Abstract
Introduction: High physical activity (PA) at old age indicates good functional capacity enabling independent living. We investigated how different disease conditions are associated with measured PA indicators in old women and men, and whether they recognize this association.
Materials and methods: This cross-sectional twin cohort study in Finland comprised 779 individuals (276 complete twin pairs, including 117 monozygotic pairs), who participated in hip-worn accelerometer monitoring of PA and responded to questions on diseases and mobility limitations at mean age of 73 (range 71–75).
Results: Of the participants, 23.2% reported having a disease restricting mobility. With sex and age in the regression model, the reported disease restricting mobility explained 11.8% of the variation in moderate-to-vigorous PA (MVPA) and 10.4% of the variation in daily steps. Adding stepwise other self-reported diseases and body mass index to the model increased the explanatory power for MVPA up to 18.5% and 25.5%, and for daily steps up to 16.0% and 20.7%, respectively. In the co-twin control analysis the PA differences were smaller in disease-discordant monozygotic than dizygotic pairs.
Conclusions: Chronic disease conditions are associated with low PA, which individuals may not always recognize. Shared genetic factors may explain part of the associations.
Among community-dwelling older men and women one-fourth of the variation in objectively measured moderate-to-vigorous physical activity is accounted for by age, sex, body mass index and self-reported diseases.
Occurrence of chronic diseases is associated with low physical activity and individuals do not always recognize this.
Healthcare professionals should pay attention to the low physical activity and mobility of individuals with chronic disease conditions before these result in limitations in independent living.
Key messages
Introduction
Ageing-related overall degenerative processes or biological physiological aging as such [Citation1], reduction of physical activity (PA) and development of specific chronic degenerative diseases [Citation2–5] are all associated with the development of disability and mobility limitations. Exercise-based rehabilitation has been shown to be a good means in improving both the measured and self-rated function among individuals with different chronic disease [Citation6] and exercise interventions prevent the occurrence of disability among the aged [Citation7]. High participation in moderate-to-vigorous physical activity (MVPA) at older age is an indicator of good physical fitness and health, and consequently predicts low risk of disability and death among older individuals [Citation8,Citation9].
While overlapping among older people, PA level as such is not a direct indicator of mobility limitations, but high PA level quite directly indicates that the individual does not have significant physical mobility limitations. The prevalence of mobility limitations depends on the definition used [Citation10]. Difficulties related to walking or climbing stairs [Citation11–13] as well as walking outdoors [Citation14] are common indicators used to quantify physical mobility capabilities.
In addition to low PA level, sedentary behaviour (SB) has been suggested to be detrimental to health in particular among those with low MVPA [Citation15–17]. Occurrence and progression of chronic diseases may lead to more sedentary lifestyle.
Inter-individual genetic differences in the liability to develop obesity and many chronic diseases are substantial [Citation18,Citation19]. Likewise, genetic factors affect fitness and participation in PA [Citation20,Citation21]. However, in addition to genetic factors, there may also be factors related to childhood environment that predispose people to different clusters of these factors [Citation8]. Genetic pleiotropy [Citation8,Citation19] means that shared genetic factors may underlie both PA and disease risk providing an alternative explanation than a causal one to the observed association between PA and later disease.
By studying outcomes in twin pairs discordant for exposure to different health habits and health outcomes, the possible confounding contributions of genetic and shared early childhood experiences can be taken into account. Twin pairs nearly always share the same childhood family environment. Dizygotic (DZ) pairs share, on average, half of their segregating genes (like siblings), while monozygotic (MZ) pairs are genetically identical at the sequence level. The co-twin control analyses among discordant monozygotic twin pairs, in particular, allow stronger estimates on causal influences compared to associations seen in unrelated individuals [Citation22].
In our 40-year longitudinal twin cohort study (MOBILETWIN study) we have previously analyzed the long-term predictors of later life PA but have identified only mid-life smoking as a factor causally influencing later life PA [Citation23]. Much of the association between mid-life and later life PA was explained by shared genetic factors [Citation23]. As it is largely unknown how we can influence the genetically determined ageing process we now investigated how chronic disease conditions are associated with objectively measured PA and SB in community-dwelling twins with mean age of 73 years. 1) First, we compared the ability of a simple question on diseases restricting mobility against other more specific questionnaire items in detecting mobility restrictions. 2) Next, we analyzed how the question on disease-related mobility restrictions was associated with objectively measured PA and SB characteristics. 3) Then, we studied how other reported chronic diseases were associated with objectively measured PA and SB in addition to the self-reported disease-related mobility restrictions. 4) Finally, we analyzed whether members of DZ and MZ twin pairs discordant for self-reported diseases restricting mobility differed in their objectively measured PA and SB.
Materials and methods
This MOBILETWIN study [Citation23] is an ancillary to the older Finnish Twin Cohort Study [Citation24]. Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of the Hospital District of Southwest Finland on 20 May 2014.
Participant inclusion and study design
The study is based on a nationwide sample of all same-sex twin pairs born before 1958 with both co-twins alive in 1975 [Citation24]. A baseline questionnaire was sent to all twin candidates in 1975. Among those whose addresses could be identified (93.5% of subjects) in 1975, the response rate for twins was 87.6%. A subsequent questionnaire was mailed in 1981 to all the verified twins. The corresponding response rate among those responding in 1975 and alive in 1981 was 90.7%.
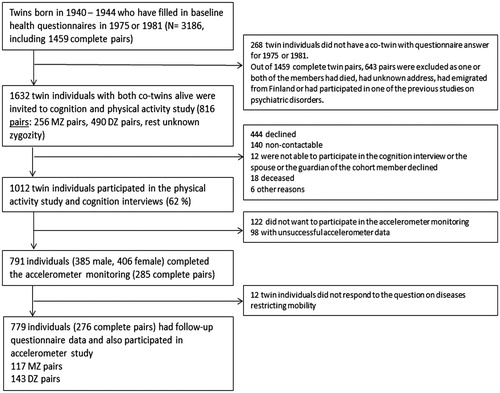
For the current PA study (MOBILETWIN), twins from 1940 to 1944 birth cohorts were selected (). Altogether 3186 twin individuals belonged to these birth cohorts and had responded to at least one of the questionnaires (1975 or 1981). A total of 145 twin individuals were excluded because they had participated in one of the previous studies on psychiatric disorders (schizophrenia and bipolar studies). All remaining 816 (256 MZ, 490 DZ and 70 with unknown zygosity) complete twin pairs, that is, both alive and contactable, were invited to participate in the present study. The twins were sent an invitation letter in which they chose whether to participate in health and cognition telephone interview and/or accelerometer study completed with physical functioning questionnaire. Altogether 1012 (61.9%) twin individuals participated in the telephone interview, 791 twin individuals wore the accelerometer, and 817 individuals filled in the whole questionnaire on physical functioning. The target group of this analysis were those 779 individuals (276 complete twin pairs; 117 MZ, 143 DZ and 16 pairs with unknown zygosity) who participated in telephone interview, objective PA monitoring and responded to the question on diseases restricting mobility. So, of the 1614 individuals alive on the day of the follow-up, 140 were not contactable (not community dwelling) and of the remaining 1474 individuals, 779 (53%) participated in all parts of this follow-up study with required responses (for more details, see ). Data were collected from 2014 to 2016 starting from the oldest birth cohort. The accelerometer and the questionnaire were mailed to each volunteering participants after the interview. The median time difference between the interview and accelerometer measurement was 17.5 days. According to the mid-life questionnaire data on PA, those who participated in this follow-up study and who did not participate had similar leisure-time PA levels [Citation23], but at follow-up, the target group was a selected community-dwelling group as those less healthy individuals living in nursing homes or long-term institutional care were not contactable.
Physician-diagnosed diseases and mobility limitations
The participants were interviewed on their health and cognition and they responded to a questionnaire on mobility limitations. The questionnaire included a structured question ‘Do you have any physician-diagnosed disease which restricts your mobility?’ The response alternatives were ‘no’ and ‘yes’ followed by an open question on the disease restricting the mobility most. The questionnaire also included questions on whether the participant was able to walk a distance of 2 km and whether the participant was able to climb one flight of stairs. In both of these structured questions there were five response alternatives [Citation25] as follows: 1) able to manage without difficulty, 2) able to manage with some difficulty, 3) able to manage with great deal of difficulty, 4) able to manage only with help of another person and 5) unable to manage even with help. Those responding to alternatives 2, 3, 4 or 5 were classified as having difficulty. We also asked the participants to estimate with 0.5 km accuracy how many kilometres altogether they have walked or jogged outside during the past 7 days. Our telephone interview and/or questionnaire included a list of common physician-diagnosed chronic diseases, which may compromise a person’s ability to be physically active. The interview included hypertension, coronary heart disease/myocardial infarction, stroke, peripheral arterial disease, heart failure, atrial fibrillation, diabetes, Parkinson’s disease, dementia, depression and diseases causing visual impairments and the questionnaire included separate items for rheumatoid arthritis, knee osteoarthritis, hip osteoarthritis, osteoporosis and arterial disease causing claudication.
Body mass index (BMI) was calculated using self-reported (questionnaire) height and weight.
Accelerometer data collection
PA was measured with a hip-worn, light triaxial accelerometer (Hookie AM20, Traxmeet Ltd, Espoo). The device with the instructions for use was mailed to the participants, who were asked to use the accelerometer during waking hours (excluding activities in water) for seven consecutive days. Participants mailed the device back to the UKK Institute for data analysis. The analysis of raw acceleration (milligravity; 1 mg = 0.00981 m/s2) data was based on novel algorithms that employ the mean amplitude deviation (MAD) of the resultant acceleration in 6 s epochs and the angle for posture estimation of the body, metrics that provide consistent and accurate assessments of the intensity, volume and daily distribution for PA and separately the volume and its daily distribution for sedentary and stationary behaviours [Citation26–28].
The MAD metric has been validated through directly measured incident oxygen uptake (VO2) during walking or running on an indoor track [Citation27]. This strong association allowed for conversion of MAD values to incident energy consumption (MET). The MET were smoothed by calculating the one-minute exponential moving average of MAD values for each 6s epoch. According to recommended use [Citation29] cut-points for different activities were set as 1.5–3 MET for light activities, 3–6 MET for moderate activities, and over 6 MET for vigorous activities, and corresponding mean daily total times were determined. As vigorous activity was very low in this age-group, MVPA variable was constructed by summing up the time of moderate and vigorous activities. Mean daily sedentary time was defined as MET under 1.5 during lying down or sitting. Mean daily standing time was analyzed separately. Average daily step count and the most intensive 10-minute period of PA (Peak-10min MET) during the monitoring week were also documented. When determining the Peak-10min MET, the accelerometer value of each epoch is first converted to MET value. Then, the Peak-10min MET value is the highest 10 min moving average MET value of the measurement week.
Altogether, 791 twin individuals wore the accelerometer for at least 600 min per day for 4 days. Final target group for this study was those individuals (N = 779, mean age 73 years, range 71–75 years) who participated in objective PA monitoring and also responded to the structured question: Do you have a physician-diagnosed disease which restricts your mobility? On average, these 779 individuals wore the device for a mean of 6.73 (95% CI 6.69–6.77) days and mean 841 min (95% CI 835–847 min) per day.
Statistical methods
First, bootstrapping (1000 samples) was used to calculate statistical significances when comparing the questionnaire item on diseases restricting mobility against other questionnaire items on mobility difficulties and PA level.
Our main analysis strategy was to investigate how much of the variation in PA was explained by the diseases, which the participants reported to restrict their mobility and to investigate whether reported chronic diseases not reported to restrict mobility were associated with low PA level also among those participants who did not report any mobility restricting disease. In addition, we analyzed the additional contribution of BMI in explaining the variation in PA.
Results were calculated with bootstrapping (1000 samples unless otherwise noted) and are given as medians and their 95% confidence intervals (CIs) (Stata 12.0; Stata Corp., College Station, TX). We used linear regression analyses to define R squared (R2) as a measure of variance accounted for. First, the analyses were done with twins treated as individuals; however, because of the clustering of twins within pairs (as the sampling unit was the twin pair), the observations obtained from twin pairs may be correlated, robust estimators of variance (the cluster option in Stata) were used [Citation30]. All basic analyses yielding R2 values were adjusted for age and sex. Square root-transformation for MVPA and log-transformation for Peak-10min MET were used for regression analyses because these variables were not normally distributed. Pairwise analyses among twin pairs (all pairs, DZ pairs and MZ pairs separately) were done using Wilcoxon matched-pairs signed-rank test to discover if pairs discordant for reported diseases restricting mobility differed in the objectively measured PA or SB variables.
Results
The mean BMI of the participants was 26.11 kg/m2 (SD 3.92) with 15.2% of the participants having a BMI over 30 kg/m2.
Questionnaire reports on diseases restricting mobility and physical mobility limitations
Among the participants, nearly every fourth participant (23.2%, 181 of 779) reported to have a disease that restricted their mobility. In the following open question the most common such diseases were musculoskeletal disease in 60.2% of cases (14% of all participants; osteoarthritis being the most common specific condition with 35.4% of the cases), followed by cardiovascular (18.8%), neurological (7.7%) and pulmonary (7.7%) diseases ().
Table 1. Self-reported physician-diagnosed diseases restricting most mobility among those 181 participants reporting a mobility restricting disease.
Of the participants, almost every fifth (19.3%) reported at least some limitations in walking 2 km. Among those who did not report diseases restricting mobility, 92.3% of participants reported no limitations in walking 2 km while this proportion was 43.1% in participants reporting restricting diseases (p < .0001). Nearly every fourth participant (23.3%) reported at least some limitations in stair climbing. Among those who did not report diseases restricting mobility, 95.6% reported no limitations in climbing stairs, compared with 63.3% in participants reporting mobility restricting diseases (p < .0001).
Participants who reported having diseases restricting mobility reported their mean weekly distance of walking or jogging being 11.5 km (95% CI 9.9.-13.3 km) compared to 18.4 km (95% CI 17.2–19.6 km) in those reporting no restricting diseases (mean difference 6.9 km (95% CI 4.7–9.1 km). As a validity test, we calculated (using bootstrapping) the correlation between the reported weekly distance of walking or jogging and objectively measured daily steps the Pearson correlation being r = .61 (95% CI .55–.66; p < .0001).
Reported disease restricting mobility, other chronic diseases and objective PA measurement; individual-based analyses
Distributions of the PA and SB variables in the target cohort are shown in Supplementary Table I. Expectedly, among those who reported diseases restricting their mobility, the objectively measured sedentary time (lying and sitting) was longer and both light PA and MVPA were lower as were the daily steps compared with those reporting no such diseases (). Also, the intensity of the most intensive 10-minute PA period during the measurement week (Peak-10min MET) was lower among those reporting mobility restricting diseases. The mean difference in daily MVPA was 18 min (95% CI 14–22 min) and in daily steps 2247 (95% CI 1746–2746 steps) in favour of those not reporting a disease restricting their mobility. All the differences were rather similar among men and women () except for standing time with borderline statistically significant sex interaction (p = .050). There was no difference in the daily measurement time between the groups.
Table 2. Daily physical activity and sedentary behavior characteristics according to self-reported physician-diagnosed diseases restricting mobility and sex.Table Footnotea
When looking at the physician-diagnosed diseases reported by the participants in the interview or questionnaire, expectedly there was substantial variation in the prevalence of the diseases by reporting any disease restricting mobility (Supplementary Table II). For example, 44.0% of those not reporting a disease restricting mobility reported having hypertension compared to 60.3% among those reporting restricting disease. The corresponding percentages were 4.4% versus 14.2% for heart failure and 14.4% versus 40.9% for knee osteoarthritis (Supplementary Table II).
Using linear regression analysis we estimated how much the reported diseases accounted for the variation in the PA characteristics. With sex and age in the model, the reported disease restricting mobility explained 11.8% of the variation in MVPA and 10.4% of the variation in daily steps (). When adding different self-reported diseases separately to the regression model, each of eight diseases (hypertension, coronary heart disease or myocardial infarction, heart failure, atrial fibrillation, diabetes, rheumatoid arthritis, knee osteoarthritis and hip osteoarthritis) increased the explanatory power in the variation of MVPA more than 0.1%. For the association between these selected reported diseases and MVPA, see Supplementary Table III, and daily steps, see Supplementary Table IV when the question on their mobility restrictions is ignored. In addition, increased number of reported diseases was associated with low PA (Supplementary Table V). After adding all these eight conditions to the previous model the fraction of variance accounted for increased to 18.5% for MVPA and 16.0% for daily steps. The explanatory fraction tended to be higher for women than men but the sex interaction was statistically non-significant (). Adding BMI to the model further increased the fraction of variance accounted for MVPA to 25.5% and for daily steps to 20.7%. For results on other PA and SB variables, see .
Table 3. Reported diseases and body-mass index explaining variation in daily physical activity/sedentary behavior characteristics.
When we combined the above-mentioned eight diseases common in the older individuals but not always considered as a disease restricting mobility, we found that participants reporting these diseases, also among the group who did not report diseases restricting their mobility, had lower MVPA, daily steps and other PA level indicators than participants not reporting these diseases (Supplementary Table VI).
Pairwise analysis
There were 100 (63 DZ, 32 MZ and 5 with unknown zygosity) same-sex twin pairs who were discordant for reported chronic diseases restricting their mobility (). Among these pairs the objectively measured MVPA was higher in co-twins not reporting diseases restricting mobility compared to their co-twins with restricting disease [pairwise difference in median daily MVPA 11 min for all pairs (p < .001 for pairwise difference), 15 min for DZ pairs (p < .001) and 5 min for MZ pairs (p = .009)]. Also for other PA and SB characteristics the differences were smaller for MZ than for DZ pairs (). SB was higher among the co-twins with restricting disease (p < .001).
Table 4. Daily physical activity and sedentary behavior in twin pairs discordant for self-reported physician-diagnosed disease restricting mobility.
Discussion
Among community-dwelling older men and women one-fourth of the variation in objectively measured MVPA was explained by age, sex, BMI and self-reported diseases. These variables explained more of the variation in MVPA than that of the low-intensity activity or SB. Diseases that the participants recognized as reducing mobility accounted for only part of the variability and adding other reported diseases to a regression model increased the explanatory power of the model. Hence, participants did not always recognize that their diseases were associated with low PA level.
Our study results are consistent with several earlier reports. The review by Brown and Flood [Citation2] reported the risk factors most frequently identified as being independently associated with mobility limitation noted by five or more observational studies include older age, low PA, obesity, strength or balance impairments, and having chronic diseases such as diabetes or arthritis. Welmer et al. [Citation3] found that BMI, coronary heart disease and heart failure are associated with mobility limitations. In addition to diseases, which according to the participants’ reports restrict their mobility, there were various common chronic diseases which associated with lower PA levels and increased sedentariness. In addition, higher number of disease conditions associated with lower PA levels. Finally, we note that Smith et al. [Citation31] found that chronic health status predicts nonparticipation in PA also after adjustment for self-reported health status. The associations of diseases with SB were weaker than those with PA, however, reducing SB may be an important and feasible way of maintaining mobility among aged with severe disease conditions.
In our study cohort, we had questionnaire data on the leisure-time PA of the participants in mid-life (in 1975, 1980 and 1990) [Citation23]. The participants reporting diseases causing mobility restrictions at follow-up did not differ significantly in leisure-time PA in mid-life from those reporting no diseases restricting mobility at follow-up (for more details, see Supplementary Table VII), supporting the direct relation between the development of disease and mobility restrictions. Pairwise analyses showed that in the co-twin control design the differences in PA variables were smaller among MZ than among DZ pairs discordant for self-reported diseases restricting mobility. This is consistent with the previous finding that genetic factors contribute to the aetiology of PA limitations [Citation23]. These findings together also suggest that reverse causality analyses are needed in studies evaluating the associations between PA and occurrence of diseases [Citation8].
It is important to pay attention to the declined fitness and PA level of older individuals, in particular, if they have chronic diseases, as the slowly progressing diseases in the long-term are related with physical inactivity and further with physical disability. Either by maintaining PA levels or by using tailored exercise interventions [Citation6,Citation7] it is possible to improve the physical function of these individuals. In Finland, physicians are advised to recommend PA for patients who have diabetes or other chronic diseases, which may attenuate the associations seen in this study, but obviously there is an urgent need to increase the exercise counselling of patients with chronic disease to both maintain mobility and slow the progression of the diseases [Citation6,Citation32].
Our results were also in agreement with the findings that overweight and obesity are associated with low PA and increased incidence of mobility limitations [Citation33,Citation34]. There is accumulating data on that genetic pleiotropy may contribute to the co-existence of low PA, obesity and chronic disease [Citation8,Citation19,Citation35]. Our pairwise analyses also support the contribution of genetic pleiotropy contributing to the association between SB, PA and chronic disease, however, there was a difference in MVPA between the members of the disease-discordant MZ twin pairs. The contribution of genetic factors for PA level was shown by the previous finding that shared genetic factors explained most of the association between mid-life and later life PA levels [Citation23]. Also, there is the possibility for reverse causality, as some of the diseases such as hypertension do not cause immediate mobility limitations. Identifying chronic disease conditions and reductions in PA is most probably essential in identifying older risk persons needing preventive measures such as exercise-based rehabilitation to maintain their mobility.
The strengths of our study include sampling from a large and population-based twin cohort including men and women and successful modern objective monitoring of PA and SB characteristics. The limitations of our study include that our data on chronic diseases relied on self-reports although they had to be physician-diagnosed. In order to maximize the participation rate, the participants were not invited to travel to laboratory examinations, which means that we have not measured their anthropometry or physical function at laboratory. Mobility limitations increase with increasing disease severity, but in our survey, we were not able to do a specific severity classification of the diseases. At follow-up, all twins were community dwelling, therefore individuals with severe mobility limitations were rare, which needs to be taken into account when generalizing the results.
In conclusion, the occurrence of chronic diseases is associated with low PA and individuals do not always recognize this. Different modes of exercise therapy have previously been shown to be effective in improving both measured and self-reported functioning [Citation6], and further, independent mobility. Healthcare professionals should pay attention to low PA and mobility of individuals with chronic disease conditions before these cause limitations in independent living.
Supplemental Material
Download MS Word (41.7 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author, U.M.K., upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Laudani L, Vannozzi G, Sawacha Z, et al. Associaiton between physical activity levels and physiological factors underlying mobility in young, middle-aged and older individuals living in a city district. PLoS One. 2013;8:e74227.
- Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310:1168–1170.
- Welmer A-K, Angleman S, Rydwik E, et al. Association of cardiovascular burden with mobility among elderly people: A population-based study. PLoS One. 2013;8:e65815.
- GBD 2013 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191.
- Vancampfort D, Koyanagi A, Ward PB, et al. Chronic physical conditions, multimorbidity and physical activity across 46 low- and middle-income countries. Int J Behav Nutr Physical Activity 2017;14:6.
- Pasanen T, Tolvanen S, Heinonen A, et al. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomized controlled trials. Br J Sports Med. 2017;51:1459–1465.
- Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396.
- Kujala UM. Physical activity, genes, and lifetime predisposition to chronic diseases. Eur Rev Aging Phys Act. 2011;8:31–36.
- Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699.
- Webber SC, Porter MM, Menec VH. Mobility in older adults: a comprehensive framework. Gerontologist. 2010;50:443–450.
- Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561.
- Shumway-Cook A, Patla A, Stewart A, et al. Environmental components of mobility disability in community-living older persons. J Am Geriatr Soc. 2003;51:393–398.
- Simonsick EM, Newman AB, Visser M, et al. Mobility limitation in self-described well-functioning older adults: Importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63:841–847.
- Taradsen K, Granat MH, Helbostad JL. Quantification of outdoor mobility by use of accelerometer-measured physical behaviour. Biomed Res Int. 2015;2015:910259.
- Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162:123–132.
- Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–1310.
- Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: s systematic review and dose-response meta-analysis. Eur J Epidemiol. 2018;33:811–829.
- Polderman TJ, Benyamin B, de Leeuw CA, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015;47:702–709.
- Visscher PM, Wray NR, Zhang Q, et al. 10 years of GWAS discovery: Biology, function, and translation. Am J Hum Genet. 2017;101:5–22.
- Stubbe JH, Boomsma DI, Vink JM, et al. Genetic influences on exercise participation in 37.051 twin pairs from seven countries. PLoS One. 2006;1:e22.
- Lightfoot JT, de Geus EJC, Booth FW, et al. Biological/genetic regulation of physical activity level: consensus from GenBioPAC. Med Sci Sports Exerc. 2018;50:863–873.
- van Dongen J, Slagboom PE, Draisma HH, et al. The continuing value of twin studies in the omics era. Nat Rev Genet. 2012;13:640–653.
- Waller K, Vähä-Ypyä H, Törmäkangas T, et al. Long-term, leisure-time physical activity and other health habits as predictors of objectively monitored late-life physical activity – a 40-year twin study. Sci Rep. 2018;8:9400.
- Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res. 2002;5:358–365.
- Mänty M, Heinonen A, Leinonen R, et al. Construct and predictive validity of a self-reported measure of preclinical mobility limitation. Arch Phys Med Rehabil. 2007;88:1108–1113.
- Vähä-Ypyä H, Vasankari T, Husu P, et al. A universal, accurate intensity-based classification of different physical activities using raw data of accelerometer. Clin Physiol Funct Imaging. 2015;35:64–70.
- Vähä-Ypyä H, Vasankari T, Husu P, et al. Validation of cut-points for evaluating the intensity of physical activity with accelerometry-based mean amplitude deviation (MAD). PLoS One. 2015;10:e0134813.
- Vähä-Ypyä H, Husu P, Suni J, et al. Reliable recognition of lying, sitting, and standing with a hip-worn accelerometer. Scand J Med Sci Sports. 2018;28:1092–1102.
- Sievänen H, Kujala UM. Accelerometry – simple, but challenging. Scand J Med Sci Sports. 2017;27:574–578.
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646.
- Smith KL, Carr K, Wiseman A, et al. Barriers are not the limiting factor to participation in physical activity in Canadian seniors. J Aging Res. 2012;2012:890679.
- Kujala UM. Evidence on the effects of exercise therapy in the treatment of chronic disease. Br J Sports Med. 2009;43:550–555.
- Houston DK, Ding JD, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults. The Health, Aging and Body Composition Study. Am J Epidemiol. 2009;169:927–936.
- Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11:568–579.
- Lee H, Ash GI, Angelopoulos TJ, et al. Obesity-related genetic variants and their association with physical activity. Sports Med Open. 2015;1:34.