Abstract
Aim
Post-transplant diabetes mellitus (PTDM) is one of the main complications after kidney transplantation. It is known that leptin plays an important role in glucose metabolism and mutations in the leptin receptor gene (LEPR) are responsible for different complications in renal transplant recipients. We aimed to analyse the association of polymorphisms in LEPR with the development of PTDM in these patients.
Methods
A total of 315 renal transplant recipients were genotyped for the Lys109Arg, Gln223Arg and Lys656Asn polymorphisms. The impact of these genetic variables together with other clinical and demographic parameters on PTDM risk was evaluated in a multivariate regression analysis.
Results
The 223Arg variant showed a significant association with PTDM risk [OR = 3.26 (1.35–7.85), p = 0.009] after correcting for multiple testing. Carriers of this variant also showed higher BMI values (26.95 ± 4.23) than non-carriers (25.67 ± 4.43, p = 0.025). In addition, it was BMI at transplant and not the BMI increment in the first year after grafting that was associated with PTDM (p > 0.00001). Haplotype analyses did not reveal significant associations.
Conclusions
Our result show, for the first time to our knowledge, that genetic variability in the LEPR may contribute significantly to the risk for PTDM in renal transplant recipients.
The LEPR Gln223Arg polymorphism significantly contributes to the development of PTDM in renal transplant recipients.
The effect of the 223Arg variant on PTDM is strongly modulated by the age of the recipient.
The 223Arg variant in the leptin receptor is related to higher BMI in renal transplant recipients.
KEY MESSAGES
Introduction
Post-transplant diabetes mellitus (PTDM) is one of the most common complications in renal transplant recipients [Citation1]. Its incidence ranges between 4 and 25% in these patients [Citation2], who will require insulin treatment in 50% of the cases [Citation3]. PTDM is also associated with further complications in renal transplantation, such as cardiovascular events, graft loss and increased risk of death [Citation4–7].
A number of risk factors have been described in association with PTDM, including age, ethnicity, obesity, certain immunosuppressive drugs, viral infections and a family history of diabetes [Citation8–10]. In addition, others and we have highlighted the importance of genetic factors in the development of this complication [Citation11–13].
Leptin is a cytokine produced in the white adipose tissue whose main role is to act on the hypothalamus to induce satiety and reduce fat reserves. In addition to this and other general functions, there is accumulating evidence indicating that input from afferent signals, such as leptin, together with other nutrient-related and hormonal signals, generate responses that control insulin sensitivity and glucose uptake in order to keep energy balance and glucose homeostasis [Citation14]. These functions have consequently prompted significant research efforts aimed to establish leptin involvement in diabetes [Citation15].
Recent studies have highlighted the clinical relevance of genetic variability in leptin genes in the renal transplant setting. Thus, it is known that mutations in the LEPR gene (coding for the leptin receptor) may induce cardiovascular complications, which are commonplace in these patients [Citation16,Citation17]. However, to our knowledge, there are no studies that have analysed the connection of these genetic variants with the incidence of PTDM.
The aim of this study was, therefore, to analyse common polymorphisms in the LEPR gene, which have previously been associated with changes in leptin levels or function and/or pathologies that are common in renal transplant recipients [Citation18–22], in order to find out putative associations with the development of PTDM.
Patients and methods
Study design
The study group consisted of 345 Caucasian renal transplant recipients who received a single kidney from deceased donors at the Badajoz University Hospital (Spain). Thirty of these patients were ruled out from the study as they had a history of diabetes mellitus prior to transplant and hence the final number of patients analysed was 315. All of the subjects provided written consent for their participation in the study, which was approved by the Ethics Committee of the hospital and was conducted in accordance with the Declaration of Helsinki and its subsequent revisions.
The treatment for the patients consisted of a triple immunosuppressive therapy with corticoids (500 mg IV methylprednisolone at the time of surgery, 125 mg intravenously the following day and then 20 mg of oral prednisone daily, progressively tapered to 5 mg daily at 2 months after transplantation), mycophenolate mofetil (MMF) (2 g/d) and calcineurin inhibitors (cyclosporine or tacrolimus).
Tacrolimus and cyclosporine initial doses were 0.1 mg/kg and 4–10 mg/kg/d divided into two administrations, respectively. Doses were routinely adjusted according to blood concentrations measured by an immunoassay performed on a Cobas Mira Plus analyser (Roche Diagnostics, Basel, Switzerland). Concentration to dose ratios (CDRs) (ng/ml per mg/kg/day) were calculated by dividing trough blood levels (ng/ml) by the previous daily weight-adjusted dose (mg/kg).
Assessment of clinical variables
Clinical records were retrospectively reviewed to establish the occurrence of PTDM according to the American Diabetes Association criteria and were defined as two fasting plasma glucose values ≥6.99 mmol/l or symptoms of diabetes plus casual plasma glucose concentrations ≥11.10 mmol/l throughout the first year [Citation23].
Acute allograft rejection was established by histological findings in renal biopsies according to the Banff classification and/or by clinical evaluation as previously described [Citation24,Citation25]. Delayed graft function (DGF) was defined as the need for dialysis within the first week after transplantation [Citation26]. Renal function was assessed by estimating the glomerular filtration rate (eGFR) from serum creatinine using the modification of diet in renal disease (MDRD) formula [Citation27].
Genotype analysis
Genomic DNA was isolated from whole blood samples using a standard phenol-chloroform extraction method. Three common, functional SNPs in the LEPR gene first reported by Chung et al. [Citation28], namely Lys109Arg (rs1137100), Gln223Arg (rs1137101) and Lys656Asn (rs1805094), were screened for by RT-PCR using commercially available probes from Life Technologies (Rockville, MD). Gln223Arg is supposed to affect receptor activity, as it is located in a key domain of the protein [Citation29]. Furthermore, this SNP and Lys109Arg have also been related to decreased levels of soluble LEPR, a special isoform of leptin receptor in the circulation that may be used as a surrogate of the cell surface expression levels of LEPR [Citation30]. The last SNP, Lys656Asn is located in the extracellular domain and produces an amino acid substitution resulting in changes in charge, which suggests functional consequences [Citation28].
Previously, sequenced samples were used as negative and positive controls to rule out possible genotyping errors. Likewise, the analysis of 5% of the samples was duplicated and confirmed by direct sequencing (ABI 3700 DNA Analyzer, Applied Biosystems. Foster City, CA). Genotyping was successful in 100% of the samples.
Statistical analyses
Fisher’s exact or Pearson’s χ2 test were used for the univariate analysis of the associations between categorical data. The t-Student’s/ANOVA or Mann–Whitney/Kruskal–Wallis tests, as appropriate, were utilized in order to compare mean values of quantitative variables between groups. Multivariate regression analysis was used to evaluate the impact of different genetic, clinical and demographic parameters on PTDM risk in our series. After analysing all models of inheritance, multivariate genetic association analyses were carried out with a dominant model (carriers vs. non-carriers) in order to balance the number of individuals in each group. Genotype was hence coded as a dichotomous variable (0 for wild type homozygotes and 1 for carriers of variant alleles. For the haplotype study, the SNPassoc package (Barcelona, Spain) [Citation31] in the R environment was utilized to estimate the frequency of the resulting haplotypes and to determine the effect of these allele combinations on PTDM risk controlling for significant covariates. Frequency threshold for rare haplotypes was set at 0.01.
The statistical power of the study was evaluated with a genetic model established analysing the frequency for carriers of the variant alleles with an arbitrarily effect size (expressed as odds ratio) set at 2.5 (type I error= 0.05), as previously described [Citation13,Citation32]. With the available sample size and the reported incidence of PTDM, the power for detecting associations ranged from 0.79 to 0.86 depending on the minor allele frequency (Quanto Software version 1.2.4, USC, Los Angeles, CA). Statistical analyses were performed with IBM SPSS statistics version 22 (Chicago, IL) and the SNPassoc R package. After correction for multiple testing (three univariate and one multivariate analyses), differences were considered to be significant when p values were lower than .0125.
Results
A cohort of 315 renal transplant recipients (196 men and 119 women) without a prior history of diabetes mellitus and a mean age of 47.58 ± 13.52 years was retrospectively studied. Most frequent primary kidney diseases in our series were glomerulonephritis (37.6%), polycystic kidney disease (16.8%) and chronic interstitial nephritis (12.1%); several other conditions accounted for 14.1% of the cases. The specific condition could not be determined in 19.4% of patients. A statistical trend was observed regarding the influence of polycystic kidney disease on PTDM [OR = 1.61 (0.80–3.26), p = 0.129].
The number of recipients who developed PTDM in the first year after grafting was 57 (18.1%), which is consistent with other studies in renal transplantation [Citation1].
Doses and trough concentrations of immunosuppressants, body mass index (BMI), weight, creatinine serum concentrations and creatinine clearance were measured at four different times after transplant: 1 week; 1 month; 5 months and 1 year. Additional clinical characteristics of the patients are shown in .
Table 1. Clinical and demographic parameters of the study population.
Genetic study
The three LEPR SNPs studied showed frequencies that did not differ from those expected by the Hardy Weinberg equilibrium (p > 0.05 in all cases). Minor allele frequencies observed ranged from 0.181 to 0.422 ().
Table 2. Genotypic and allelic frequencies in 315 renal transplant recipients.
The association of these polymorphisms with the risk of PTDM was evaluated in a crude analysis, using a dominant model of inheritance, i.e. carriers of the variant allele vs. non-carriers (). A statistical trend towards increased risk of PTDM was observed for carriers of the LEPR 223Arg variant allele [OR = 1.94 (0.98–3.86), p = 0.058]. The results for other models of inheritance are shown in Supplementary Table S1.
Table 3. Crude analyses for the association between polymorphisms in the LEPR gene with the development of post-transplant diabetes mellitus (PTDM) in renal transplant recipients.
After performing univariate analyses, several clinical and demographic covariates were included in a logistic regression model to re-evaluate the risk conferred by these SNPs. The results showed how the 223Arg variant was significantly associated with PTMD risk after correcting for multiple testing [OR = 3.26 (1.35–7.85), p = 0.009] (). None of the other two SNPs showed any effect on the susceptibility to PTDM in our series.
Table 4. Multivariate logistic regression analysis for the risk of post-transplant diabetes mellitus in renal transplant recipients.
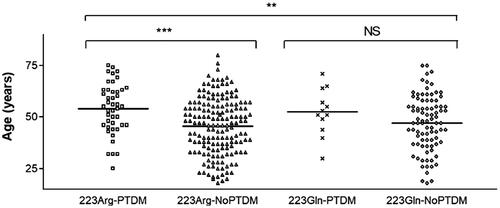
The fact that the 223Arg variant only displayed a statistical trend towards increased risk of PTDM in univariate analyses but was regarded as highly significant in the multivariate model, prompted us to investigate which of the other covariates allowed the variant to gain significance as PTDM predictor. Additional logistic regression analyses with forward and backward methods (data not shown) pointed to “age of the recipient” as being key for this increase. We then carried out additional analyses to further investigate a possible effect of the 223Arg*Age interaction. First, we show in and Supplementary Table S2 the distribution of recipient age across four different groups, namely carriers and non-carriers of the 223Arg variant in patients with or without PTDM. There was indeed a statistically significant age difference (p = 0.0004) between carriers of the 223Arg with or without PTDM (). In contrast, this difference was not significant amongst non-carriers. Second, interestingly enough, Supplementary Table S3 shows that the interaction 223Arg*Age was also an independent risk factor for PTDM in multivariate analysis.
Figure 1. Distribution of the age of the recipient across four different population groups according to post-transplant diabetes mellitus (PTDM) and 223Arg status. **p = .002; ***p = .0004; NS: non-significant. Note: Analyses of differences between the first and third groups, and between the second and fourth groups produced non-significant results.
Risk of PTDM associated with demographic and clinical variables
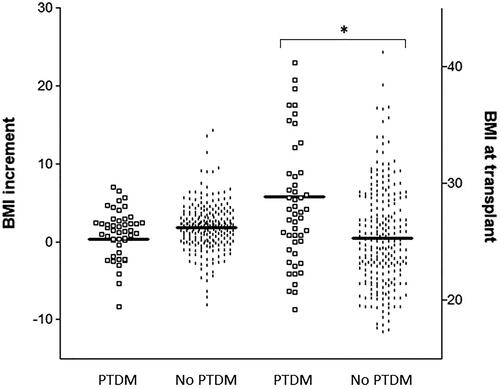
Different non-genetic factors were analysed to investigate their impact on PTDM. The age of the recipient and BMI showed a strong association with this complication. Mean age and standard deviation of the recipients without PTDM were 46.19 ± 13.49 years vs. 53.88 ± 11.85 years for patients with PTDM (p < 0.0001). BMI values at all-time points considered were significantly higher in the PTDM group (Supplementary Figure S1), although the statistical significance of the association between BMI and PTDM was lost in multivariate analysis (). We also aimed to determine whether it was BMI at transplant or the increment of BMI in the first year after grafting that it was associated with PTDM. shows that BMI values at transplant were far higher in patients with new-onset diabetes (28.81 ± 7.08) than in patients without the complication (25.3 ± 4.28, p < 0.00001); however, BMI increments in the first year after transplant were fairly similar between both groups (0.36 ± 5.61 vs. 1.78 ± 3.04, p = .185).
Figure 2. Body mass index (BMI) at transplant and increment of BMI during the first year after transplant, in renal transplant recipients with and without post-transplant diabetes mellitus (PTDM). *p < .00001.
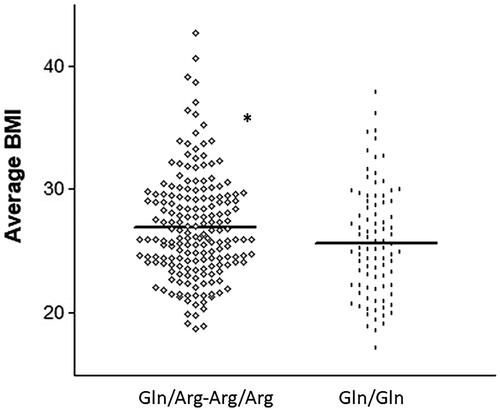
Of note, the same 223Arg variant that was associated with PTDM was observed to be significantly more frequent among patients with higher BMIs. The average BMI in the first year after grafting was 26.95 ± 4.23 for carriers of the Arg variant allele vs. 25.67 ± 4.43 for non-carriers (p = 0.025) (). The other two SNPs did not have a significant impact on BMI values (p > 0.05 in both cases).
Figure 3. Average body mass index (BMI) in the first year after transplant for carriers and non-carriers of the 223Arg variant. *p < .05.
The type of immunosuppressant (tacrolimus or cyclosporine) utilized did not influence the development of PTDM, as the incidence of the complication in both groups was fairly similar [18.5 and 16.7%, respectively, OR = 1.13 (0.55–2.33), p = 0.735]. In the same manner, Supplementary Figure S2 shows that the levels of immunosuppressant were not significantly different between patients with and without PTDM.
Finally, among other clinical variables, DGF and acute rejection showed associations with PTDM risk in univariate analyses [OR = 1.78 (0.96–3.30), p = 0.05] and [OR = 2.38 (1.17–4.85), p = .016], respectively.
Haplotype analysis
Haplotype analyses were carried out in order to determine if allele combinations could display a more profound effect than that observed in the single-SNP approach. Three haplotypes were identified with a frequency over 0.100. Haplotype *1 AAG (Lys109Arg/Gln223Arg/Lys656Asn) was the most frequent, as it was carried by almost 40% of the patients. None of the haplotypes showed significant associations with PTDM after controlling for the same covariates previously described in the single-SNP approach (). Linkage disequilibrium information, frequencies observed and OR values obtained are depicted in Supplementary Figure S3.
Discussion
PTDM, usually developing in the first months after transplantation, may severely impact both short- and long-term outcomes of kidney transplant recipients in terms of graft and patient survival, but it often goes undiagnosed, is underestimated or poorly managed [Citation1]. Furthermore, the incidence of PTDM is likely to increase in the next years due to the growing prevalence of obesity among renal transplant recipients combined with improved access to transplantation for older patients [Citation33]. Therefore, identifying new risk factors for this complication may be very important for improving its management. In this regard, the main finding of the present work is that genetic variability in the leptin receptor gene (LEPR) increased the risk for developing PTDM in renal transplant recipients. Using logistic regression modelling, we observed that carriers of the LEPR 223Arg variant allele had over two-fold risk of PTDM than non-carriers. This finding is in line with the growing evidence suggesting that leptin plays an important role in glucose metabolism. Indeed, early rodent models of leptin deficiency were characterized by insulin resistance and diabetes [Citation34,Citation35], conditions that have been shown to be ameliorated with leptin administration both in rodents and humans [Citation36,Citation37]. Moreover, a very recent report argued that leptin levels could be used as a marker for PTDM risk in renal transplant recipients [Citation38].
The Gln223Arg SNP in the LEPR is a widely studied mutation that has been related to metabolic, infectious and malignant pathologies [Citation39–42]. More relevant for the aims of this study, this SNP has also been examined in relation to the incidence of diabetes mellitus. Consistent with our results, a meta-analysis totalizing 3649 type-2 diabetes mellitus (T2DM) cases and 2844 controls reported that the Gln223Arg SNP was associated with T2DM using the same dominant model of inheritance utilized in the present work [Citation43]. It should be mentioned, however, that there are other studies that do not support this effect of this Gln223Arg SNP [Citation44]. Interestingly, this polymorphism has also been associated with higher BMI values [Citation45] and obesity [Citation46], which are known risk factors for diabetes. In this regard, our results also confirm that the SNP was associated with higher BMIs in our group of transplant recipients.
The functional consequences of this polymorphism, resulting in a glutamine-to-arginine substitution at residue 223, are not clearly established yet, although it does take place in a domain that is crucial for receptor activity [Citation29]. Differences in affinity and kinetics of leptin binding are, however, unlikely to explain mechanistically the different phenotypes that have been linked to this SNP [Citation47]. One of the hypotheses proposed to explain the observed phenotypes is that the presence of variants in the LEPR gene locus may lead to impaired tyrosine phosphorylation in the receptor and subsequently reduced leptin signalling [Citation48,Citation49]. We, therefore, propose that this deficient leptin signalling would also reflect in an impaired glucose-lowering function. Indeed, leptin has been shown to display these glucose-lowering effects in several models [Citation50–52] and has even been proposed as a potentially useful adjunct to insulin treatment in the management of diabetes [Citation15]. Of interest to our study population, there are also indications arguing for an important role of leptin in renal transplant recipients. Nicoletto et al. reported that leptin levels decrease in the first year after transplant, which is accompanied by insulin resistance and further complications [Citation53]. Our hypothesis linking polymorphisms in leptin-related genes to PTDM is not unprecedented. Romanowski et al. have shown in 323 renal transplant recipients that the rs2167270 SNP of the LEP gene, coding for the leptin protein, was also associated with increased risk of PTDM [Citation54], which is consistent with our view that variability in leptin genes can be important for the susceptibility to diabetes onset after kidney transplant.
Finally, our results also showed that there exists an interaction between the age of the recipient and the presence of the 223Arg variant, as this allele modified the strength of association between age and PTDM. It should be noted, however, that the low number of patients with PTDM (n = 12) who did not carry the 223Arg variant may have hampered the detection of age differences in this group of non-carriers.
Determining the role of LEPR genetic variability in PTDM was the main objective of this study, but there are other non-genetic risk factors that have been formulated, although they vary greatly across patients series [Citation5,Citation55–58]. In our population, the age of the recipient showed one of the strongest associations with PTDM, which is in agreement with the general consensus on the relevance of this parameter [Citation57]. A high BMI has also been regarded as traditional risk factor for new-onset diabetes after renal transplantation. We and others have pointed to pre-transplant anthropometric measures as the most influential for PTDM development [Citation13,Citation56,Citation59], whilst others argue for a greater importance of weight gain after transplant [Citation57,Citation60]. This study is consistent with the former theory, as clearly shows how pre-transplant BMI was considerably higher in recipients with PTDM, whereas the increment in BMI during the first year after grafting was fairly comparable between both groups of patients. In any case, it should be noted that the effect of BMI on PTDM lost significance in multivariate analysis, which could have to do with the already described strong effect of the age of the recipient and the 223Arg variant. In fact, when these two covariates were introduced as an interaction term (Age*223Arg), as shown in Supplementary Table S3, the BMI showed a borderline p value (p = .053).
Finally, with regard to the immunosuppressant medication administered, tacrolimus has traditionally been regarded as more diabetogenic than cyclosporine [Citation5,Citation61,Citation62]. Our results did reveal a higher incidence of PTDM in tacrolimus-treated patients, but the difference did not reach statistical significance. Perhaps the comparatively small size of the group of patients on cyclosporine, with few subjects experiencing PTDM, hampered the statistical analysis. It could also be that differences between both treatments could be better assessed in the long term [Citation5,Citation56,Citation61]. In fact, Webster et al. have shown in a meta-analysis with 4102 renal transplant recipients that the relative risk for PTDM in patients on tacrolimus compared with those on cyclosporine was 1.8, twelve months after the transplant but increased up to almost four three years later [Citation63].
In addition to the aforementioned relatively small size of the cyclosporine group, there were other limitations in this retrospective study, the most important of which was that leptin concentrations were not available for the transplant recipients. Having this data would have helped elucidate some of the mechanisms by which polymorphisms in leptin-related genes affect the development of PTDM. Furthermore, even though the sample was large enough to provide an adequate statistical power, the inclusion of more individuals would have allowed the formal analysis of the influence of variant homozygous genotypes, which were not carried by enough patients in this study.
In summary, we have shown for the first time to our knowledge that genetic variability in the LEPR significantly contributed to the development of PTDM in renal transplant recipients after controlling for other relevant variables. Therefore, genotyping for the LEPR Gln223Arg could be useful in identifying patients at-risk for PTDM. However, it should be remarked that BMI and especially age are factors that may affect this genotype-phenotype association.
These findings add to the growing body of research suggesting an important role for leptin in diabetes and more specifically new-onset diabetes after transplant. Notwithstanding, further studies addressing this question in larger cohorts are warranted to confirm the findings described herein.
Supplemental Material
Download PDF (229.4 KB)Acknowledgements
The authors want to thank the technical and human support provided by the Facility of Bioscience Applied Techniques of SAIUEx (financed by UEX, Junta de Extremadura, MICINN, FEDER and FSE).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Conte C, Secchi A. Post-transplantation diabetes in kidney transplant recipients: an update on management and prevention. Acta Diabetol. 2018;55:763–779.
- Pham PT, Pham PM, Pham SV, et al. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes. 2011;4:175–186.
- Zelle DM, Corpeleijn E, Deinum J, et al. Pancreatic beta-cell dysfunction and risk of new-onset diabetes after kidney transplantation. Diabetes Care. 2013;36:1926–1932.
- Israni AK, Snyder JJ, Skeans MA, et al. Predicting coronary heart disease after kidney transplantation: patient outcomes in renal transplantation (PORT) study. Am J Transplant. 2010;10:338–353.
- Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185.
- Bonato V, Cataldo D, Dotta F, et al. Diagnosis and approach to post-transplant diabetes. Curr Diab Rep. 2009;9:317–323.
- Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. 2016;37:37–61.
- Mora PF. New-onset diabetes after renal transplantation. J Investig Med. 2010;58:755–763.
- Yates CJ, Fourlanos S, Hjelmesaeth J, et al. New-onset diabetes after kidney transplantation-changes and challenges. Am J Transplant. 2012;12:820–828.
- Luan FL, Steffick DE, Ojo AO. New-onset diabetes mellitus in kidney transplant recipients discharged on steroid-free immunosuppression. Transplantation. 2011;91:334–341.
- Kang ES, Kim MS, Kim YS, et al. A variant of the transcription factor 7-like 2 (TCF7L2) gene and the risk of posttransplantation diabetes mellitus in renal allograft recipients. Diabetes Care. 2008;31:63–68.
- Sarno G, Muscogiuri G, De Rosa P. New-onset diabetes after kidney transplantation: prevalence, risk factors, and management. Transplantation. 2012;93:1189–1195.
- Gervasini G, Luna E, Garcia-Cerrada M, et al. Risk factors for post-transplant diabetes mellitus in renal transplant: role of genetic variability in the CYP450-mediated arachidonic acid metabolism. Mol Cell Endocrinol. 2016;419:158–164.
- Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389–411.
- Meek TH, Morton GJ. The role of leptin in diabetes: metabolic effects. Diabetologia. 2016;59:928–932.
- Lian Y, Tang Z, Xie Y, et al. Leptin receptor gene polymorphisms and risk of hypertension: a meta-analysis. Int J Clin Exp Med. 2015;8:14277–14282.
- Aijala M, Santaniemi M, Bloigu R, et al. Leptin receptor Arg109 homozygotes display decreased total mortality as well as lower incidence of cardiovascular disease and related death. Gene. 2014;534:88–92.
- Li YY, Wang H, Yang XX, et al. LEPR gene Gln223Arg polymorphism and type 2 diabetes mellitus: a meta-analysis of 3367 subjects. Oncotarget. 2017;8:61927–61934.
- Wu L, Sun D. Leptin receptor gene polymorphism and the risk of cardiovascular disease: a systemic review and meta-analysis. Int J Environ Res Public Health. 2017;14:pii: E375.
- Chavarria-Avila E, Vazquez-Del Mercado M, Gomez-Banuelos E, et al. The impact of LEP G-2548A and LEPR Gln223Arg polymorphisms on adiposity, leptin, and leptin-receptor serum levels in a Mexican mestizo population. Biomed Res Int. 2015;2015:539408.
- Suriyaprom K, Tungtrongchitr R, Thawnasom K. Measurement of the levels of leptin, BDNF associated with polymorphisms LEP G2548A, LEPR Gln223Arg and BDNF Val66Met in Thai with metabolic syndrome. Diabetol Metab Syndr. 2014;6:6.
- de Luis DA, Aller R, Izaola O, et al. Influence of Lys656Asn polymorphism of leptin receptor gene on leptin response secondary to two hypocaloric diets: a randomized clinical trial. Ann Nutr Metab. 2008;52:209–214.
- Davidson JA, Wilkinson A. New-onset diabetes after transplantation 2003 international consensus guidelines: an endocrinologist’s view. Diabetes Care. 2004;27:805–812.
- Joosten SA, Sijpkens YW, van Kooten C, et al. Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int. 2005;68:1–13.
- Gervasini G, Garcia M, Macias RM, et al. CYP2C8*3 polymorphism and donor age are associated with allograft dysfunction in kidney transplant recipients treated with calcineurin inhibitors. J Clin Pharmacol. 2013;53:427–434.
- Perico N, Cattaneo D, Sayegh MH, et al. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827.
- Levey AS, Greene T, Kuske J, et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A.
- Chung WK, Power-Kehoe L, Chua M, et al. Exonic and intronic sequence variation in the human leptin receptor gene (LEPR). Diabetes. 1997;46:1509–1511.
- Stratigopoulos G, LeDuc CA, Matsuoka N, et al. Functional consequences of the human leptin receptor (LEPR) Q223R transversion. Obesity (Silver Spring). 2009;17:126–135.
- Sun Q, Cornelis MC, Kraft P, et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum Mol Genet. 2010;19:1846–1855.
- Gonzalez JR, Armengol L, Sole X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23:644–645.
- Gervasini G, Garcia-Cerrada M, Vergara E, et al. Polymorphisms in CYP-mediated arachidonic acid routes affect the outcome of renal transplantation. Eur J Clin Invest. 2015;45:1060–1068.
- McCaughan JA, McKnight AJ, Maxwell AP. Genetics of new-onset diabetes after transplantation. J Am Soc Nephrol. 2014;25:1037–1049.
- Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metab Clin Exp. 1976;25:1567–1574.
- Wyse BM, Dulin WE. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia. 1970;6:268–273.
- Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350.
- Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543.
- Dedinska I, Mackova N, Kantarova D, et al. Leptin – a new marker for development of post-transplant diabetes mellitus? J Diabetes Complications. 2018;32:863–869.
- Jackson KG, Delgado-Lista J, Gill R, et al. The leptin receptor Gln223Arg polymorphism (rs1137101) mediates the postprandial lipaemic response, but only in males. Atherosclerosis. 2012;225:135–141.
- Abdu Allah AM, El-Hefnway SM, Alhanafy AM, et al. Leptin receptor gene (A/G) polymorphism rs1137101 and renal cell carcinoma. Mol Cell Biochem. 2018;448:137–144.
- Mackey-Lawrence NM, Guo X, Sturdevant DE, et al. Effect of the leptin receptor Q223R polymorphism on the host transcriptome following infection with entamoeba histolytica. Infect Immun. 2013;81:1460–1470.
- Madan R, Guo X, Naylor C, et al. Role of leptin-mediated colonic inflammation in defense against clostridium difficile colitis. Infect Immun. 2014;82:341–349.
- Yang MM, Wang J, Fan JJ, et al. Variations in the obesity gene “LEPR” contribute to risk of type 2 diabetes mellitus: evidence from a meta-analysis. J Diabetes Res. 2016;2016:5412084.
- Yang Y, Niu T. A meta-analysis of associations of LEPR Q223R and K109R polymorphisms with type 2 diabetes risk. PLoS One. 2018;13:e0189366.
- Murugesan D, Arunachalam T, Ramamurthy V, et al. Association of polymorphisms in leptin receptor gene with obesity and type 2 diabetes in the local population of Coimbatore. Indian J Hum Genet. 2010;16:72–77.
- Yiannakouris N, Yannakoulia M, Melistas L, et al. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86:4434–4439.
- Verkerke H, Naylor C, Zabeau L, et al. Kinetics of leptin binding to the Q223R leptin receptor. PLoS One. 2014;9:e94843.
- Banks AS, Davis SM, Bates SH, et al. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572.
- Pan W, Allison MB, Sabatini P, et al. Transcriptional and physiological roles for STAT proteins in leptin action. Mol Metab. 2019;22:121–131.
- Havel PJ, Uriu-Hare JY, Liu T, et al. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274:R1482–1491.
- Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492.
- Yu X, Park BH, Wang MY, et al. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105:14070–14075.
- Nicoletto BB, Souza GC, Goncalves LF, et al. Leptin, insulin resistance, and metabolic changes 5 years after renal transplantation. J Ren Nutr. 2012;22:440–449.
- Romanowski M, Dziedziejko V, Maciejewska-Karlowska A, et al. Adiponectin and leptin gene polymorphisms in patients with post-transplant diabetes mellitus. Pharmacogenomics. 2015;16:1243–1251.
- Gaynor JJ, Ciancio G, Guerra G, et al. Multivariable risk of developing new onset diabetes after transplant-results from a single-center study of 481 adult, primary kidney transplant recipients. Clin Transplant. 2015;29:301–310.
- Marrero D, Hernandez D, Tamajon LP, et al. Pre-transplant weight but not weight gain is associated with new-onset diabetes after transplantation: a multi-centre cohort Spanish study. NDT Plus. 2010;3:ii15–ii20.
- Cosio FG, Kudva Y, van der Velde M, et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–2421.
- Numakura K, Satoh S, Tsuchiya N, et al. Clinical and genetic risk factors for post-transplant diabetes mellitus in adult renal transplant recipients treated with tacrolimus. Transplantation. 2005;80:1419–1424.
- Cole EH, Prasad GV, Cardella CJ, et al. A pilot study of reduced dose cyclosporine and corticosteroids to reduce new onset diabetes mellitus and acute rejection in kidney transplant recipients. Transplant Res. 2013;2:1.
- Courivaud C, Ladriere M, Toupance O, et al. Impact of pre-transplant dialysis modality on post-transplant diabetes mellitus after kidney transplantation. Clin Transplant. 2011;25:794–799.
- Woodward RS, Schnitzler MA, Baty J, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3:590–598.
- Vincenti F, Schena FP, Paraskevas S, et al. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8:307–316.
- Webster AC, Woodroffe RC, Taylor RS, et al. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810.
