Abstract
Fibromyalgia (FM) is a chronic non-degenerative disease, whose nutritional therapy seems controversial. This systematic review aimed to synthesize the knowledge about the effect of dietary interventions on patient-reported outcomes (PRO) and inflammation in patients with FM. Six electronic databases – PubMed, BioMed Central, Cochrane library, EMBASE, LILACS and ISI – were searched for clinical trials, in which a dietary intervention in patients with FM diagnosed was conducted. Quality of evidence assessment was measured in accordance with GRADE methodology. Seven clinical trials – 3 randomized controlled trials, 1 unrandomized clinical trial and 3 uncontrolled clinical trials were identified. Dietary approaches included gluten-free diet (n = 1), raw vegetarian diet (n = 2), low Fermentable oligo-, di- and monossacharides, alcohols and polyols (FODMAPs) diet (n = 1), hypocaloric diet (n = 2) and monosodium glutamate- and aspartame-free diet interventions (n = 1). The major PRO were pain and functional repercussion, with 5 out of 7 studies reporting an improvement. The progress in secondary outcomes was reported for fatigue (2/5 studies), sleep quality (2/3 studies), depression and anxiety (3/6 studies), quality of life (4/5 studies), gastrointestinal symptoms (1/2 studies) and inflammatory biomarkers (1/1 study). However, according to Cochrane Risk of Bias, these studies had poor statistical quality. Well-designed studies should be performed to investigate the dietary interventions effect on FM.
Fibromyalgia (FM) is a chronic non-degenerative disease, whose nutritional therapy seems controversial but promising.
Pain and functional repercussion in FM patients seem to improve with a hypocaloric diet, a raw vegetarian diet or a low FODMAPs diet, as much as quality of life, quality of sleep, anxiety and depression and inflammatory biomarkers.
Existing studies in this subject are scarce and low quality, which does not allow conclusions to be drawn.
Key messages
Introduction
Fibromyalgia (FM) is a chronic non-degenerative disease of unknown aetiology that mostly affects women, with a prevalence range between 0.5 and 2% worldwide [Citation1] estimated at 1.7% in Portugal [Citation2]. The diagnosis is based on the criteria of Rome III of the American College of Rheumatology (ACR), reviewed in 2010 [Citation3].
The main symptoms of FM are musculoskeletal pain and chronic fatigue. Patients usually also refer nonrestorative sleep, morning stiffness, depression, anxiety [Citation1] and gastrointestinal (GI) symptoms similar to irritable bowel syndrome (IBS) [Citation4], strongly compromising their quality of life. All these patient’s reported outcomes (PRO) are evaluated in clinical practice, through questionnaires which are considered subjective. However, changes in biomarkers, particularly inflammatory cytokines were also described. A meta-analysis with 25 clinical trials and 1255 FM patients revealed a higher plasma interleukin (IL)-6 in these patients, compared to healthy controls [Citation5]. Additionally, several studies showed an association between FM and intestinal inflammation [Citation4,Citation6–8], through a slight but significant plasma pro-inflammatory cytokines increase [Citation9], suggesting a low-grade inflammation in these patients, associated with altered intestinal microbiota and dysbiosis [Citation10,Citation11].
Medical therapy consists mainly of analgesic, muscle relaxants and non-steroids anti-inflammatory drugs (NSAID), but it seems not to completely resolve the symptoms of the disease [Citation1,Citation12]. Additionally, the modification of intestinal microbiota composition described in these patients, emerges as an opportunity to intervene through dietary approaches. However, according to the literature, the effect of nutritional interventions on FM remain controversial. Thus, the aim of this systematic review was to synthesize the knowledge about the effect of nutritional interventions on the PRO and inflammation in patients with FM.
Material and methods
This review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [Citation13].
Data sources and study selection
A systematic search was conducted by three independent researchers (AS, AC and PS) in PubMed, BioMed Central, Cochrane library, EMBASE, LILACS and ISI databases, using as keywords the terms “fibromyalgia [all fields] or “fibromyalgia” [MeSH Terms] and “diet” or “diet therapy” or “nutrition” or “sibo” or “small intestinal bacterial overgrowth” or “microflora” or “microbiota” or “intestinal microbiota”.
Intervention studies investigating the association between diet and FM that were published from January 1990 to April 2018 were included. The reference lists of included articles were screened manually for additional studies. Disagreements were resolved by consensus.
The last search was conducted on 14 May 2018.
Study design and eligibility criteria
Clinical trials including adult human populations with FM diagnosed according to ACR criteria revised in 2010, within which a dietary intervention was implemented, were considered eligible. No restrictions were imposed on language.
Studies with interventions other than only dietary interventions, such as acupuncture, physical exercise, quiropractice, pharmaceutical interventions, among others, were considered not eligible. Studies including dietary supplementation were excluded. Studies that included patients diagnosed with FM combined with other disorders, such as rheumatoid arthritis, lupus or irritable bowel syndrome, were also excluded.
Data extraction
After selecting the eligible studies, the following information was extracted from each study: name of the first author, year of publication, study design, sample size, characteristics of participants (sex and age), dietary intervention protocol, outcomes and results.
The primary PRO of interest for this study were pain and functional repercussion. The secondary outcomes were fatigue, quality of sleep, quality of life, anxiety and depression and GI symptoms. The presence of inflammation assessed with biomarkers was also an outcome of interest.
Risk of bias and grading system
The risk of bias of the individual studies was assessed through The Cochrane Collaboration’s tool for Assessing Risk of Bias in Randomized Studies (ROBIS) [Citation14] and Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS) [Citation15]. Aspects of methodological quality, such as participant selection, classification of interventions and deviations from intended protocol, measurement of outcomes and selection of reported results were evaluated.
Risk of bias was included in the GRADE assessment, in order to assess the quality of evidence, evaluating inconsistency, indirectness of evidence, imprecision and publication bias.
Results
Overview of included studies
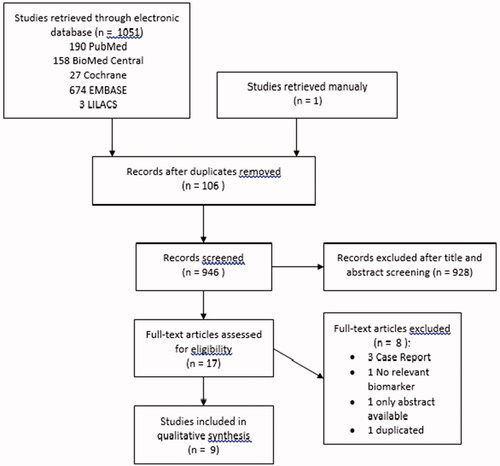
After removing duplicates (n = 206), a total of 972 studies were identified. Of these, 954 were excluded due to a non-clinical trial (n = 86), to include patients with other diseases besides FM (n = 797), to include animals (n = 4) or children (n = 1) and to use other interventions besides dietary therapy (n = 66). Eighteen complete articles were included and evaluated, among which 11 were excluded for the following reasons: non-eligible study type (case report, n = 3; only abstract publication, n = 1), duplicated study (n = 3), selected outcomes not included (n = 1), no dietary intervention (n = 1) and patients with other diseases besides FM (n = 2). A total of 7 clinical trials were included. These results are presented in the Summary of Evidence Search and Selection, which is based on PRISMA flow chart ().
From the 7 clinical trials included in this review, 3 were Randomized Controlled Trials (RCT) [Citation16–18], 1 was a Controlled Clinical Trial, Unrandomized (CCT) [Citation19], and 3 were Uncontrolled Clinical Trials (UCT) [Citation20–22].
Included study characteristics
The controlled studies included 266 FM patients (132 in the intervention group and 134 in the control group), of which 255 were women. The UCT studies included 82 FM patients, all women. The mean sample size was 49.7 FM patients, and the time of follow up ranged from 4 weeks to 7 months. The age of the patients ranged from 39.5 to 54.5 years.
The 7 included studies presented distinct dietary interventions: diet low in foods rich in FODMAPs (fermentable oligo-, di- and monossacharides, alcohols and polyols) [Citation20]; gluten-free diet [Citation16]; monosodium glutamate- and aspartame-free diet [Citation17]; hypocaloric diet [Citation18,Citation22]; and raw vegetarian diet [Citation19,Citation21]. The studies used different methods to evaluate the effect of the intervention in PRO and biomarkers parameters. summarizes the characteristics of each intervention, in respect to sample size, study design, methodology and results.
Table 1. Characteristics and results of included studies.
The low FODMAPs diet intervention was characterized by an exclusion of all dairy products; all cereals except rice; cashew; all fruit other than banana, citrus, pineapple, red berries, strawberries and kiwi; all vegetables other than pumpkin, cabbage, lettuce, tomato, carrot and cucumber, for a 4 week period [Citation20]. The aim of this study was to examine the effects of a low FODMAPs diet in the PRO, mainly pain, quality of life and GI symptoms.
The hypocaloric diet interventions considered the hypothesis that a weight loss could beneficiate FM symptoms, particularly pain. This intervention was characterized by an ingestion of 1200 kcal/d distributed as 20% protein, 50% carbohydrates and 30% of fats, in the form of vegetables, fruit, whole cereals and light dairy. One study used a group approach methodology [Citation22], and the other used a regular personal dietary plan implementation [Citation18].
In the gluten-free diet intervention [Citation16], patients were randomly distributed in two groups: intervention group engaged in a gluten-free diet for 6 months, avoiding wheat, rye, barley and oat; control group underwent a hypocaloric diet, described previously.
In the vegetarian diet interventions, patients were instructed to embrace a raw, low-salt ingestion of vegetables, legumes, fruit, whole cereals and nuts. In one study patients were distributed, according to their own preference, in two groups: intervention group and control group, which continued to have an omnivorous diet [Citation19]. The other study had no control group [Citation21].
In the monosodium glutamate- and aspartame-free diet [Citation17], patients were randomly distributed in two groups: intervention group employed monosodium glutamate- and aspartame-free diet for 3 months, and the control group was placed on a waiting list.
Overview of the outcomes (PRO and biomarkers)
Five of the 7 clinical trials evaluated the pain and functional repercussion as primary efficacy variable, through Fibromyalgia Impact Questionnaire (FIQ) [Citation18,Citation21,Citation22] or Revised Fibromyalgia Impact Questionnaire (FIQR) [Citation16,Citation20]. To evaluate the intensity of pain, 2 studies applied Visual Analogic Scale (VAS) [Citation17,Citation19], other Multidimensional Pain Inventory (MPI) [Citation22], and other the Brief Pain Inventory (BPI) [Citation16]. Examination of Tender Points (TP), was also assessed by 2 studies [Citation18,Citation19].
Secondary outcomes varied according to each study. Fatigue was not evaluated through a specific tool by any study. However, it was considered in 5 studies, as it is referred in one question of FIQ [Citation16,Citation18,Citation20–22]. Quality of sleep was assessed through Pittsburg Sleep Quality Inventory (PSQI) by 2 studies [Citation16,Citation18]. Four studies assessed depression through Beck Depression Inventory (BDI) [Citation16,Citation18,Citation19,Citation22] and 2 assessed anxiety through State-Trait Anxiety Inventory (STAI-I) [Citation16,Citation22]; 1 study assessed both variables through the Five Dimensions Euro – Quality of Life (EQ-5D) [Citation20]. The quality of life was assessed in 5 studies, through EQ-5D [Citation20], Short-Form (SF)-12 [Citation16] or SF-36 [Citation21] and Health Assessment Questionnaire [Citation19,Citation22]. Two studies evaluated GI symptoms through Irritable Bowel Syndrome – Symptom Severity Scale (IBS-SSS) [Citation20] and through a classification of a list of common symptoms based on current literature of Non-Coeliac Gluten Sensitivity (NCGS) [Citation16]. Additionally, 1 study assessed inflammatory biomarkers parameters, namely Interleukin (IL)-6 and C reactive protein (CRP) [Citation18].
Four of the 7 studies applied measures to control the diet compliance, such as food record [Citation17,Citation19,Citation22] or food frequency questionnaire (FFQ) [Citation21].
Effect on pain and functional repercussion
Despite the differences between the dietary approaches, the results in every single study are similar regarding the impact of intervention on these PRO, except for 2 studies, the aspartame- and monosodium glutamate-free diet [Citation17] and the gluten-free interventions [Citation16], which revealed no significant differences between the intervention and the control group.
Intervention with a low FODMAPs diet [Citation20] reduced significantly pain associated with FM: there was a reduction both in FIQR (61.6 vs. 48.1, p < .01) and FSQ scores (21.8 vs. 16.9, p < .01), between the beginning and the end of the intervention. Additionally, this study showed a significant and positive correlation (r = 0.36, p < .05) between FIQR and IBS-SSS.
Similarly, hypocaloric diet interventions also showed a significant reduction in pain. In 1 study [Citation22], FIQ scores decreased from 56.7 ± 14.9 to 46.2 ± 18.3 (p < .001), after 5 months of the intervention. Also, MPI decreased from 3.8 ± 1.1 to 3.3 ± 1.4 (p = .04). In addition, this study showed a direct significant correlation between weight and both MPI (r = 0.31, p < .05) and HAQ (r = 0.35, p < .05) [Citation22] at baseline. In the other study [Citation18], after 6 months of hypocaloric diet, the intervention group presented significantly improved FIQ scores compared to the control group (51.6 ± 9.4 vs. 47.0 ± 5.1, p = .007).
The vegetarian interventions also revealed an improvement in pain. One vegetarian diet intervention [Citation21] showed a significant reduction in FIQR, after 7 months of intervention (51.4 ± 14.2 vs. 27.6 ± 19.0, p < .001). In the other vegetarian study, the authors describe a significant reduction in VAS (p < .005), however the score values obtained were not published [Citation19].
Effect on fatigue
As mentioned, fatigue was considered in 5 studies, as it is referred in one question of FIQ [Citation16,Citation18,Citation20–22]. One of the hypocaloric diet studies showed a lower score of fatigue dimension of FIQ after 6 months of intervention, compared to control group (4.7 ± 1.8 vs. 5.8 ± 1.9, p = .008) at the endpoint [Citation18]. A vegetarian diet also revealed similar results after 7 months of intervention (7.8 ± 3.2 vs. 4.4 ± 2.8, p < .05) [Citation21]. The remaining interventions did not show any significant differences between diet and fatigue.
Effect on sleep quality
After 6 months of intervention with a hypocaloric diet, intervention group showed significantly lower PSQI score compared to control group (4.0 ± 1.9 vs. 5.3 ± 2.4, p = .006) [Citation18]. In parallel, a gluten-free intervention showed no significant effect in sleep quality [Citation16].
Another intervention, including a vegetarian raw diet for 3 months, which assessed the quality of sleep through a non-identified questionnaire, reported significant differences after intervention (p < .001) [Citation19]. However, the study protocol did not reveal the tool’s details, so this result was not considered.
Effect on depression and anxiety
Three studies reported an improvement in depression and anxiety. One hypocaloric approach showed a decrease in BDI scores (17.8 ± 11.2 vs. 9.7 ± 8.4, p < .001) and in STAI scores (42.8 ± 11.7 vs. 35.8 ± 11.3, p < .001) after intervention, in comparison with the initial scores [Citation22]. Similarly, the other hypocaloric trial revealed a significant difference between intervention and control groups after a 6 months intervention (12.8 ± 5.8 vs. 17.6 ± 7.7, p = .002) in BDI [Citation18]. Regarding vegetarian studies, 1 revealed a significant decrease after 7 months intervention, in the depression (5.0 ± 3.0 vs. 2.4 ± 2.5, p < .05) and anxiety (5.7 ± 2.7 vs. 3.0 ± 2.3, p < .05) dimensions of FIQ [Citation21]. The gluten-free diet [Citation16], low FODMAPs diet [Citation20] and the other vegetarian diet [Citation19] interventions did not show a significant effect on anxiety and depression.
Effect on quality of life
In low FODMAPs diet study [Citation20], EQ-5D score differences before and after intervention had no significant differences. However, the dimensions Mobility and Pain significantly improved (2.7 vs. 2.3, p = .02 and 3.5 vs 2.8, p < .01, respectively). In a raw vegetarian diet study [Citation21], patients revealed parameters improvement comparing the begin and endpoint, regarding vitality (18.0 ± 14.4 vs. 48.0 ± 28.9, p < .001), mobility (36.3 ± 24.3 vs. 60.3 ± 26.7, p < .001), emotional health (25.0 ± 26.5 vs. 75.0 ± 25.7, p < .001) and mental health (57.2 ± 23.1 vs. 77.0 ± 15.3, p < .001) in SF-36 questionnaire. Additionally, there was an improvement in HAQ scores, from 3.9 to 4.7 (p < .05) after 7 months, in the same study [Citation21]. On another vegetarian study, although the score values obtained were not published, the authors reported that the intervention group had better autonomy (p = .03) and morning stiffness (p < .001) [Citation19] compared with control group, assessed through HAQ. Hypocaloric diet showed a significant improvement in quality of life, assessed through QOL (33.2 ± 26.7 vs. 44.6 ± 29.8, p = .01), but no significant differences in HAQ, after 5 months intervention [Citation22]. Gluten-free diet intervention showed no significant differences in SF-12 questionnaire [Citation16].
Effect on gastrointestinal symptoms
Two of the 7 studies evaluated GI disturbances among FM patients, and reported the impact of a dietary intervention in these symptoms [Citation16,Citation20]. A low FODMAPs diet showed a reduction in gastric pain and intestinal changes in IBS-SSS (275.3 vs. 158.1, p < .01), with a reduction in 50% of symptoms after a 4 week-intervention [Citation20]. The gluten-free diet showed no significant differences in GI symptoms between intervention and control groups, at the end of the intervention [Citation16].
Inflammatory biomarkers
Only 1 study measured inflammatory biomarkers parameters, namely IL6 and CRP. After a hypocaloric diet, intervention group showed significantly lower inflammation biomarkers compared to control group, namely IL6 (4.1 ± 1.5 pg/ml vs. 3.4 ± 1.4 pg/ml, p = .03) and CRP (2.6 ± 1.1 mg/dL vs. 2.0 ± 1.1 mg/dL, p < .001) at the end of the intervention [Citation18].
Risk of bias and GRADE
The results after applying the Cochrane Risk of Bias Tool are presented in . Although the quality of evidence of the studies varied, the Risk of Bias analyzed allowed us to verify a poor statistical quality in most of them. The majority of the studies have a high risk of bias, which decrease the quality of evidence. The risk of bias was integrated into GRADE profile.
Table 2. Cochrane risk of bias of included studies.
GRADE methodology allowed an analysis of the included studies, according to the risk of bias, inconsistency, indirectness of evidence, imprecision and publication bias. According to the nature of a dietary study, it is not possible to blind population. As so, this was not considered a factor to downgrade studies. However, there were some reasons that downgraded the included studies, such as: the small sample size and optimal information size (OIS) never estimated [Citation16–22]; some studies had no control group [Citation20–22] or no randomization [Citation19] or did not use an independent control group (two different interventions are applied) [Citation16]; some studies did not apply intention-to-treat analysis, despite presenting >5% of loss of follow up [Citation19,Citation20,Citation22]; some studies presented heterogeneity of the population in pain level [Citation19] or medical therapy [Citation16], at baseline. This analysis resulted in an evaluation of low to very low uncertainty of evidence, except for one study [Citation18], considered of moderate uncertainty. shows a summary of GRADE profile for studies representing each outcome. Risk of bias classification justification is shown in .
Table 3. GRADE Profile for studies evaluating the impact of dietary interventions on PRO and inflammation in FM patients.
Table 4. Summary of author’s justification of risk of bias classification.
Given the diversity of studies, it was not possible to conduct a meta-analysis.
Discussion
This study reviewed the evidence of dietary interventions effect in PRO and inflammatory biomarkers of FM patients and identified 7 studies that fulfilled the inclusion criteria. To the best of our knowledge this is the first systematic review on dietary interventions effect in this population. According to the results of this review, a hypocaloric diet, a raw vegetarian diet or a low FODMAPs diet may improve pain and functional repercussion in FM patients. However, the fact that the improvement was achieved with different dietary approaches, may lead to the hypothesis that the psychosomatic component of the disease must be taken into account. On the other hand, FM symptoms appear to be associated with several metabolic alterations, namely with regard to changes in the composition of the intestinal microbiota and consequent existence of Small Intestinal Bacterial Overgrowth (SIBO) [Citation4,Citation10,Citation11], changes in the hypothalamic axis and increase of cortisol [Citation23,Citation24], mitochondrial dysfunction and oxidative stress [Citation24–27] and alterations in the Central Nervous System, with activation of glial cells in cerebrospinal fluid [Citation28]. In this perspective, a combination of several dietary approaches that could interfere in each metabolic alteration could be a better way to improve the disease symptomatology.
Patients with FM often report specific food intolerances and undertake dietary approaches, seeking an improvement of their symptoms and a better quality of life [Citation29]. In this review, most of the included studies used tools that assess not only the pain associated with FM, but also other common PRO, namely fatigue, quality of sleep, anxiety and depression, general quality of life, and GI symptoms, whereas only one assessed inflammatory biomarkers. This reveals an attempt to better understand the disease and its symptoms, and to meet the needs of these patients, since medical treatment does not appear to be fully effective in eliminating symptoms. The included clinical trials showed a significant improvement in the quality of life [Citation21,Citation22], quality of sleep [Citation18,Citation19], and anxiety and depression [Citation18,Citation21,Citation22]. Additionally, a hypocaloric study showed a reduction in IL6 and CRP after 6 months [Citation18], which reveals an objective positive impact of a weight reduction in decreasing inflammation.
In parallel, high body mass index has been directly and significantly correlated to pain and functional repercussion in FM patients [Citation22], suggesting that obesity could influence the symptoms of the disease. Other authors have postulated that fact previously [Citation30], since adipocytes produce pro-inflammatory cytokines that could prorogate the pain. Furthermore, some studies pointed the existence of an association between FM and intestinal inflammation [Citation1,Citation6,Citation7,Citation31], which suggests that in addition to weight reduction, a diet with an anti-inflammatory potential could contribute to improve disease symptoms.
Moreover, the decrease in GI symptoms associated with a low FODMAPs diet intervention was related with a decrease in pain and functional repercussion [Citation20], revealing a possible association of these symptoms and intestinal microbiota changes.
It is already known that GI symptoms, such as nausea, vomiting and dyspepsia, are very common in patients with FM [Citation1,Citation32,Citation33]. Various authors suggested that the persistence of the described symptoms, along with sleep quality changes, depression and pain, may be related to modifications of intestinal microbiota [Citation1], and consequent existence of SIBO [Citation34–36].
However, it is worth mentioning that the included studies have relevant bias that may limit the interpretation of the results. Given the nature of dietary intervention, it is always impossible to perform a double-blind intervention, which increases the risk of bias. In addition, some studies have other parameters that decrease the quality of the design, namely regarding the lack of control group (n = 3) [Citation20–22] and non-randomization of the sample (n = 1) [Citation19], which allows less control over possible confounding variables.
Furthermore, not only the small size of the total sample, but also the divergences in the methodology used among studies, contribute to the difficulty of obtaining conclusive results. In addition, the fact that the follow-up time for each intervention is diverse, increases the probability of obtaining different effects on the measured outcomes further contributing to inconsistent results, which may hamper a conclusion based on a summary measure of the various studies. Additionally, although the same variables were evaluated in different studies, diverse methods were used to evaluate each of them. This factor may influence results and, consequently, the conclusions, as some methods may enable a more specific or a more comprehensive assessment of a given parameter.
The majority of studies did not take into account possible confounding variables, such as sex, pain level at baseline and medication, which may potentially confound the association between diet and disease-related variables. Also, in three studies [Citation16,Citation18,Citation20], no methods of controlling diet compliance were applied, which means that is not possible to exclude the hypothesis that the diet has not been fully attained.
In general, the risk of bias allowed to assume a poor statistical quality in most of these studies. Since that, the positive associations between the different dietary interventions and the outcomes should be regarded as potential associations that deserve to be further studied.
Although, dietary interventions seem to be promising as complementary therapies in FM, the results of this review should be interpreted with caution. Well-designed studies are lacking to conclude about the effect of the nutritional interventions on the progression and symptoms of FM.
Conclusion
Pain and functional repercussion in FM patients seem to improve with a hypocaloric diet, a raw vegetarian diet or a low FODMAPs diet. Other PRO, such as quality of life, quality of sleep, anxiety and depression and inflammatory biomarkers also showed a significant improvement with these interventions. However, due to the low quality of the included studies, these promising results should be interpreted with caution, and no quantitative and objective conclusions should be drawn.
The development of well-designed clinical trials in FM patients are needed to conclude about the effect of the dietary interventions on FM patients. Dietary interventions based on scientific evidence, combined with medical therapy could be a strategic approach, in the treatment of FM.
Disclosure statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors report no financial or personal conflicts of interest.
References
- Clauw DJ. Fibromyalgia: an overview. Am J Med. 2009;122:S3–S13.
- Branco JC, Rodrigues AM, Gouveia N, et al. Prevalence of rheumatic and musculoskeletal diseases and their impact on health-related quality of life, physical function and mental health in Portugal: results from EpiReumaPt- a national health survey. RMD Open. 2016;2:e000166.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610.
- Wallace DJ, Hallegua DS. Fibromyalgia: the gastrointestinal link. Curr Pain Headache Rep. 2004;8:364–368.
- Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskeletal Disorders. 2011;12:245.
- Clauw DJ. Fibromyalgia and related conditions. Mayo Clin Proc. 2015;90:680–692.
- Feng B, La JH, Schwartz ES, et al. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1085–G1098.
- Alam MS, Choudhary V, Zeeshan M, et al. Interaction of Plasmodium vivax tryptophan-rich antigen PvTRAg38 with band 3 on human erythrocyte surface facilitates parasite growth. J Biol Chem. 2015;290:20257–20272.
- Bazzichi L, Rossi A, Massimetti G, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25:225–230.
- Triadafilopoulos G, Simms RW, Goldenberg DL. Bowel dysfunction in fibromyalgia syndrome. Dig Dis Sci. 1991;36:59–64.
- Carding S, Verbeke K, Vipond DT, et al. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191.
- Atzeni F, Masala IF, Salaffi F, et al. Pain in systemic inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2015;29:42–52.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928.
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. 2016;355:i4919.
- Slim M, Calandre EP, Garcia-Leiva JM, et al. The effects of a gluten-free diet versus a hypocaloric diet among patients with fibromyalgia experiencing gluten sensitivity-like symptoms: a pilot, open-label randomized clinical trial. J Clin Gastroenterol. 2017;51:500–507.
- Vellisca MY, Latorre JI. Monosodium glutamate and aspartame in perceived pain in fibromyalgia. Rheumatol Int. 2014;34:1011–1013.
- Senna MK, Sallam RA, Ashour HS, et al. Effect of weight reduction on the quality of life in obese patients with fibromyalgia syndrome: a randomized controlled trial. Clin Rheumatol. 2012;31:1591–1597.
- Kaartinen K, Lammi K, Hypen M, et al. Vegan diet alleviates fibromyalgia symptoms. Scand J Rheumatol. 2000;29:308–313.
- Marum AP, Moreira C, Saraiva F, et al. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand J Pain. 2016;13:166–172.
- Donaldson MS, Speight N, Loomis S. Fibromyalgia syndrome improved using a mostly raw vegetarian diet: an observational study. BMC Complem Altern Med. 2001;1:7.
- Shapiro JR, Anderson DA, Danoff-Burg S. A pilot study of the effects of behavioral weight loss treatment on fibromyalgia symptoms. J Psychosom Res. 2005;59:275–282.
- Riva R, Mork PJ, Westgaard RH, et al. Fibromyalgia syndrome is associated with hypocortisolism. Intj Behav Med. 2010;17:223–233.
- Romano GF, Tomassi S, Russell A, et al. Fibromyalgia and chronic fatigue: the underlying biology and related theoretical issues. Adv Psychosom Med. 2015;34:61–77.
- Cordero MD, Diaz-Parrado E, Carrion AM, et al. Is inflammation a mitochondrial dysfunction-dependent event in fibromyalgia? Antioxid Redox Signal. 2013;18:800–807.
- Cordero MD, De Miguel M, Moreno Fernandez AM, et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease. Arthritis Res Ther. 2010;12:R17.
- Cordero MD, de Miguel M, Carmona-Lopez I, et al. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol Lett. 2010;31:169–173.
- Kadetoff D, Lampa J, Westman M, et al. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol. 2012;242:33–38.
- Arranz LI, Canela MA, Rafecas M. Dietary aspects in fibromyalgia patients: results of a survey on food awareness, allergies, and nutritional supplementation. Rheumatol Int. 2012;32:2615–2621.
- Cordero MD, Alcocer-Gomez E, Cano-Garcia FJ, et al. Clinical symptoms in fibromyalgia are associated to overweight and lipid profile. Rheumatol Int. 2014;34:419–422.
- Buskila D, Odes LR, Neumann L, et al. Fibromyalgia in inflammatory bowel disease. J Rheumatol. 1999;26:1167–1171.
- Slim M, Calandre EP, Rico-Villademoros F. An insight into the gastrointestinal component of fibromyalgia: clinical manifestations and potential underlying mechanisms. Rheumatol Int. 2015;35:433–444.
- Mahdi AA, Fatima G. A quest for better understanding of biochemical changes in fibromyalgia syndrome. Ind J Clin Biochem. 2014;29:1–2.
- Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr. 2016;55:897–906.
- Othman M, Aguero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24:11–16.
- Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450–452.