Abstract
Objective
Total knee replacement (TKA) is an effective way to treat teratogenic and disabling knee diseases such as advanced osteoarthritis. Tourniquets are often used in TKA to reduce bleeding and to get a better visualization of the surgical field, while it is related to safety concerns. We did this network meta-analysis to comprehensively compare the efficacy and safety of various tourniquet application strategies.
Method
PubMed, Embase, Cochrane Library, CNKI, and WanFang Database were systematically searched from January 1990 to May 2020. A network meta-analysis with a frequentist framework was done to assess the relative efficacy and safety by comparing seven clinical important endpoints.
Results
38 eligible studies that assessed 3007 participants who underwent TKA were included in this network meta-analysis. Tourniquet inflation before osteotomy then deflation after wound closure effectively reduce perioperative bleeding (WMD compared with control group −234.66, 95% CI [–409.19 to −60.13]), while shortening the operation time (WMD −8.98, 95%CI [–14.07 to −3.88]) and reducing postoperative complications, including DVT (OR −0.58, 95%CI [–1.19 to 0.03]) and minor wound complications (OR −1.38, 95%CI [–3.00 to 0.25]). No difference was found in the late postoperative knee pain and function outcomes.
Conclusions
Using tourniquets during the entire operation can effectively reduce blood loss, but it also can cause many safety problems, including DVTs, wound oozing, delayed healing, and serious wound complications. Tourniquet inflation before osteotomy then deflation after wound closure effectively can reduce perioperative bleeding while shortening the operation time and reducing postoperative complications, so it could be the ideal tourniquet application strategy in TKA.
This is the first study that comprehensively compared different tourniquet application strategies to evaluate their impact on postoperative recovery following TKA, and five clinically important endpoints were assessed in this study: perioperative blood loss, operation time, postoperative pain and function, and complications.
We conclude that tourniquet inflation before osteotomy then deflation after wound closure could be the ideal tourniquet application strategy in TKA.
Key messages
1. Introduction
Knee osteoarthritis (KOA) and rheumatoid arthritis (RA) are common knee diseases that cause joint pain and loss of function. Pharmacological treatments may be used early to improve symptoms, but for end-stage patients, total knee replacement (TKA) is often the only effective intervention [Citation1,Citation2].
Some studies have found significantly more intraoperative bleeding [Citation3], which can further lead to the deterioration of surgical visualization, the extension of surgical time, and can interfere with the bonding effect of bone cement. To solve these difficulties, tourniquets are widely used during TKA. However, it was associated with a higher risk of complications, including Thigh pain and swelling, paresthaesia, vascular injury, and venous thromboembolism, subcutaneous fat necrosis, postoperative stiffness, poor wound healing, and delayed recovery of quadriceps strength [Citation4–7].
Previous studies have confirmed that the prolonged application of a tourniquet is a key factor in complications that may be associated with prolonged ischaemic time for tissues [Citation7–9]. More and more researchers have begun to minimize tourniquet time to determine whether the limited application of tourniquets in TKA can reduce complications and promote postoperative recovery.
Kvederas G et al. [Citation10] compared the efficacy and safety of three tourniquet application strategies (Tourniquet inflation before incision then deflation after wound closure; inflation before incision then deflation after cement hardening; and inflation just during cementing). Their study demonstrated that tourniquet inflation before incision then deflation after cement hardening tends to give better outcomes. Inflation only during cementing was associated with the greatest total blood loss following TKA. Tai TW et al. [Citation11] compared perioperative blood loss and postoperative pain between two groups with or without a tourniquet. They reported that the use of a tourniquet during TKA effectively reduces perioperative blood loss but was related to slightly more postoperative pain that did not affect recovery. A shorter duration of tourniquet application resulted in a faster recovery and less pain during the early postoperative period and a lower incidence of complications following TKA [Citation12,Citation13], but was associated with a significantly greater risk of blood loss and transfusion [Citation14]. Therefore, the optimal timing of tourniquet application is still highly controversial and should be balanced with the increased blood loss and risk of transfusion with postoperative complications when using the tourniquet in TKA.
Until now, six different tourniquet applications have been proposed clinically, including non-use of a tourniquet (NTU); tourniquet inflation before incision than deflation after wound closure (throughout the operation, TTO); tourniquet inflation before incision and deflation after cement hardening (first half of operation, FHO); tourniquet inflation before osteotomy and deflation after wound closure (before osteotomy in operation, BOO); tourniquet inflation after osteotomy and deflation after wound closure (second half of operation, SHO); and tourniquet inflation only during cementing (middle of operation, MO).
Previously, numerous meta-analyses in this field have been published. For instance, Alcelik I et al. [Citation15] conducted a meta-analysis of outcomes with and without a tourniquet in TKA, whereas Wang C et al. [Citation16] presented a meta-analysis comparing tourniquet application only during cementation and long-duration tourniquet application in TKA. However, there still has been no systematic review evaluating the relative efficacy and safety of the various tourniquet application strategies or comparisons of these strategies to cases without tourniquet application in TKA. Accordingly, to comprehensively quantify the clinical efficacy and safety of different tourniquet applications and select the best strategy, we designed and conducted this network meta-analysis.
2. Method
2.1. Data Sources and searches
This network meta-analysis was designed and conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [Citation17].
PubMed, Embase, Cochrane Library, CNKI, and WanFang Database were systematically searched from January 1990 to May 2020, with the search terms ((“tourniquet” OR “tourniquet time” OR “tourniquet application” OR “tourniquet release”) OR (“ischaemic” OR “ischaemic injury” OR “tourniquet related ischaemic injury”) AND (“knee arthroplasty” OR “knee replacement” OR “TKA”)). Reference lists of relevant systematic reviews and identified articles also were reviewed to find additional eligible studies as completely as possible. No restriction was placed on the language of publication.
2.2. Study selection
Any article which met the following inclusion criteria were included: 1. The patients included in the study underwent total knee replacement or arthroplasty; 2. The study included a comparison among two or more different tourniquet release strategies; 3. The study used a randomized clinical trial design, whether blinded or not; 4. The study reported at least one desirable outcome: operation time, blood loss, functional rehabilitation, pain relief, the incidence of DVT, or other complications.
The exclusion criteria for this study were: 1. The study included another type of surgery, including unicompartmental knee arthroplasty or osteotomy; 2. Low-quality studies according to the Cochrane Handbook; 3. All animal studies and cadaver studies; 4. All reviews, systematic reviews and meta-analyses, conference abstracts, letters, and those without original study data.
2.3. Data extraction and quality assessment
Two authors reviewed the full manuscripts of all eligible studies and extracted relevant data from the studies, including author name, publication year, patient number, diagnosis, mean age, gender, surgical approach, whether to use tranexamic acid, whether to use drainage, whether to use anticoagulation and outcomes data. To avoid the influence of withdrawal bias, we preferred to extract data from the intention-to-treat analysis if available.
Two other authors conducted the methodological quality and bias assessment of studies using the Cochrane risk of the bias assessment tool [Citation18]. The following indices were evaluated and ranked the as low risk of bias, unclear risk of bias, or high risk of bias: Sequence generation, allocation concealment, blinding, incomplete outcome data, selection outcome reporting, and other sources of bias. All disputes were resolved through discussion.
2.4. Outcome measures
Operation time, blood loss, postoperative pain, and function scale and safety endpoints were chosen as outcome measurements considered important for decision making. Blood loss included intraoperative, postoperative, and total blood loss, which were compared among groups to identify the efficacy of each tourniquet application strategy. The weighted mean difference (WMD) with 95% confidence intervals (CI) was used to measure the operation time and blood loss.
Early postoperative pain/function status (within one week after surgery) and late status (at least one month after surgery) were compared. No restriction was placed on the types of questionnaire used in pain evaluation. The function subscales of the Western Ontario and McMaster Universities Arthritis Index (WOMAC) were used to evaluate postoperative functional status. If the WOMAC function score was not measured or reported, the Lequesne Index or other functional measurement scales were used. The standardized mean difference (SMD) was used because results from different scales were included in the same network. Additionally, the range of knee flexion was compared to reflect the postoperative functional recovery.
Safety endpoints comprised DVT, minor and major wound complications. Minor wound complications included wound oozing, erythema, cellulitis, minor dehiscence, and superficial infection. Major wound complications included any condition that led to surgical failure, required a second surgery, or caused disability or death. The odds ratio [OR] with 95% confidence intervals (CI) was used to measure relative safety.
2.5. Statistical analysis
Traditional pairwise meta-analysis was conducted to compare the efficacy of the target drugs with placebo using RevMan (Review Manager. Version 5.3, Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The heterogeneity across studies was tested by the Q and I2 statistics, in which p < .05 or I2 > 50% implies significant heterogeneity. A random-effects model was used if significant heterogeneity across studies was found, otherwise, a fixed-effects model was preferred.
The random-effects network meta-analysis was conducted using Stata/MP (version 14.0) in a frequentist method. The multivariate random-effects meta-regression was used to pool data with a proportional variance-covariance matrix, and a restricted maximum-likelihood method was used to assess model fit [Citation19]. Global inconsistency tests and node-split tests were conducted, and the consistency model was adopted if no inconsistency was reported (p-value of Z-test >.05). If available, loop-specific inconsistency tests were also conducted to evaluate the inconsistency between direct and indirect comparisons within every closed triangle or quadratic loop. Inconsistency factors (IFs) and their 95% CI reported by loop-specific inconsistency tests reflect the consistency [Citation20]. If inconsistency was reported in any network, the sensitivity analysis was used to identify the source of inconsistency and exclude these studies from this network. Funnel plots and Egger’s tests were conducted in Stata/MP to detect publication bias for each endpoint. The trim and filling method was used for further analyses to confirm whether significant publication bias was present [Citation21]. The surface under the cumulative ranking (SUCRA) was used to rank the efficacy and safety of different drugs [Citation22]. To select the most effective and safest drug simultaneously, cluster-ranking plots were constructed. Significant differences were considered between treatments when the corresponding 95% CI did not contain 1 for OR. p < .05 was considered statistically significant.
3. Result
3.1. Literature selection
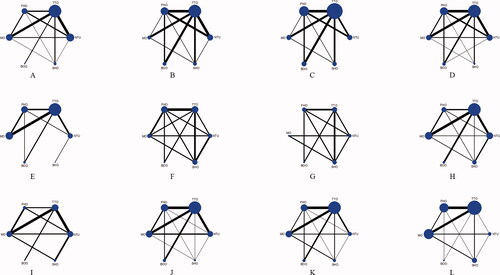
Thirty-eight eligible studies were identified [Citation10–13, Citation23–56]. The PRISMA flow diagram is shown in Supplementary figure 1. Six different tourniquet strategies (NTU, TTO, FHO, MO, BOO, and SHO) were analyzed, and the NTU was selected as the standard control group. The network plot is presented in .
3.2. Study characteristics
The mean age of the 3007 patients included in this study was 67.61 years (IQR 64.66 to 70.50 years) and the proportion of male patients was 31.79% (ranged from 10.91% to 53.23%) across studies. Most participants received primary TKA due to KOA or RA, except for five patients who underwent revision surgery. The medial parapatellar approach was the most common surgical approach (26 articles), followed by the mid-vastus approach (4 articles) and sub-vastus approach (1 article). Seven articles did not report the surgical approach used. Other baseline characteristics are presented in Supplementary Table 1. The details of quality and bias-risk assessments are presented in Supplementary Table 2. Based on these results, the main contributing factors to the risk of bias were performance bias, selection bias, and attrition bias.
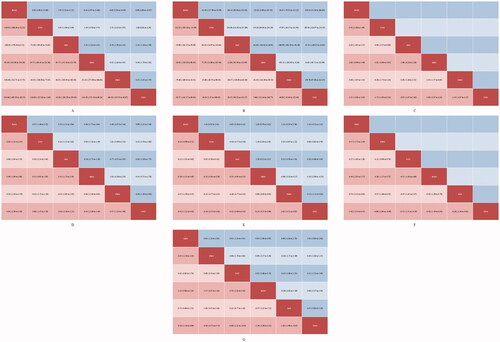
Publication bias was not reported in any network according to the results of funnel plots and Egger’s tests (Supplementary Figure 2). Results of cluster-rank analyses are presented in Supplementary Figure 3. The detailed results of conventional pair-wise meta-analyses are presented in . The forest plots of network meta-analyses are presented in . The league plots which showed the relative effects between different groups are presented in . Detailed SURCA values were presented in Supplementary Tables 3–5.
Table 1. The detailed results of conventional pair-wise meta-analyses.
3.3. Operation time
3.3.1. Conventional pair-wise meta-analysis
Significant heterogeneity of the included studies and interventions was reported (I2 = 79.9%), so the random-effects model was adopted. The use of a tourniquet overall was associated with a significantly shorter operation time than non-use (WMD −4.00, 95% CI [–7.14 to −0.84]).
3.3.2. Network meta-analysis
A total of 33 articles were analyzed in this network. However, significant inconsistency was reported by the global inconsistency test (p < 0.05). In the node-split test and loop-specific inconsistency tests, inconsistency was found in the NTU – FHO – BOO loop (IF 2.32, 95%CI [1.45 to 3.18], p = .000), NTU – TTO – BOO loop (IF 1.94, 95%CI [1.20 to 2.68], p = .000) and NTU – TTO – FHO loop (IF 1.29, 95%CI [0.08 to 2.50], p = .037). Sensitivity analysis was conducted, and four studies were excluded from this network (study 5,9,16,22). Following this, no inconsistency was reported in the reconstructed network and the consistency model was adopted.
The BOO group had the shortest operation time (WMD compared with NTU −8.98, 95%CI [–14.07 to −3.88], SURCA 99.8%), followed by the TTO group (WMD −2.06, 95%CI [–3.29 to −0.82], SURCA 72.9%). No significant differences were found between other tourniquet application groups and the NTU group.
3.4. Blood loss
3.4.1. Conventional pair-wise meta-analysis
Significant heterogeneity was found in intraoperative blood loss (I2 = 88.2%), postoperative blood loss (I2 = 59.2%) and total blood loss (I2 = 92.2%) so the random-effects model was adopted.
The use of a tourniquet overall was associated with a significantly decreased intraoperative blood loss compared to non-use of a tourniquet (WMD −142.86, 95% CI [–175.05 to −110.67]), but also with increased postoperative blood loss (WMD 81.28, 95% CI [35.23 to 127.33]). No significant difference was found in total blood loss (WMD −79.28, 95% CI [–177.34 to 18.78]).
3.4.2. Network meta-analysis
Thirty-three articles were assessed in this network. No significant inconsistency was reported by the global inconsistency tests, node-split tests, or loop-specific inconsistency tests for the three blood loss indicators. The consistency model was more statistically suitable than the inconsistency model.
Apart from the MO group (WMD 47.21, 95% CI [–54.65 to 149.07]) and SHO group (WMD −34.63, 95%CI [–132.99 to 63.72]) which were not significantly different than NTU, other groups all demonstrated less intraoperative blood loss. The TTO group had the largest probability of having the least intraoperative blood loss (WMD −187.65, 95%CI [–254.33 to −120.97], SURCA 93.9%), followed by the BOO group (WMD −156.25, 95%CI [–260.93 to −51.56], SURCA 76.6%) and the FHO group (WMD −139.78, 95%CI [–212.47 to −67.08], SURCA 68.6%).
Only the TTO (WMD 84.95, 95% CI [1.47 to 168.43]) group showed more postoperative blood loss than the NTU group. No significant differences were found for any other groups compared with NTU. BOO (WMD −115.32, 95% CI [–195.56 to −35.08]) also was superior to TTU. The top three effective groups were BOO (SURCA = 84.1%), NTU (SURCA = 66.9%), and SHO (SURCA = 61.2%).
Similarly, the BOO (WMD −234.66, 95% CI [–409.19 to −60.13]) group and the TTO (WMD −116.63, 95% CI [–227.62 to −5.63]) group had less total blood loss than the NTU group. According to the SURCA value, the best strategy option was BOO (SUCRA = 92.8%), followed by SHO (SURCA = 64.7%) and TTO (SURCA = 60.2%). MO (SURCA = 66.9%) might be the least effective strategy other than NTU.
3.5. Pain
3.5.1. Conventional pair-wise meta-analysis
Significant heterogeneity was found for both early (I2 = 97.3%) and late (I2 = 73.4%) postoperative pain so the random-effects model was adopted. No significant differences were found in early postoperative pain (SMD 0.23, 95% CI [–0.73 to 1.19]) or late postoperative pain (SMD −0.04, 95% CI [–0.34 to 0.27]).
3.5.2. Network meta-analysis
Seventeen articles were assessed in this network. The consistency model was adopted because no significant inconsistency was reported by global inconsistency, node-split, or loop-specific inconsistency tests.
The SHO group demonstrated the most obvious early postoperative pain compared with the NTU group (SMD 1.10, 95% CI [0.13 to 2.08]) and any of the other tourniquet application groups. The BOO group had the greatest probability of being the best option (SUCRA = 96.5%), followed by SHO (SURCA = 61.8%) and TTO (SURCA = 53.9%).
No significant differences were found in the late postoperative pain network. BOO had the largest probability of being the best option (SMD −0.42, 95% CI [–1.25 to 0.42], SUCRA = 79.1%), followed by MO (SMD −0.21, 95% CI [–0.66 to 0.24], SURCA = 61.8%) and TTO (SMD −0.18, 95% CI [–0.55 to 0.20], SURCA = 63.2%). The greatest late postoperative pain was in the SHO group (SMD 0.14, 95% CI [–0.37 to 0.66], SURCA = 22.6%), but no significant difference was found compared with the NTU group.
3.6. Function
3.6.1. Conventional pair-wise meta-analysis
Heterogeneity was found only in early postoperative function (I2 = 71.6%). The fixed-effects model was used for late postoperative function (I2 = 0.00%) and knee flexion range (I2 = 39.3%), and the random-effects model was adopted for early postoperative function. No significant difference was found in any functional outcome. The use or absence of tourniquets does not influence the patient’s early postoperative functional status (SMD −0.30, 95%CI [–0.682 to 0.073]), early postoperative functional status (SMD 0.05, 95%CI [–0.186 to 0.277]) or knee flexion range (WMD −0.79, 95%CI [–2.598 to 1.028]).
3.6.2. Network meta-analysis
Seventeen articles with postoperative functional status and 15 articles with postoperative knee flexion range were assessed in this network. The consistency model was adopted for the early/late postoperative functional status because no inconsistency was reported in the global inconsistency, node-split, or loop-specific inconsistency tests. However, significant inconsistency was found in the postoperative knee flexion range. Based on the loop-specific inconsistency test, inconsistency was found in the TTO – MO – SHO loop (IF 25.39, 95%CI [21.53 to 29.25], p = .000) and the NTU – TTO – SHO loop (IF 20.82, 95%CI [12.92 to 28.71], p = .000). Sensitivity analysis was conducted, and one study was excluded from this network (study 37). Following this, no inconsistency was reported in the reconstructed network and the consistency model was adopted.
Similar to the results obtained by pair-wise meta-analysis, network meta-analysis did not find any significant differences in functional outcomes. According to the SURCA rank, theoretically, the best strategies for positive functional outcomes were SHO (knee flexion range, WMD 1.09, 95%CI [–2.07 to 4.25], SURCA = 85.4%), and FHO (early postoperative function, SMD 0.49, 95%CI [–0.82 to 1.80], SURCA = 89.1%; late postoperative function, SMD 0.37, 95%CI [–1.24 to 1.99], SURCA = 69.2%). The worst outcomes were TTO (knee flexion range, WMD −1.72, 95%CI [–3.93 to 0.50], SURCA = 20.9%), BOO (early postoperative function, SMD −0.99, 95%CI [–3.20 to 1.21], SURCA = 23.8%) and SHO (late postoperative function, SMD −0.45, 95%CI [–2.06 to 1.16], SURCA = 27.7%), respectively.
3.7. Safety outcome
3.7.1. Conventional pair-wise meta-analysis
No heterogeneity was reported in safety outcomes (I2 = 0.00%) so the fixed-effects model was used. No significant differences were found between the use or absence of the tourniquet on the incidence of DVT (OR 1.39, 95%CI [0.879 to 2.209]), minor complications (OR 1.31, 95%CI [0.846 to 2.017]) or major complications (OR 0.95, 95%CI [0.251 to 3.555]).
3.7.2. Network meta-analysis
Twenty-two articles were included in the safety network. The consistency model was adopted because no significant inconsistency was reported. Considering the actual clinical situation, the TTO group was chosen as the control group for this part of the analysis.
Consistent with expected results, the TTO group had a higher DVT incidence than any other group (the differences were all statistically significant). The SHO group had the lowest risk of postoperative DVT (OR −1.02, 95%CI [–2.52 to 0.47], SURCA = 72.2%) followed by FHO (OR −0.86, 95%CI [–1.66 to −0.06], SURCA = 71.3%) and NTU (OR −0.75, 95%CI [–1.31 to −0.19], SURCA = 64.9%).
Similar to the DVT results, the TTO group had a greater minor complication incidence than any other group (the differences were all statistically significant). The BOO group had the lowest risk of postoperative minor complications (OR-1.38, 95%CI [–3.00 to 0.25], SURCA = 83.9%) followed by MO (OR −1.00, 95%CI [–1.98 to −0.03], SURCA = 76.7%) and NTU (OR −0.68, 95%CI [–1.32 to −0.04], SURCA = 63.6%).
The TTO group had the highest risk for postoperative major complications (SURCA = 34.3%), but no significant difference was found when was compared with BOO (OR −0.09, 95%CI [–3.56 to 3.37]) or NTU (OR −0.12, 95%CI [–1.48 to 1.23]). The three strategies with lower risk were SHO (OR –1.03, 95%CI [–2.90 to 0.84], SURCA = 77.4%), MO (OR −0.37, 95%CI [–1.58 to 0.84], SURCA = 55.5%) and FHO (OR –0.19, 95%CI [–1.37 to 1.00], SURCA = 45.7%).
4. Discussion
This is the first study that comprehensively compared different tourniquet application strategies to evaluate their impact on postoperative recovery following TKA.
Previous systematic reviews have indicated that the use of tourniquets can reduce surgical bleeding to a certain extent, but can cause vascular endothelial injury or ischemia-reperfusion injury of the lower limbs, resulting in poor wound healing, DVT, swelling, increased postoperative pain, and loss of function.
To comprehensively weigh the pros and cons of tourniquet use to help clinicians make decisions, five clinically important endpoints were assessed in this study: perioperative blood loss, operation time, postoperative pain and function, and complications. All relevant RCTs were screened and 38 eligible RCTs were included in the study. A frequentist framework network meta-analysis was conducted. This method integrates all available direct or indirect evidence from RCTs comparing different tourniquet application strategies, thereby augmenting the number of studies within each comparison, which increases the power of the study. For instance, only five of the included studies compared SHO with other strategies, but through the network, there were 38 studies allowing six indirect comparisons.
The main results of this analysis revealed that: (1) BOO and TTO result in shorter operation times. Probably for this reason they also had less intraoperative and total blood loss; (2) TTO had more postoperative blood loss, while BOO had the least postoperative bleeding; (3) SHO may cause more early postoperative pain, but no difference was found in late postoperative pain; (4) BOO had the lowest early and late postoperative pain, but could negatively affect early functional recovery following TKA; (5) TTO was related to a greater incidence of DVT, as well as minor and major complications. BOO had the lowest risk of postoperative minor complications, whereas SHO had the lowest risk of postoperative DVT and major complications; (6) Regardless of whether a tourniquet is used or how it is used, the late knee pain and function following TKA will not be affected; (7) According to the results of the cluster-rank analysis, it is safer and more effective to use the BOO strategy to prevent DVT and to reduce bleeding and minor complications simultaneously, but SHO is more suitable for patients who are at high risk of major complications.
Alcelik I et al. [Citation15] supposed that using a tourniquet to decrease blood loss may involve two main effects. First, bleeding at the surgical site might be reduced, consequently reducing blood loss during surgery. Second, if there is little bleeding from the wound, the surgeon could spend less time controlling the bleeding, so the operation time should be shortened. According to our results, TTO and BOO were associated with shorter operation time and less intraoperative and total blood loss. This is in line with Alcelik I’s speculation, but we believe that shortening the operation time as much as possible and closing the wound in advance also has a positive effect on reducing intraoperative blood loss. Theoretically, the use of a tourniquet may cause calcification of the vascular endothelium, blood flow stasis of the lower limbs, and ischemia-reperfusion injury to potentially increase the risk of postoperative complications. Previous studies have proven that a higher rate of complications was related to longer tourniquet times [Citation57–59]. Olivecrona C et al. found that every additional 10 min of tourniquet time was associated with an increased risk for complications [Citation60]. Because using a tourniquet throughout the surgery can reduce blood loss effectively, it is indeed related to many safety issues. Thus, it is crucial to find a balance between reducing surgical bleeding and shortening surgical time, i.e. the best tourniquet application strategy. According to our results, tourniquet inflation before osteotomy then deflation after wound closure may be the ideal choice, with the lowest perioperative blood loss and consequently the shortest operation time and the lowest risk of complications.
There are several limitations to this study. First, there are some inevitable confounding factors that may affect the stability of the results. For instance, the skills of the surgeons will undoubtedly affect the operation time and blood loss. It is an uncontrollable variable when comparing these studies and is impossible to control or eliminate by statistical means. Second, considering the unmanageable confounding factors in observational studies, only RCTs were included even though observational studies also can play an important role in evaluating the effectiveness and safety of tourniquet application following TKA. This may contribute to the small number of studies we could include. Although funnel plots and Egger’s tests were performed in this study and no significant publication bias was found, bias can be a potential problem especially when the funnel plots showed a dubious asymmetry. Finally, the DVT and major complication networks inevitably involved rare event rates. Although the Cochrane Handbook recommends the omission of studies with no events in both arms, it is still controversial whether this exclusion reduces the coverage rates of confidence intervals and increases the bias estimates. Previous studies have shown that including such trials produces unbiased estimation with narrow confidence intervals, improving the accuracy of combined estimation in a meta-analysis. The results of this should be interpreted cautiously. More high-quality trials are needed.
5. Conclusion
Using tourniquets during the entire operation can effectively reduce blood loss, but it also can cause many safety problems, including DVTs and wound complications. Tourniquet inflation before osteotomy then deflation after wound closure effectively can reduce perioperative bleeding while shortening the operation time and reducing postoperative complications. We conclude that tourniquet inflation before osteotomy then deflation after wound closure could be the ideal tourniquet application strategy in TKA.
Author contributions
YJL and JHW conceived the study, participated in its design and coordination, and critically revised the manuscript. QXL and JHW searched the databases. JG and YJL reviewed the full manuscripts of all eligible studies and finished the data collection. YJL and ZQC conducted the quality assessment of studies. ZQC and YJL finished the data analysis. ZQC drafted the manuscript. YJL, JHW, and ZQC had full access to all the data collection, analysis, and interpretation. All authors read and approved the final manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
| Abbreviations | ||
| BOO | = | before osteotomy in operation |
| CI | = | confidence intervals |
| DVT | = | deep vein thrombosis |
| FHO | = | first half of operation |
| IFs | = | inconsistency factors |
| IQR | = | interquartile range |
| KOA | = | knee osteoarthritis |
| MO | = | middle of operation |
| NTU | = | non-use of a tourniquet |
| OR | = | odds ratio |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines |
| RA | = | rheumatoid arthritis |
| SMD | = | standardized mean difference |
| SHO | = | second half of operation |
| SUCRA | = | surface under the cumulative ranking |
| TKA | = | total knee arthroplasty |
| TTO | = | throughout the operation |
| WMD | = | weighted mean difference |
| WOMAC | = | Western Ontario and McMaster Universities Arthritis Index |
Supplemental Material
Download ()Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided. This manuscript is not under review with any other journal now and has not been published in any other journal previously.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149–162.
- Quinn RH, Murray J, Pezold R. The American Academy of Orthopaedic Surgeons appropriate use criteria for surgical management of osteoarthritis of the knee. J Bone Joint Surg Am. 2017;99(8):697–699.
- Noticewala MS, Nyce JD, Wang W, et al. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961–967.
- Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
- Silver R, de la Garza J, Rang M, et al. Limb swelling after release of a tourniquet. Clin Orthop Relat Res. 1986;(206):86–89.
- Kumar SN, Chapman JA, Rawlins I. Vascular injuries in total knee arthroplasty. A review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211–216.
- Saunders KC, Louis DL, Weingarden SI, et al. Effect of tourniquet time on postoperative quadriceps function. Clin Orthop Relat Res. 1979;(143):194–199.
- Horlocker TT, Hebl JR, Gali B, et al. Anesthetic, patient, and surgical risk factors for neurologic complications after prolonged total tourniquet time during total knee arthroplasty. Anesth Analg. 2006;102(3):950–955.
- Christodoulou AG, Ploumis AL, Terzidis IP, et al. The role of timing of tourniquet release and cementing on perioperative blood loss in total knee replacement. Knee. 2004;11(4):313–317.
- Kvederas G, Porvaneckas N, Andrijauskas A, et al. A randomized double-blind clinical trial of tourniquet application strategies for total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2790–2799.
- Tai TW, Chang CW, Lai KA, et al. Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2209–2215.
- Rathod P, Deshmukh A, Robinson J, et al. Does tourniquet time in primary total knee arthroplasty influence clinical recovery? J Knee Surg. 2015;28(4):335–342.
- Ejaz A, Laursen AC, Kappel A, et al. Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop. 2014;85(4):422–426.
- Mittal R, Ko V, Adie S, et al. Tourniquet application only during cement fixation in total knee arthroplasty: a double-blind, randomized controlled trial. ANZ J Surg. 2012;82(6):428–433.
- Alcelik I, Pollock RD, Sukeik M, et al. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(3):331–340.
- Wang C, Zhou C, Qu H, et al. Comparison of tourniquet application only during cementation and long-duration tourniquet application in total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2018;13(1):216.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- Higgins JPT, Thomas J, Chandler J, et al (Eds.). Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: John Wiley & Sons; 2019.
- White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111–125.
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–944.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463.
- Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58.
- Hasanain MS, Apostu D, Alrefaee A, et al. Comparing the effect of tourniquet vs tourniquet-less in simultaneous bilateral total knee Arthroplasties. J Arthroplasty. 2018;33(7):2119–2124.
- Huang ZY, Pei FX, Ma J, et al. Comparison of three different tourniquet application strategies for minimally invasive total knee arthroplasty: a prospective non-randomized clinical trial. Arch Orthop Trauma Surg. 2014;134(4):561–570.
- Ishii Y, Matsuda Y. Effect of the timing of tourniquet release on perioperative blood loss associated with cementless total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(8):977–983.
- Fukuda A, Hasegawa M, Kato K, et al. Effect of tourniquet application on deep vein thrombosis after total knee arthroplasty. Arch Orthop Trauma Surg. 2007;127(8):671–675.
- Aglietti P, Baldini A, Vena LM, et al. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res. 2000;(371):169–177.
- Harvey EJ, Leclerc J, Brooks CE, et al. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291–296.
- Zhang Y, Li D, Liu P, et al. Effects of different methods of using pneumatic tourniquet in patients undergoing total knee arthroplasty: a randomized control trial. Ir J Med Sci. 2017;186(4):953–959.
- Kageyama K, Nakajima Y, Shibasaki M, et al. Increased platelet, leukocyte, and endothelial cell activity are associated with increased coagulability in patients after total knee arthroplasty. J Thromb Haemost. 2007;5(4):738–745.
- Zhou K, Ling T, Wang H, et al. Influence of tourniquet use in primary total knee arthroplasty with drainage: a prospective randomised controlled trial. J Orthop Surg Res. 2017;12(1):172.
- Hernandez-Castanos DM, Ponce VV, Gil F. Release of ischaemia prior to wound closure in total knee arthroplasty: a better method? Int Orthop. 2008;32(5):635–638.
- Hakkalamani S, Clark V, Pradhan N. Short versus standard duration tourniquet use during total knee replacement: a pilot study. Acta Orthop Belg. 2015;81(1):52–56.
- Widman J, Isacson J. Surgical hemostasis after tourniquet release does not reduce blood loss in knee replacement. A prospective randomized study of 81 patients. Acta Orthop Scand. 1999;70(3):268–270.
- Vandenbussche E, Duranthon LD, Couturier M, et al. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306–309.
- Li B, Wen Y, Wu H, et al. The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop. 2009;33(5):1263–1268.
- Tetro AM, Rudan JF. The effects of a pneumatic tourniquet on blood loss in total knee arthroplasty. Can J Surg. 2001;44(1):33–38.
- Wang K, Ni S, Li Z, et al. The effects of tourniquet use in total knee arthroplasty: a randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2849–2857.
- Chen S, Li J, Peng H, et al. The influence of a half-course tourniquet strategy on peri-operative blood loss and early functional recovery in primary total knee arthroplasty. Int Orthop. 2014;38(2):355–359.
- Yavarikia A, Amjad GG, Davoudpour K. The influence of tourniquet use and timing of its release on blood loss in total knee arthroplasty. Pak J Biol Sci. 2010;13(5):249–252.
- Hersekli MA, Akpinar S, Ozkoc G, et al. The timing of tourniquet release and its influence on blood loss after total knee arthroplasty. Int Orthop. 2004;28(3):138–141.
- Dennis DA, Kittelson AJ, Yang CC, et al. Does tourniquet use in TKA affect recovery of lower extremity strength and function? A randomized trial. Clin Orthop Relat Res. 2016;474(1):69–77.
- Yin D, Delisle J, Banica A, et al. Tourniquet and closed-suction drains in total knee arthroplasty. No beneficial effects on bleeding management and knee function at a higher cost. Orthop Traumatol Surg Res. 2017;103(4):583–589.
- Ejaz A, Laursen AC, Kappel A, et al. Tourniquet induced ischemia and changes in metabolism during TKA: a randomized study using microdialysis. BMC Musculoskelet Disord. 2015;16:326.
- Goel R, Rondon AJ, Sydnor K, et al. Tourniquet use does not affect functional outcomes or pain after total knee arthroplasty: a prospective, double-blinded, randomized controlled trial.J Bone Joint Surg. 2019;101(20):1821–1828.
- Mori N, Kimura S, Onodera T, et al. Use of a pneumatic tourniquet in total knee arthroplasty increases the risk of distal deep vein thrombosis: a prospective, randomized study. Knee. 2016;23(5):887–889.
- Schnettler T, Papillon N, Rees H. Use of a tourniquet in total knee arthroplasty causes a paradoxical increase in total blood loss. J Bone Joint Surg Am. 2017;99(16):1331–1336.
- Huiling G, Jie X. Clinical comparison on the different uses of pneumatic tourniquet in total knee arthroplasty. Fujian University of Medicine; 2013. (In Chinese).
- Mengjian Z, Zongsheng Y. Effects of different app1ication methods of pneumatic tourniquet on total knee arthroplasty. Anhui Medical University; 2016. (In Chinese).
- Xinling W, Biao T, Jiandong Y, et al. Restrictive use and non-use tourniquets during total knee arthroplasty. Chinese J Tissue Eng Res. 2019;23(28):4456–4460.
- Jun F. Comprehensive effect of half tourniquet to stop bleeding during total knee arthroplasty and its influence on early functional recovery. Modern Instr Med Treat. 2016;22(05):26–27.
- Weishan L, Bingling Y, Xiaonie C, et al. Comparison of the effect of applying pneumatic tourniquet at different times in total knee arthroplasty. Modern Hospital. 2018;18(03):417–419.
- Qiang Z, Xifu S. Comparison of effect of tourniquet in different periods of total knee arthroplasty. China J Modern Med. 2017;27(16):86–89.
- Qi Z, Jiyuan D, Ke G, et al. Effects of tourniquet use on perioperative outcome in total knee arthroplasty. Chinese J Reparative Reconstruct Surg. 2016;30(04):421–425.
- Tarwala R, Dorr LD, Gilbert PK, et al. Tourniquet use during cementation only during total knee arthroplasty: a randomized trial. Clin Orthop Relat Res. 2014;472(1):169–174.
- Fan Y, Jin J, Sun Z, et al. The limited use of a tourniquet during total knee arthroplasty: a randomized controlled trial. Knee. 2014;21(6):1263–1268.
- Butt U, Ahmad R, Aspros D, et al. Factors affecting wound ooze in total knee replacement. Ann R Coll Surg Engl. 2011;93(1):54–56.
- Jacob AK, Mantilla CB, Sviggum HP, et al. Perioperative nerve injury after total knee arthroplasty: regional anesthesia risk during a 20-year cohort study. Anesthesiology. 2011;114(2):311–317.
- Tai TW, Lin CJ, Jou IM, et al. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1121–1130.
- Olivecrona C, Lapidus LJ, Benson L, et al. Tourniquet time affects postoperative complications after knee arthroplasty. Int Orthop. 2013;37(5):827–832.