, Wakabayashi H, Yamada M, et al. Interventions for treating sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc. 2017;18:553 e1–e16.
- Deutz NE, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759–768.
- Engelen MP, Safar AM, Bartter T, et al. High anabolic potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann Oncol. 2015;26:1960–1966.
- Engelen M, Klimberg VS, Allasia A, et al. Presence of early stage cancer does not impair the early protein metabolic response to major surgery. J Cachexia Sarcopenia Muscle. 2017;8:447–456.
- Jonker R, Deutz NE, Erbland ML, et al. Effectiveness of essential amino acid supplementation in stimulating whole body net protein anabolism is comparable between COPD patients and healthy older adults. Metabolism. 2017;69:120–129.
- Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–532.
- Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36.
- Earthman CP. Body composition tools for assessment of adult malnutrition at the bedside: a tutorial on research considerations and clinical applications. JPEN J Parenter Enteral Nutr. 2015;39:787–822.
- Sallinen J, Stenholm S, Rantanen T, et al . Hand-Grip Strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58:1721–1726.
- Mauricio SF, Xiao J, Prado CM, et al. Different nutritional assessment tools as predictors of postoperative complications in patients undergoing colorectal cancer resection. Clin Nutr. 2017. DOI:10.1016/j.clnu.2017.08.026
- Fearon KCH, Argiles JM, Baracos VE, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. 2015;6:272–274.
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
- Laban MM. Atrophy and clinical weakness of the iliopsoas muscle: a manifestation of hip osteoarthritis. Am J Phys Med Rehabil. 2006;85:629.
- Cooper RG, Forbes WSC, Jayson MIV. Radiographic demonstration of paraspinal muscle wasting in patients with chronic low back pain. Rheumatology. 1992;31:389–394.
- Avrutin E, Moisey LL, Zhang R, et al. Clinically practical approach for screening of low muscularity using electronic linear measures on computed tomography images in critically ill patients. JPEN J Parenter Enteral Nutr. 2018;42:885–891.
- Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle. 2017;8:527–528.
- Abe T, Kawakami Y, Suzuki Y, et al. Effects of 20 days bed rest on muscle morphology. J Gravit Physiol. 1997;4:S10–S14.
- Brewster DJ, Strauss BJ, Crozier TM. Measuring visceral fat, subcutaneous fat and skeletal muscle area changes by computed tomography in acute pancreatitis: a retrospective, single-centre study. Crit Care Resusc. 2014;16:42–47.
- Paris M, Mourtzakis M. Assessment of skeletal muscle mass in critically ill patients: considerations for the utility of computed tomography imaging and ultrasonography. Curr Opin Clin Nutr Metab Care. 2016;19:125–130.
- Beaudart C, Dawson A, Shaw SC, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28:1817–1833.
- ClinicalTrials.gov. Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy (MENAC). NCT02330926 January 2017 [cited 2018 Aug 24].
- Clinicaltrials.gov. NCT03021902: Nutrition and Exercise in Critical Illness (NEXIS). Bethesda, MD; 2017 [cited 2018 Aug 24].
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Jco. 2013;31:1539–1547.
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763.
- Delmonico MJ, Harris TB, Lee J-S, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774.
- Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609.
- Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56:1710–1715.
- Janssen I, Baumgartner RN, Ross R, et al. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421.