Abstract
Introduction
Clinical and pharmacological characteristics of elderly patients hospitalized for bleeding and in-hospital mortality according to bleeding type are barely described.
Methods
Retrospective cohort study of 13,496 consecutive patients admitted to internal medicine wards. Clinical characteristics, comorbidities and pharmacological treatments were collected for each patient. Predictors of in-hospital mortality were investigated.
Results
Overall, 531 (3.9%) patients were admitted for bleeding: 189 clinically relevant non-major bleeding, 106 cerebral and 236 major non-cerebral (95.8% gastrointestinal (GI)). Among 106 cerebral bleedings, 28.3% and 24.5% were typical and atypical intracranial, respectively, and 47.2% were subdural haemorrhages. Most of patients with GI bleeding presented with anaemia (90.7%). A similar rate of GI bleeding was found in aspirin-treated patients taking or not proton pump inhibitors (PPI). In-hospital mortality was 9.98%. Age ≥80 years (odds ratio (OR) 2.513, p=.005), cerebral bleeding (OR 5.373, p<.001), eGFR <30 ml/min/m2 (OR 2.388, p=.035) and COPD (OR 2.362, p=.024) were positively associated with mortality, while ACE inhibitors/ARBs use was negatively associated (OR 0.383, p=.028).
Conclusions
The most frequent type of major haemorrhage was GI bleeding, which was not modified by the use of PPI in patients taking aspirin. Cerebral bleeding increased all-cause death, which was lower in ACE inhibitors/ARBs users.
Gastrointestinal (GI) bleeding was the most common reason for hospital admission.
The rate of GI bleeding was similar in patients on aspirin using or not PPI.
Cerebral bleeding increased in-hospital mortality, which was lower in patients taking ACE inhibitors/ARBs.
KEY MESSAGE
1. Introduction
Elderly patients represent a subgroup of fragile patients at particularly elevated risk for hospitalization. These patients usually have multiple comorbidities and are prescribed on several medications. Among drugs, antiplatelet and oral anticoagulant (OAC) drugs for secondary prevention of ischaemic heart disease, treatment of venous thromboembolism or for the thromboprophylaxis of atrial fibrillation (AF)-related ischaemic stroke are frequently present. As a consequence, the risk of bleeding may increase, mostly from gastrointestinal (GI) tract or at cerebral site [Citation1–3], and hospitalization for bleeding and management of its sequelae are associated with significantly high health-care related costs [Citation4,Citation5]. In particular, the rate of cerebral bleeding in the general population is 24.6 per 100,000 person-years, while the incidence of bleeding from upper and lower GI bleeding ranges from 78.4 to 60.6 per 100,000 person-years and from 41.8 to 35.7 per 100,000 person-years, respectively [Citation6,Citation7].
In particular, in patients treated with OAC the rate of bleeding is higher compared to the general population, with an annual incidence rate ranging from 1.3% to 7.2% according to different reports [Citation8]. In particular, intracranial haemorrhage (ICH) and subdural haemorrhage represent serious and life-threatening complications [Citation9]. For example, the risk of ICH related to warfarin therapy is increased by 7- to 10-fold with respect to spontaneous ICH, occurring in about 1% of patients on OAC [Citation10]. In addition, bleeding is also a frequent reason for stopping antithrombotic therapy such as oral anticoagulation, as shown by the ORBIT study [Citation11], in which patients with AF were more likely to withdrawn anticoagulation after an hospitalization due to bleeding.
However, characteristics of patients requiring hospital admission for a major bleeding, especially cerebral haemorrhage, have been barely investigated. The aim of this study is to investigate clinical, pharmacological and biochemical characteristics of consecutive patients admitted for a bleeding event and to investigate the risk of in-hospital mortality in this clinical setting.
2. Methods
2.1. Cohort identification and data collection
We performed a retrospective cohort single-centre study including all consecutive patients admitted to the Internal Medicine Units of the Niguarda Hospital in Milan for bleeding-related complications from 1 January 2015 to 31 December 2018.
The study cohort was identified from administrative medical billing codes of the International Classification of Diseases (ICD-9) from patient’s hospital discharge data. Codes considered for the identification of bleeding have been listed in Supplementary Table 1. Three independent investigators have analysed and collected data through electronic medical records. Information regarding medical history and medication exposure of selected patient were recorded. Medication exposure was defined as the therapy assumed by the patient at the moment of admission. We also recorded data about intra-hospital mortality and laboratory values as follows: haemoglobin (Hb, g/dl), platelet count, glomerular filtration rate by the MDRD formula, and alanine transaminase (U/l). Anaemia was defined as an Hb <13 g/dl in men and <12 g/dl in women. Inflammatory bowel disease and diverticulosis are grouped under “gastrointestinal disease”.
Previous cardiovascular disease included history of myocardial infarction or coronary revascularization (stent or coronary artery bypass graft surgery). Polytherapy was defined by concomitant chronic use of ≥5 drugs [Citation12]. Cognitive impairment was defined by a positive personal medical history of cognitive decline at admission.
We evaluated complexity of patients by calculating the Charlson comorbidity index (CCI) [Citation13]: history of definite myocardial infarction (one point); congestive heart failure (one point); peripheral vascular disease (one point); cerebrovascular accident or transient ischaemic attack (one point); chronic cognitive deficit (one point); chronic obstructive pulmonary disease (one point); connective tissue disease (one point); peptic ulcer disease (one point); chronic liver disease (1–2 points if severe); diabetes (1–2 points if complicated); hemiplegia (one point); moderate to severe chronic kidney disease (severe = dialysis, transplant, uraemia; moderate = creatinine >3 mg/dL; two points); localized solid tumour/leukaemia/lymphoma (two points each); metastatic solid tumour (six points); AIDS (six points); age (one point for every decade age 50 years and over, maximum four points). We also considered the version of CCI implemented by Gagne et al. [Citation14] and the Drug Derived Complexity Index (DDCI) [Citation15], a score predictive of mortality and hospitalization in the overall population based on prescription patterns indicative of chronic diseases.
2.2. Bleeding definition
We classified bleeding events as follows: clinically relevant non-major bleedings (CRNMB), major non-cerebral bleedings and cerebral bleedings. Cerebral bleedings include intraparenchymal, subarachnoid and subdural haemorrhages. Events were classified in major and CRNMB according to ISTH criteria [Citation16,Citation17]. An event is defined as major if one of the following conditions has occurred: (1) death, (2) fall in Hb level of 2 g/dL or the transfusion of two or more units of whole blood or packed cells and (3) symptomatic bleeding in a critical area or organ (i.e. intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial). We considered all other events as CRNMB. Non-cerebral bleeding was further divided according to the bleeding site; GI bleedings were grouped into upper and lower if they were deemed to evolve below the Ligamentum of Treitz or not.
2.3. Statistical analysis
Categorical variables were reported as counts (percentage). Continuous variables were expressed as mean ± standard deviation (SD), and two-sided t-test was used to compare means. Pearson’s chi-square test was used to compare proportions. Comparisons among groups were performed with ANOVA with post hoc LSD analysis. We performed a descriptive analysis of clinical and pharmacological characteristics of patients according to bleeding type (CRNMB, major non-cerebral and cerebral).
We performed a multivariable logistic regression analysis, to calculate the adjusted odds ratios (ORs) of factors associated with in-hospital mortality. In the multivariable model, only variables with a p value <.100 at univariate analysis were included, and the final model was built using a stepwise forward procedure. Linear variables were categorized when possible for the logistic regression analysis. The Hosmer–Lemeshow (HL) test was used for goodness of fit for logistic regression models.
All tests were two-tailed and analyses were performed using computer software packages (SPSS-25.0, SPSS Inc., Chicago, IL). Only p values <.05 were considered as statistically significant.
Given the retrospective design of the study, written informed consent from patients was waived and a notification to Hospital Ethical Committee was done (AIFA guidelines G.U. 76 published on 31 March 2008).
3. Results
Among 13,496 consecutive admissions to internal medicine, 606 patients had a diagnosis of bleeding. Of these, 75 were excluded as bleeding was not the reason for hospitalization, but the event occurred during the hospital staying. This resulted in a final cohort of 531 patients. Thus, bleeding accounted for 3.9% (2.5% considering only major bleedings) of all consecutive hospital admissions.
Mean age was 77.0 ± 13.1 years (46.9% aged ≥80 years) and 39.7% of patients were women. Bleedings were cerebral in 106, major non-cerebral in 236 and CRNMB in 189 patients. Among major non-cerebral bleeding, there were 226 (95.8%) GI, of which 111 from upper and 115 from lower GI tract. Characteristics of patients according to bleeding type are shown in .
Table 1. Characteristics of patients according to bleeding type.
3.1. Cerebral bleeding
Patients with cerebral bleeding were more likely to be older and more frequently women than patients with major non-cerebral bleeding. In particular, 56.6% of the patients were aged ≥80 years.
Among 106 cerebral bleedings, 30 (28.3%) were typical ICH, 26 (24.5%) were atypical ICH and 50 (47.2%) were subdural haemorrhages.
In this group, 14.3% of patients were on OAC, 11.4% on warfarin and 2.9% on NOAC. Furthermore, a significantly lower proportion of NOAC use was found in patients with cerebral bleeding compared to other groups (). Three patients on warfarin received plasma infusion as reversal strategy at admission.
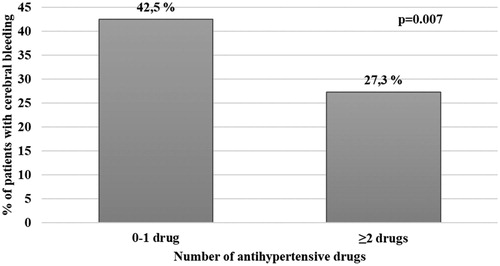
In this group, 58.5% of patients had a history of cardiovascular disease, but only 13.3% were receiving a treatment with statins. When we calculated the proportion of cerebral bleeding according to the number of anti-hypertensive drugs (including ACEi, ARBs, calcium channel antagonists, beta blockers and diuretics), we found a significantly lower rate of cerebral bleeding in patients taking ≥2 drugs as compared to those taking 0–1 drug (p=.007, ).
3.2. Gastrointestinal bleeding
reports the characteristics of patients with GI bleeding, and according to upper and lower GI tract. The majority of patients admitted for GI bleeding presented with anaemia (90.7%), that was more frequent in patients with bleeding from upper than lower GI tract (p=.002).
Table 2. Characteristics of patients with major lower or upper gastrointestinal (GI) bleeding.
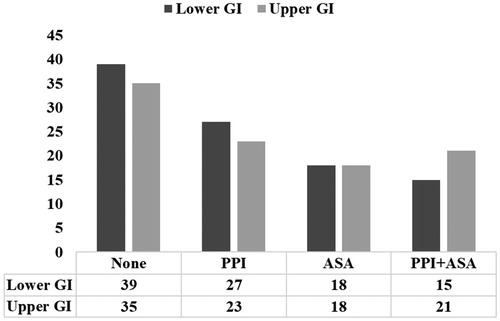
The use of proton pump inhibitors (PPI) was detected in 45.6% of these patients, with no difference between upper and lower GI bleeding groups ().
After excluding patients taking non-aspirin antiplatelet agents (n = 30), we did not find any difference in the proportion of upper and lower GI bleeding in patients taking PPI and/or aspirin (p=.679, ).
3.3. Anticoagulation and atrial fibrillation
In the whole cohort, 112 patients were treated with OACs: 84 were on warfarin; eight on Dabigatran, 11 on Apixaban, five on Rivaroxaban and four on Edoxaban. The most common indication for OAc was represented by AF in 78 (69.9%) cases. Among 141 patients with AF, 26 (18.4%) were not treated with OACs, 28 (19.9%) were receiving antiplatelet agents and 87 (61.7%) were on oral anticoagulation (62 on warfarin and 25 on NOACs).
3.4. In-hospital mortality
During the hospital stay, 53 (9.98%) patients died. Of these, 33 deaths were bleeding-related, six were due to cancer, four acute kidney diseases, three acute respiratory failures, one endocarditis, three ischaemic strokes and one acute heart failure; two unknown causes.
Mortality rates among CRMB, major non-cerebral and cerebral bleeding were respectively 4.8%, 8.9% and 21.7%. Considering only patients with major bleeding, the proportion of death was higher in patients with cerebral as compared to those with GI bleeding (p=.004).
The proportion of death was 16.7%, 30.8% and 20.0% in patients with typical and atypical ICH and subdural haemorrhage, respectively. Characteristics of patients who died are reported in . Patients who died were more frequently elderly, with a higher proportion of severe renal disease, cognitive impairment and COPD. Conversely, a lower prevalence of GI disease and a lower use of ACE inhibitors/ARBs was found.
Table 3. Characteristics of patients who died during hospital staying.
Multivariable logistic regression analysis showed that age ≥80 years (OR 2.513, 95% confidence interval (CI) 1.314–4.808, p=.005), cerebral (vs. non cerebral) bleeding (OR 5.373, 95%CI 2.264–12.748, p<.001), eGFR <30 ml/min/m2 (OR 2.388 95%CI 1.062–5.370, p=.035) and COPD (OR 2.362, 95%CI 1.121–4.979, p=.024) were positively associated with mortality, while the use of ACE inhibitors/ARBs was negatively associated (OR 0.383, 95%CI 0.163–0.901, p=.028) (). The HL confirmed the goodness of fit for the model (p=.956).
Table 4. Univariable and multivariable logistic regression analyses of factors associated with in-hospital mortality.
4. Discussion
In this real-world retrospective cohort study, we have observed that hospitalization for bleeding events in the internal medicine ward accounted for the 4% of all hospitalizations. Overall, 20% were cerebral haemorrhages, and 46.7% of these patients were on therapy with antiplatelet drugs.
In literature, conflicting data exist regarding the rate of major bleeding of very elderly patients [Citation18–20]. Despite similar use of anticoagulants (11.4% on warfarin and 2.9% on NOAC in our study vs. 10.6% and 3.5% respectively in the study by Inohara et al. [Citation10]), patients experiencing cerebral bleeding in our study were characterized by a more advanced age (80.4 vs. 68.3 years) and a higher prevalence of antiplatelet agents (46.7% vs. ≈30%) [Citation10]. In the recent REstart or STop Antithrombotics Randomized Trial (RESTART) trial [Citation21], evaluating antiplatelet therapy effect on recurrent intracerebral haemorrhage, the prevalence of patients on therapy with aspirin at the onset of the first cerebral bleeding was similar to that found in our study (approximately 50%).
Regarding the type of cerebral bleeding, half of patients admitted to our Internal Medicine wards presented with subdural haematoma, confirming a high prevalence of this type of bleeding in very elderly patients, which is often secondary to trauma or falls in these patients [Citation22].
Another interesting finding of our study relates the use of PPI, which was common in our population (43.9%). We found a similar proportion of upper and lower GI bleedings in patients treated with both PPI and low dose aspirin versus those taking aspirin alone. These data represent a novelty in literature, in fact studies describing increased risk of gastroduodenal complication of patients treated with low dose aspirin plus placebo or PPIs (HR 10.6 95%CI 1.3–86.1), only focuses on secondary prevention [Citation23,Citation24], while our cohort study describes factors associated with GI bleedings occurring in general population, not only in those who previously had an event. A recent work including 6985 new aspirin users, 5545 chronic users and 48,908 non-users, showed that the risk of GI bleeding between new and chronic users of aspirin was evident in the first 6 months but not afterwards, with PPI inversely associated with GI bleeding (HR 0.46, 95%CI 0.36–0.58) [Citation25]. These findings suggest that the use of PPI may be beneficial in the first period after starting treatment with aspirin. On the other hand, the authors did not find a reduction of the incidence of GI bleeding over time, despite a very high proportion of patients treated with gastroprotective agents (>90%) [Citation25], and reported an in-hospital mortality of 9.6% both in new and chronic users, which is very similar to our study (9%). Thus, the usefulness of PPI for the long-term prevention of GI bleeding deserves further investigation.
Other important findings of our study relate to therapeutic appropriateness, in fact, the 37.9% of patients with AF were not in therapy with an anticoagulant and almost half of them were assuming an antiplatelet agent at the time of bleeding. Furthermore, despite a high prevalence of CVD, very few patients were treated with statins, which is even less than previously reported data on statin underuse [Citation26,Citation27]. All those evidence regarding inappropriateness of prescriptions in elderly patients raises the question of how much this can lead to serious adverse event and fatal outcomes in an already fragile population.
4.1. Mortality
In our study, we found an overall mortality rate of 9.98%, rising to 21.7% in patients with cerebral bleeding. Previous studies on this topic reported a risk of death in patients with cerebral bleeding ranging from 35 to 52%, but most of these studies considered 30-day or long-term mortality [Citation28–30]. In a retrospective study evaluating intra-hospital mortality after cerebral bleedings, the authors found an in-hospital mortality of 22.5% in patients not on OAC, 26.5% and 32.6% in patients taking warfarin or NOAC, respectively [Citation10].
Predictors of mortality in our study were age ≥80 years, cerebral bleeding and history of COPD. Exposition to ACE inhibitors/ARBs was found to be protective against mortality; this finding is in keeping with previous evidence from a meta-analysis of 20 cardiovascular morbidity–mortality trials including 158,998 patients with hypertension which showed an association between renin–angiotensin system inhibition and all-cause mortality reduction (HR: 0.95, 95%CI: 0.91–1.00, p=.032) [Citation31]. Also in patient with heart failure, the use of ACE inhibitors is associated with significative reduction in total mortality (OR: 0.77; 95%CI: 0.67–0.88, p<.001) [Citation32]. A possible explanation of the protective role of ACEi/ARBs could lie in their better control of hypertension which, is associated with a better outcome after intracerebral haemorrhage (untreated-hypertension showed to predict increased intra-hospital mortality when compared with normotensive individuals, HR: 3.41, p<.01) [Citation33]. This is indirectly suggested by the lower rate of cerebral bleeding in patients taking two or more anti-hypertensive drugs, as compared to those taking one or no drug.
4.2. Implications and limitations
Our study has clinical implications for the management of elderly patients admitted with bleeding. The most frequent bleeding was the GI, which had as most frequent clinical presentation the presence of anaemia (>90% patients). Thus, GI bleeding should always be suspected in case of unexplained new-onset anaemia. Moreover, the similar use of PPI in aspirin users with or without GI bleeding, suggest that the use of PPI for the prevention of GI bleeding should be carefully evaluated and PPI should be prescribed only in high-risk patients who could benefit from this therapy. Second, cerebral bleeding was associated with an increased in-hospital mortality, along with advanced age >80 years. The inverse association of ACEi/ARBs with cerebral bleeding and mortality indicates that these drugs may be beneficial for the reduction of mortality also in this group of patients. Finally, the underuse of statins in our study suggests that in the real world secondary prevention strategies need to be implemented.
This study has limitations, first of all the retrospective nature design which has precluded us from collecting information regarding mortality after hospital discharge. Second, we analysed only patients admitted to Internal Medicine Units, thus the prevalence of different types of bleeding and mortality rate may be different considering patients admitted to other specialty units, such as neurology, neurosurgery, gastroenterology and intensive care units. Finally, despite there was no apparent excessive use of warfarin in the group of cerebral bleeding compared to other bleeding groups, it would be useful to have INR value at the moment of bleeding onset to better understand this finding.
In conclusion, this study shows that patients hospitalized for bleeding events have a fivefold increased risk of mortality if the site of bleeding is cerebral and brings to light an inverse association between ACE inhibitors/ARBs use and intra-hospital mortality.
Supplemental Material
Download MS Word (34 KB)Acknowledgements
The authors very much appreciate the support received by Michaela Bertuzzi (Department of Quality and Risk Management – ASST GOM Niguarda) for the identification of patients from the administrative database and Loredana Lupica (Clinical Research Unit – ASST GOM Niguarda) for her continuous efforts in supporting our research.
Disclosure statement
All authors declare no conflicts of interest related to the manuscript.
References
- Quilliam BJ, Lapane KL, Eaton CB, et al. Effect of antiplatelet and anticoagulant agents on risk of hospitalization for bleeding among a population of elderly nursing home stroke survivors. Stroke. 2001;32(10):2299–2304.
- Hreinsson JP, Kalaitzakis E, Gudmundsson S, et al. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol. 2013;48(4):439–447.
- Ahsberg K, Hoglund P, Kim WH, et al. Impact of aspirin, NSAIDs, warfarin, corticosteroids and SSRIs on the site and outcome of non-variceal upper and lower gastrointestinal bleeding. Scand J Gastroenterol. 2010;45(12):1404–1415.
- Ghate SR, Biskupiak J, Ye X, et al. All-cause and bleeding-related health care costs in warfarin-treated patients with atrial fibrillation. J Manag Care Pharm. 2011;17(9):672–684.
- Deitelzweig S, Luo X, Gupta K, et al. All-cause, stroke/systemic embolism-, and major bleeding-related health-care costs among elderly patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Clin Appl Thromb Hemost. 2018;24(4):602–611.
- Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14(4):300–306.
- Laine L, Yang H, Chang SC, et al. Trends for incidence of hospitalization and death due to GI complications in the United States from. Am J Gastroenterol. 2001;107(2009):1190–1195.
- Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients. Executive Summary of a Position Document from the European Heart Rhythm Association [EHRA], endorsed by the European Society of Cardiology [ESC] Working Group on Thrombosis. Thromb Haemost. 2011;106(6):997–1011.
- Hawkes MA, Rabinstein AA. Anticoagulation for atrial fibrillation after intracranial hemorrhage: a systematic review. Neurol Clin Pract. 2018;8(1):48–57.
- Inohara T, Xian Y, Liang L, et al. Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA. 2018;319(5):463–473.
- Steinberg BA, Simon DN, Thomas L, et al. Management of major bleeding in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants compared with warfarin in clinical practice (from phase II of the outcomes registry for better informed treatment of atrial fibrillation [ORBIT-AF II]). Am J Cardiol. 2017;119(10):1590–1595.
- Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- Robusto F, Lepore V, D'Ettorre A, et al. The Drug Derived Complexity Index (DDCI) predicts mortality, unplanned hospitalization and hospital readmissions at the population level. PLoS One. 2016;11(2):e0149203.
- Kaatz S, Ahmad D, Spyropoulos AC, et al. Subcommittee on Control of A. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119–2126.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747.
- Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689–2696.
- Ono A, Kawamura I, Fujita T. Letter regarding article by Hylek et al., "Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation". Circulation. 2007;116(20):e538.
- Poli D, Antonucci E, Grifoni E, et al. Bleeding risk during oral anticoagulation in atrial fibrillation patients older than 80 years. J Am Coll Cardiol. 2009;54(11):999–1002.
- Collaboration R. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open-label trial. Lancet. 2019;393(10191):2613–2623.
- Benko MJ, Abdulla SG, Cuoco JA, et al. Short- and long-term geriatric mortality after acute traumatic subdural hemorrhage. World Neurosurg. 2019;130:e350–e355.
- Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346(26):2033–2038.
- Maiden LP, Harris AW. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin. N Engl J Med. 2002;347(20):1623–1624.
- Guo CG, Cheung KS, Zhang F, et al. Incidences, temporal trends and risks of hospitalisation for gastrointestinal bleeding in new or chronic low-dose aspirin users after treatment for Helicobacter pylori: a territory-wide cohort study. Gut. 2020;69(3):445–452.
- Tonstad S, Rosvold EO, Furu K, et al. Undertreatment and overtreatment with statins: the Oslo Health Study 2000–2001. J Intern Med. 2004;255(4):494–502.
- Ruscica M, Macchi C, Pavanello C, et al. Appropriateness of statin prescription in the elderly. Eur J Intern Med. 2018;50:33–40.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151(3):713–719.
- Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66(8):1182–1186.
- Fogelholm R, Murros K, Rissanen A, et al. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry. 2005;76(11):1534–1538.
- van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33(16):2088–2097.
- Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273(18):1450–1456.
- Hevesi M, Bershad EM, Jafari M, et al. Untreated hypertension as predictor of in-hospital mortality in intracerebral hemorrhage: a multi-center study. J Crit Care. 2018;43:235–239.