Abstract
Persistent reservoirs of multidrug-resistant microorganisms (MDRO) that are prevalent in hospital settings and communities can lead to the spread of MDRO. Currently, there are no effective decolonization strategies, especially non-pharmacological strategies without antibiotic regimens. Our aim was to evaluate the efficacy and safety of fecal microbiota transplantation (FMT) for the eradication of MDRO. A systematic literature search was performed to identify studies on the use of FMT for the decolonization of MDRO. PubMed, EMBASE, Web of Science, and Cochrane Library were searched from inception through January 2019. Of the 1395 articles identified, 20 studies met the inclusion and exclusion criteria. Overall, the efficacy of FMT for the eradication of each MDRO was 70.3% (102/146) in 121 patients from the 20 articles. The efficacy rates were 68.2% (30/44) for gram-positive bacteria and 70.6% (72/102) for gram-negative bacteria. Minor adverse events, including vomiting, diarrhea, abdominal pain, and ileus, were reported in patients who received FMT. FMT could be a promising strategy to eradicate MDRO in patients. Further studies are needed to confirm these findings and establish a comprehensive FMT protocol for standardized treatment.
The development of new antibiotics lags behind the emergence of multidrug-resistant microorganisms (MDRO). New strategies are needed.
Theoretically, fecal microbiota transplantation (FMT) might recover the diversity and function of commensal microbiota from dysbiosis in MDRO carriers and help restore colonization resistance to pathogens.
A literature review indicated that FMT could be a promising strategy to eradicate MDRO in patients.
Key messages
Introduction
Antimicrobial resistance is a significant global threat to public health [Citation1,Citation2]. The increasing prevalence of multidrug-resistant microorganisms (MDRO) is one of the most important medical challenges associated with the risk of mortality from infectious diseases for which no effective antibiotic treatment exists.
The intestinal colonization of MDRO causes the spread of MDRO infections in both hospitals and communities [Citation3,Citation4]. Antimicrobial resistance can emerge from the gut microbiome via patient-to-patient or environment-to-patient transmission of exogenous MDRO, as well as via de novo acquisition of antibiotic-resistant mutants mediated by the presence of antibiotic pressure or by gene transfer events [Citation5,Citation6].
Intestinal decontamination of MDRO might prevent further infection and transmission. Various interventions with antibiotic regimens with evidence of clinical success are available to eradicate intestinal MDRO carriage from patients [Citation7–10]. However, these interventions can occasionally be ineffective or can be associated with the risk of decolonization-associated antimicrobial resistance [Citation11–13]. Non-pharmacological approaches, such as infection control practices and antimicrobial stewardship programs, have been applied as an alternative to antibiotics for the prevention or treatment of MDRO infections [Citation14–16].
Researchers have been investigating the ability of gut microbiota in healthy humans to prevent pathogen colonization, a mechanism known as colonization resistance [Citation17]. If intestinal colonization by MDRO indicates the perturbation of the normally stable gut microbiota, a state known as dysbiosis, healthy microbiota might reverse dysbiotic changes to a healthy microbiome ecosystem, also known as resilience [Citation18].
In practice, fecal microbiota transplantation (FMT) has been shown to be a highly effective nonpharmacologic treatment for recurrent Clostridioides difficile infection (CDI) [Citation19,Citation20]. A few case reports showed that several types of MDRO were eradicated in patients undergoing FMT for recurrent CDI [Citation16]. Several studies have shown that FMT can directly decolonize non-CDI patients with MDRO infection [Citation6]. Although patients benefit from FMT, concerns regarding the long-term outcomes and adverse events remain to be addressed.
To provide clarity, we systematically reviewed the efficacy and adverse events of FMT for the decolonization of intestinal MDRO carriages by compiling previous studies regarding FMT for the eradication of MDRO.
Materials and methods
Information source and search strategy
This systematic review was performed in accordance with the guidelines of the 2009 Preferred Reporting Items for Systematic Review and Meta-analysis Statement [Citation21]. Electronic databases for the planned literature search included PubMed, EMBASE, Web of Science, and Cochrane Library for English language articles on FMT for the eradication of MDRO acquisition from the date of inception to January 2019. The last search was run on January 18, 2019.
These databases were searched using the Medical Subject Headings and keyword terms under three broad search themes of FMT—mechanisms of antimicrobial resistance, species of bacteria—and combined using a Boolean operator AND OR (Supplementary material 1). Patients of all age groups were included.
Selection of studies
Our review was designed according to the patient-intervention-comparison-outcome model, and previous studies meeting the eligibility criteria were included in our analysis. All study types with original data published in English were reviewed by three investigators. Titles, abstracts, and keywords were independently assessed by two investigators to determine the inclusion or exclusion eligibility. Both investigators (YKY and JWS) checked all the articles in accordance with the inclusion and exclusion criteria. Any discrepancies were resolved by discussion with a third investigator (JYK).
The reference lists of the included and selected articles were manually searched for additional articles that might have been missed by the database search. Original full-text articles, letters to the editor, single cases, and case series published between 1913 and 2019 were reviewed. Animal studies and non-original reports (reviews, systematic reviews, meta-analyses, abstracts of scientific conferences, and editorials) were excluded.
Our eligibility criteria for inclusion were as follows: (1) full-text articles on studies of any type on human subjects who had MDRO intestinal carriage; (2) FMT administration via any method for laboratory-confirmed MDRO intestinal carriage; and (3) reporting of any of the outcomes of interest in the article. Patients who received FMT in inpatient, outpatient, or home settings were included. The exclusion criteria were as follows: (1) studies that evaluated the efficacy or safety of FMT only for CDI; (2) letters to the editor with duplication of study subjects with a subsequent full publication; and (3) studies that did not report on any of the required outcomes.
Data collection and list of articles
Data were extracted according to the inclusion and exclusion criteria, and were cross-checked by the two independent investigators (YKY and JWS). Study items included study characteristics (first author, year of publication, study type, and length of follow-up), patients (age, gender, and pathogens), FMT procedure (pre-transplant bowel preparation, pre-transplant use of antibiotics, dose of infused stools, route of infusion, number of infusions, and stool donor relationship), and outcome data (eradication of intestinal MDRO carriage and description of adverse events within 30 days from the first FMT).
Definition
FMT was defined as the administration of a suspension of donor feces (either fresh or frozen) into the gastrointestinal tract. Bacteriotherapy with a suspension of specific bacterial groups was not regarded as FMT. Our outcomes of interest were microbiological eradication of MDRO intestinal colonization and adverse events of FMT. Microbiological eradication was defined as the absence of MDRO from the intestine after FMT during study follow-up periods, which varied by study or case report. Eradication was defined as the absence of MDRO on follow-up stool testing. Eradication failure was defined as persistence or recurrence of positive results on microbiological tests. Microbiological tests included culture methods and polymerase chain reaction detection of MDRO. The number and timing of stool testing varied by study or case report.
Adverse events were defined as any unintended and disadvantageous signs, symptoms, or diseases temporally related to FMT [Citation22]. A serious adverse event was any unexpected medical occurrence in a patient after the administration of FMT that resulted in any of the following: death, life-threatening events, new hospitalization or extension of hospital stay, significant or lasting incapacity or disability, congenital anomaly, or an important medical incident [Citation22].
Risk of bias and assessment of study quality
The Cochrane Collaboration’s Risk of Bias tool and Ottawa–Newcastle Scale were used to assess bias and study quality in the randomized controlled trial (Supplementary material 2) and observational studies (Supplementary material 3), respectively [Citation23,Citation24].
Statistical analyses
Descriptive statistics were performed on the data extracted from all included studies. Data were described as n (%) or median with interquartile range (IQR). The efficacy of FMT was evaluated as microbiological eradication of MDRO carriage defined by our study. Based on a summary of treatment efficacy in the cases, we calculated the overall treatment effect of FMT as the percentage of patients who received FMT and achieved microbiological eradication. Safety was assessed using reported and serious adverse event data. Analyses were performed using IBM SPSS Statistics version 20.0 (IBM Corporation, Armonk, NY, USA) statistical software package for data analyses.
Results
Study selection and characteristics of included studies
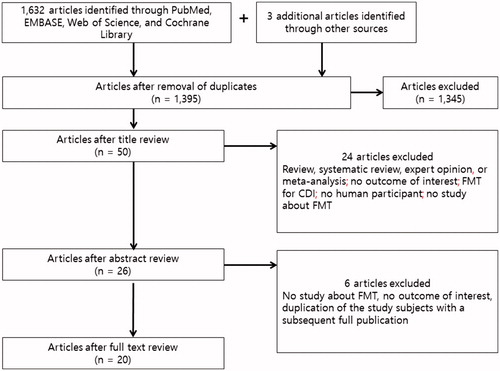
In all, 1633 eligible articles were identified using our search strategy. An additional 3 articles that were missed by the database search were identified from the reference lists of selected articles via the manual search. After duplicate articles were excluded, 1395 articles were subjected to title and abstract review (578 from PubMed, 606 from EMBASE, 168 from Web of Science, 40 from the Cochrane Library, and 3 from the manual search). Further screening of titles and abstracts led to the exclusion of 1,345 records, mainly because the topic did not pertain to FMT. Subsequently, 6 articles were excluded after a full-text review. Finally, a total of 20 articles were selected: 10 single cases [Citation25–34], 3 case series comprising 10 cases [Citation35–37], and 7 prospective studies comprising 101 cases [Citation38–44]. Of these articles, only one was a randomized controlled trial. Overall, the efficacy of FMT for each of 146 MDROs was evaluated in 121 patients, from 20 articles. The studies were published between 2014 and 2019. The literature retrieval, review, and selection process are summarized in .
Figure 1. Flowchart of the study selection showing the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) 2009 study flow diagram: Identification, screening, eligibility, and inclusion of studies. CDI: Clostridioides difficile infection; FMT: fecal microbiota transplantation.
Procedure protocol of FMT
Among the 18 patients in 12 case reports and the 101 patients in 7 prospective studies with available data, FMT was administered via the upper gastrointestinal tract (nasogastric or nasojejunal tube, jejunostomy tube, and gastrostomy tube) to 80 patients in 17 studies, via the lower gastrointestinal tract (retention enema and colonoscopy) in 17 patients in 5 studies, and via both routes of nasoduodenal tube or capsules in 22 patients in one study. Among the 116 patients with available data, bowel lavage, prescription for proton pump inhibitor, or use of antibiotics regardless of CDI treatment as preparation before FMT involved 73 (62.9%), 54 (46.6%), and 28 patients (24.1%), respectively. All donor stools in 101 patients from prospective studies and 6 donor stools (37.5%) in 16 patients from case reports were from healthy anonymous donors. In the remaining 16 patients in the case reports, the donor stools were provided by relatives. In the 86 patients with available data, the frequency of FMT episodes was once (n = 83), twice (n = 2), and three times (n = 1). The stool amount used in each dose ranged from 30 to 150 g per 250 mL.
Outcomes of microbiological eradication
Overall, 121 patients with a total of 146 MDROs were studied. The efficacy of FMT for MDRO eradication was 70.3% (102/146). The types of MDRO (44 gram-positive and 102 gram-negative bacteria) that were subjected to eradication included vancomycin-resistant Enterococci (VRE; n = 38), methicillin-resistant Staphylococcus aureus (MRSA; n = 6), extended-spectrum ß-lactamase (ESBL)-producing Enterobacteriaceae (n = 51), carbapenem-resistant Enterobacteriaceae (CRE) or carbapenemase-producing Enterobacteriaceae (CPE; n = 43), carbapenem-resistant Acinetobacter baumannii (CRAB; n = 2), carbapenem-resistant Pseudomonas aeruginosa (CRPA; n = 5), and Stenotrophomonas maltophilia (n = 1).
The efficacy of FMT for the eradication of MDRO carriage found at the last follow-up period was 68.2% (30/44) for gram-positive bacteria and 70.6% (72/102) for gram-negative bacteria. For each type of MDRO, the efficacy of FMT for the eradication of MDRO carriage found at the last follow-up period was 63.2% (24/38) for VRE, 100% (6/6) for MRSA, 67.4% (29/43) for CRE or CPE, 68.6% (35/51) for ESBL-producing Enterobacteriaceae, 100% (5/5) for CRPA, 100% (2/2) for CRAB, and 100% (1/1) for S. maltophilia.
Outcomes of microbiological eradication in single cases and case series
Data on 20 patients from 13 articles [Citation24–36] are shown in . In 11 studies presenting available data, the median age of included patients was 66 years (IQR, 34–75 years), and 35% of the patients were male. The last follow-up ranged from 100 to 182 days. Of note, 45% (9/20) had a diagnosis of recurrent or relapsing CDI, and received FMT for recurrent CDI.
Table 1. Case and case series regarding the use of FMT for patients with intestinal tract colonization with multidrug-resistant microorganisms.
In 20 MDRO cases, the efficacy of FMT for the eradication of each MDRO carriage found at the last follow-up period was 60.0% (6/10) for gram-positive bacteria and 90.0% (9/10) for gram-negative bacteria. For each type of MDRO, the efficacy of FMT for the eradication of MDRO carriage found at the last follow-up period was 20.0% (1/5) for VRE, 100% (5/5) for MRSA, 100% (6/6) for CRE or CPE, and 75.0% (3/4) for ESBL-producing Enterobacteriaceae.
Data on the delivery method of FMT were available for 18 patients. MDRO colonization could not be eradicated in 3 patients (75%) using retention enema and in one patient (8.3%) using a nasoduodenal tube ().
Data on safety were available for 15 patients. Episodes of mild abdominal discomfort and diarrhea were reported in 2 patients (13.3%). No life-threatening complications were noted ().
Outcomes of microbiological eradication in prospective studies
Data on 101 patients from 7 articles [Citation37–43] are shown in . From the data presented in 5 studies, the median age of included patients was 65 years (IQR, 56 – 76 years), and 52.5% of patients were male. The last follow-up ranged from 7 to 180 days. Of the 7 studies, only one analyzed patient received FMT for recurrent or relapsing CDI (n = 11). The patients in the remaining studies, regardless of CDI therapy, underwent FMT for MDRO eradication. Of the 101 cases in the prospective studies, 10.9% (11/101) experienced CDI.
Table 2. Prospective studies on the use of FMT for patients with intestinal tract colonization with multidrug-resistant microorganisms.
The efficacy of FMT for the eradication of MDRO carriage found at the last follow-up period was 75.7% (28/37) for gram-positive bacteria and 67.4% (60/89) for gram-negative bacteria. For each type of MDRO, the efficacy of FMT for the eradication of MDRO carriage found at the last follow-up period was 71.0% (22/31) for VRE, 100% (6/6) for MRSA, 61.1% (22/36) for CRE or CPE, 68.1% (32/47) for ESBL-producing Enterobacteriaceae, 100% (4/4) for CRPA, 100% (1/1) for CRAB, and 100% (1/1) for S. maltophilia. Of the 7 studies, there was one randomized trial examining 22 patients that showed an efficacy of 41% using FMT via nasogastric tube or capsules.
In the 5 prospective studies investigating adverse events, 3 studies reported minor adverse events, including vomiting, diarrhea, abdominal pain, and ileus in patients who received FMT, and 2 studies reported no adverse events (). No deaths were found to be directly related to FMT, but encephalopathy in a patient with liver cirrhosis was reported as a serious adverse event ().
Outcomes of microbiological eradication by host characteristics and by FMT characteristics
Data on 79 cases from 17 articles [Citation25,Citation27–39,Citation41–43] were available for outcome analysis by host characteristics and by FMT characteristics. The effect of FMT was better in immunocompromised patients than in immunocompetent patients (). Patients who received antibiotics after FMT had more frequent microbiological eradication failure than patients who did not receive antibiotics (). However, outcomes did not seem to have been affected by the delivery route of FMT, number of the FMT trials, selection of donors, or FMT pretreatment such as antibiotic use or bowel lavage ().
Table 3. Outcomes of microbiological eradication by host characteristics and by FMT characteristics.
Discussion
This study shows that FMT is likely safe with a moderate impact on the eradication of intestinal MDRO colonization, although further well-designed randomized controlled trials are required to confirm these results. Furthermore, considering the absence of a standardized protocol detailing FMT, an integrated protocol is warranted to improve the efficacy of FMT treatment.
In our study, the overall efficacy of FMT for the eradication of MDRO carriage was 70.3% (102/146) in a total of 121 patients from 20 articles. In practice, FMT has already become a widely accepted treatment for recurrent CDI with efficacy rates of 90% or even higher [Citation45–47]. CDI and MDRO colonization have similar risk factors, and patients with CDI are frequently co-colonized in tandem with MDRO. Given these facts, the effectiveness of MDRO eradication might be expected to be similar to that of CDI treatment by restoring colonization resistance [Citation48–50]. However, the efficacy of FMT for the eradication of MDRO carriage has remained moderate. Many factors that influence MDRO eradication might explain this difference, and include host characteristics, microorganisms, or environmental factors. Particularly, antibiotic use after FMT seems to be the main cause of decolonization failure in many cases included in our analysis [Citation40,Citation41,Citation43]. Conversely, the alteration of FMT according to the differences of specific MDRO might be considered to help improve the success rate of treatment.
In this context, it is necessary to evaluate the effect of FMT for the eradication of MDRO colonization according to each type of MDRO. Our study included an integrated analysis of the efficacy of FMT for eradication against each type of MDRO, unlike a recently published systematic review [Citation51]. We also focused on the intestinal colonization of MDRO. The efficacy of FMT for the eradication of MDRO carriage was 68.2% (30/44) for gram-positive bacteria and 70.6% (72/102) for gram-negative bacteria. More specifically, the efficacy of FMT for the eradication of MDRO carriage was 63.2% (24/38) for VRE, 67.4% (29/43) for CRE or CPE, and 68.6% (35/51) for ESBL-producing Enterobacteriaceae. Thus, there was no significant difference in eradication rates between VRE and CRE colonization. Most previous studies also showed no difference in the efficacy of FMT for eradication between gram-positive and gram-negative bacteria, and found no evidence for the possibility of any difference. A few studies have also shown similar median duration of spontaneous colonization of 45 to 306 days for VRE and 42 to 387 for CRE, although the results varied widely [Citation52–57]. A recently published randomized controlled trial also showed that 29% (5/17) of patients in the control group without intervention had no MDRO colonization at the last follow-up [Citation44].
Interestingly, patients who received antibiotics after FMT had more frequent microbiological eradication failure than patients who did not receive antibiotics (). Similarly, a previous study suggested that MDRO decolonization was achieved significantly more often in patients who did not receive antibiotics in the first 7 days after FMT than those who received antibiotics [79% (11/14) vs. 36% (4/11), p = .039].
In our study, the adverse events associated with FMT were mild and self-limiting and were gastrointestinal in nature. Although encephalopathy was reported in a patient with liver cirrhosis as a serious adverse event, finding a basis for the association between encephalopathy and FMT in previous studies was difficult. Our findings also identified comparable efficacy and safety of FMT for the eradication of MDRO in immunocompromised patients compared to that in immunocompetent patients, consistent with the findings of a recent study [Citation58]. Even our studies have shown better efficacy in immunosuppression, but these results should be verified in future studies. A recent meta-analysis of 50 publications that reported the adverse events of FMT suggested that adverse and serious adverse events are not rare and should be carefully monitored throughout FMT [Citation22]. Furthermore, no long-term safety data beyond 68 months are available for FMT, and unrecognized transmission of infectious diseases and antibiotic resistance genes or development of chronic diseases, such as obesity, diabetes, atherosclerosis, and autoimmune diseases, due to alteration of gut microbiota is theoretically possible [Citation59–61]. Further studies are warranted to determine the long-term adverse effects of FMT.
Although an integrated and standardized protocol might be critical to the success of FMT, no standard protocol of FMT regarding the type or dosing of stool, donor selection, FMT delivery methods, and recipient preparation was found in the literature review. The review revealed diverse delivery methods of FMT, which included nasogastric or nasojejunal tubes, colonoscopy, enema, and capsules. Because no studies of MDRO decolonization have compared the diverse FMT routes of administration, the most effective delivery method of FMT is not yet clear [Citation62–64]. The use of unrelated donors to save processing time and cost was common in the prospective studies. This might be a favorable trend in real-life, where more thorough donor screening is required. Thus, if the effect of capsules for the eradication of MDRO colonization is comparable to that of other delivery methods of FMT, the use of FMT capsules is likely to be an attractive option, considering their convenience and tolerability [Citation63].
Despite the concept of adjunct procedures, our findings showed that bowel lavage or pretreatment antibiotics did not significantly affect the therapeutic effect of FMT. However, it is still unclear whether bowel lavage or pretreatment antibiotics can decrease the bacterial burden and enable healthy microbial engraftment in patients [Citation19]. A recently published randomized controlled trial showed that oral non-absorbable antibiotics followed by FMT resulted in a higher proportion of intestinal eradication of MDRO colonization during follow-up, compared with control [9/22 (41%) in the intervention arm vs. 5/17 (29%) in the control arm; odds ratio 1.7 (95% confidence interval 0.4–6.4)] [Citation44]. However, intestinal decolonization strategies with antibiotic regimen before or during FMT in patients with non-CDI can be associated with antimicrobial resistance, subsequent perturbation of intestinal commensal microbes, and disturbance of FMT-administered gut flora [Citation44]. A meta-analysis also showed that the ongoing antibiotic therapy after FMT may slow the clearance of MDRO over time and that antibiotic pretreatment may promote the development of antimicrobial resistance to decolonization antibiotics, considering that antimicrobial agents impact the gut microbiota [Citation57,Citation65,Citation66]. Presently, the frequency of FMT trials did not significantly affect the therapeutic effect of FMT. However, a few studies have suggested that repeated FMT might be more effective for the eradication of MDRO colonization [Citation40,Citation42]. More evidence is needed for concrete recommendations of FMT as the established treatment option for MDRO decolonization.
There are several limitations to our study. The first is the limited number of case reports and small sample sizes of studies with only a single randomized controlled trial. Patients, type of MDRO, and FMT procedures were heterogeneous. Particularly, in the case reports and the prospective studies, 45% (9/20) and 10.9% (11/101) of the respective cases experienced CDI. The effect of CDI on the efficacy of FMT for the MDRO eradication could not be totally excluded. Second, the follow-up period to confirm MDRO decolonization and the number of microbiological tests were inconsistent. The various follow-up periods are summarized in . The timing of follow-up evaluations might have affected the treatment outcome of FMT for MDRO eradication. Third, no study compared the efficacy of FMT for MDRO eradication with the control group. Spontaneous clearance of MDRO without treatment is not uncommon [Citation57,Citation65]. In a previous study, spontaneous eradication of VRE and CRE occurred in 48.2% of cases with a median time of 49 days [Citation57]. On some occasions, spontaneous eradication of MDRO, rather than FMT, might have had a greater impact on the MDRO decolonization. However, there was a possibility that MDRO reacquisition at a medical institution was mistaken for failure of MDRO eradication after FMT. Finally, our study could not completely exclude the possibility of the selection bias towards cases with beneficial effects of FMT.
In conclusion, this study indicates a potential benefit of FMT as a treatment option in patients with MDRO colonization with therapeutic limitations. Furthermore, FMT appears to be safe and is expected to be used for the eradication of MDRO colonization as a non-pharmacological approach to minimize collateral damage. However, further studies of larger, well-designed randomized controlled trials are needed to develop a standardized protocol of FMT for more efficient implementation.
Author contributions
Yoon YK designed the study, analyzed the data, and was the major contributor in drafting the manuscript. Suh JW and Kim JY contributed to the selection of eligible articles. Kang E-J performed the systematic review using the database. All authors read and approved the final manuscript as submitted.
Supplemental Material
Download MS Word (15.4 KB)Disclosure statement
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- World Health Organization. Worldwide country situation analysis: response to antimicrobial resistance. Geneva: WHO, 2015.
- O'Neill J. Review on antimicrobial resistance tackling drug-resistant infections globally: final report and recommendations. London: Wellcome Trust and UK Government; 2016.
- Aliyu S, Smaldone A, Larson E. Prevalence of multidrug-resistant gram-negative bacteria among nursing home residents: a systematic review and meta-analysis. Am J Infect Control. 2017;45(5):512–518.
- Pop-Vicas AE, D'Agata EM. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin Infect Dis. 2005;40(12):1792–1798.
- Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. IDR. 2018;11:1645–1658.
- Casals-Pascual C, Vergara A, Vila J. Intestinal microbiota and antibiotic resistance: perspectives and solutions. Hum Microbiome J. 2018;9:11–15.
- van Saene HK, Stoutenbeek CP, Gilbertson AA. Review of available trials of selective decontamination of the digestive tract (SDD). Infection. 1990;18(S1):S5–S9.
- Saidel-Odes L, Polachek H, Peled N, et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012;33(1):14–19.
- Cheng VC, Chen JH, Tai JW, et al. Decolonization of gastrointestinal carriage of vancomycin-resistant Enterococcus faecium: case series and review of literature. BMC Infect Dis. 2014;14(1):25248287.
- Zuckerman T, Benyamini N, Sprecher H, et al. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 2011;46(9):1226–1230.
- Katchman E, Marquez M, Bazerbachi F, et al. A comparative study of the use of selective digestive decontamination prophylaxis in living-donor liver transplant recipients. Transpl Infect Dis. 2014;16(4):539–547.
- Halaby T, Al Naiemi N, Kluytmans J, et al. Emergence of colistin resistance in Enterobacteriaceae after the introduction of selective digestive tract decontamination in an intensive care unit. Antimicrob Agents Chemother. 2013;57(7):3224–3229.
- Kronman MP, Zerr DM, Qin X, et al. Intestinal decontamination of multidrug-resistant Klebsiella pneumoniae after recurrent infections in an immunocompromised host. Diagn Microbiol Infect Dis. 2014;80(1):87–89.
- De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189–196.
- Opal SM. Non-antibiotic treatments for bacterial diseases in an era of progressive antibiotic resistance. Crit Care. 2016;20(1):397.
- Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis. 2016;48(8):587–592.
- Kamada N, Chen GY, Inohara N, et al. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14(7):685–690.
- Seekatz AM, Aas J, Gessert CE, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014;5(3):e00893–00814.
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415.
- Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315(2):142–149.
- Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535–19622551.
- Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS ONE. 2016;11(8):e0161174–27529553.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. [cited 2011 March]. Available from: http://handbook-5-1.cochrane.org/
- Wg A, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019. [cited 2019 Jan 27]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Singh R, van Nood E, Nieuwdorp M, et al. Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin Microbiol Infect. 2014;20(11):O977–O978.
- Crum-Cianflone NF, Sullivan E, Ballon-Landa G. Fecal microbiota transplantation and successful resolution of multidrug-resistant-organism colonization. J Clin Microbiol. 2015;53(6):1986–1989.
- Stripling J, Kumar R, Baddley JW, et al. Loss of vancomycin-resistant enterococcus fecal dominance in an organ transplant patient with Clostridium difficile colitis after fecal microbiota transplant. Open Forum Infect Dis. 2015;2:ofv078
- Lagier JC, Million M, Fournier PE, et al. Faecal microbiota transplantation for stool decolonization of OXA-48 carbapenemase-producing Klebsiella pneumoniae. J Hosp Infect. 2015;90(2):173–174.
- Jang MO, An JH, Jung SI, et al. Refractory Clostridium difficile infection cured with fecal microbiota transplantation in vancomycin-resistant enterococcus colonized patient. Intest Res. 2015;13(1):80–84.
- Biliński J, Grzesiowski P, Muszyński J, et al. Fecal microbiota transplantation inhibits multidrug-resistant gut pathogens: preliminary report performed in an immunocompromised host. Arch Immunol Ther Exp. 2016;64(3):255–258.
- García-Fernández S, Morosini MI, Cobo M, et al. Gut eradication of VIM-1 producing ST9 Klebsiella oxytoca after fecal microbiota transplantation for diarrhea caused by a Clostridium difficile hypervirulent R027 strain. Diagn Microbiol Infect Dis. 2016;86(4):470–471.
- Stalenhoef JE, Terveer EM, Knetsch CW, et al. Fecal microbiota transfer for multidrug-resistant gram-negatives: a clinical success combined with microbiological failure. Open Forum Infect Dis. 2017;4:ofx047.
- Ponte A, Pinho R, Mota M. Fecal microbiota transplantation: is there a role in the eradication of carbapenem-resistant Klebsiella pneumoniae intestinal carriage? Rev Esp Enferm Dig. 2017;109:28196423.
- Lahtinen P, Mattila E, Anttila VJ, et al. Faecal microbiota transplantation in patients with Clostridium difficile and significant comorbidities as well as in patients with new indications: a case series. WJG. 2017;23(39):7174–7184.
- Wei Y, Gong J, Zhu W, et al. Fecal microbiota transplantation restores dysbiosis in patients with methicillin resistant Staphylococcus aureus enterocolitis. BMC Infect Dis. 2015;15(1):265.
- Sohn KM, Cheon S, Kim YS. Can fecal microbiota transplantation (FMT) eradicate fecal colonization with vancomycin-resistant enterococci (VRE)? Infect Control Hosp Epidemiol. 2016;37(12):1519–1521.
- Dias C, Pipa S, Duarte-Ribeiro F, et al. Fecal microbiota transplantation as a potential way to eradicate multiresistant microorganisms. IDCases. 2018;13:e00432.
- Dubberke ER, Mullane KM, Gerding DN, et al. Clearance of vancomycin-resistant enterococcus concomitant with administration of a microbiota-based drug targeted at recurrent Clostridium difficile infection. Open Forum Infect Dis. 2016;3(3):ofw133.
- Davido B, Batista R, Michelon H, et al. Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage? J Hosp Infect. 2017;95(4):433–437.
- Bilinski J, Grzesiowski P, Sorensen N, et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single-center study. Clin Infect Dis. 2017;65(3):364–370.
- Dinh A, Fessi H, Duran C, et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect. 2018;99(4):481–486.
- Singh R, de Groot PF, Geerlings SE, et al. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: a proof of principle study. BMC Res Notes. 2018;11(1):29566738.
- Davido B, Batista R, Fessi H, et al. Fecal microbiota transplantation to eradicate vancomycin-resistant enterococci colonization in case of an outbreak. Med Mal Infect. 2019;49(3):214–218.
- Huttner BD, de Lastours V, Wassenberg M, R-Gnosis WP3 study group, et al. A five-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: a randomized clinical trial. Clin Microbiol Infect. 2019;25(7):830–838.
- Li YT, Cai HF, Wang ZH, et al. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016;43(4):445–457.
- Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479–493.
- Rossen NG, MacDonald JK, de Vries EM, et al. Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. WJG. 2015;21(17):5359–5371.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136(11):834–844.
- Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279(1):90–105.
- Keith JW, Pamer EG. Enlisting commensal microbes to resist antibiotic-resistant pathogens. J Exp Med. 2019;216(1):10–19.
- Saha S, Tariq R, Tosh PK, et al. Faecal microbiota transplantation for eradicating carriage of multidrug-resistant organisms: a systematic review. Clin Microbiol Infect. 2019;25(8):958–963.
- Sohn KM, Peck KR, Joo EJ, et al. Duration of colonization and risk factors for prolonged carriage of vancomycin-resistant enterococci after discharge from the hospital. Int J Infect Dis. 2013;17(4):e240–e246.
- Shenoy ES, Paras ML, Noubary F, et al. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC Infect Dis. 2014;14(1):177.
- Patel R, Allen SL, Manahan JM, et al. Natural history of vancomycin-resistant enterococcal colonization in liver and kidney transplant recipients. Liver Transpl. 2001;7(1):27–31.
- Haverkate MR, Derde LP, Brun-Buisson C, et al. Duration of colonization with antimicrobial-resistant bacteria after ICU discharge. Intensive Care Med. 2014;40(4):564–571.
- Zimmerman FS, Assous MV, Bdolah-Abram T, et al. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am J Infect Control. 2013;41(3):190–194.
- Davido B, Moussiegt A, Dinh A, et al. Germs of thrones - spontaneous decolonization of Carbapenem-Resistant Enterobacteriaceae (CRE) and Vancomycin-Resistant Enterococci (VRE) in Western Europe: is this myth or reality? Antimicrob Resist Infect Control. 2018;7(1):100.
- Shogbesan O, Poudel DR, Victor S, et al. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol. 2018;2018:1.
- Millan B, Park H, Hotte N, et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin Infect Dis. 2016;62(12):1479–1486.
- Leung V, Vincent C, Edens TJ, et al. Antimicrobial resistance gene acquisition and depletion following fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Infect Dis. 2018;66(3):456–457.
- Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. 2016;9(2):229–239.
- Narula N, Kassam Z, Yuan Y, et al. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm Bowel Dis. 2017;23(10):1702–1709.
- Ramai D, Zakhia K, Ofosu A, et al. Fecal microbiota transplantation: donor relation, fresh or frozen, delivery methods, cost-effectiveness. Ann Gastroenterol. 2019;32(1):30–38. [
- Krajicek E, Fischer M, Allegretti JR, et al. Nuts and bolts of fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2019;17(2):345–352.
- Bar-Yoseph H, Hussein K, Braun E, et al. Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(10):2729–2739.
- Rashid MU, Zaura E, Buijs MJ, et al. Determining the long-term effect of antibiotic administration on the human normal intestinal microbiota using culture and pyrosequencing methods. Clin Infect Dis. 2015; 60(suppl_2):S77–S84.
