?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Previous studies have reported a negative relationship between thyroid-stimulating hormone (TSH) and renal function in euthyroid individuals, but others have found that higher free thyroxine (FT4) was associated with an increased risk of chronic kidney disease. This study was designed to analyze the relationship between thyroid and renal function from a new perspective of sensitivity to thyroid hormone.
Methods
This retrospective study included 2831 euthyroid individuals who underwent a health examination at the First Hospital of China Medical University between January 2017 and December 2018. Parametric Thyroid Feedback Quantile-based Index (PTFQIFT4), TSH index (TSHI), thyrotroph T4 resistance index (TT4RI), free triiodothyronine to FT4 ratio (FT3/FT4), the secretory capacity of the thyroid gland (SPINA-GT) and the sum activity of peripheral deiodinases (SPINA-GD) were calculated. We also innovated the TT3RI and PTFQIFT3 indices based on FT3 and TSH. Renal function was assessed by estimated glomerular filtration rate (eGFR) CKD-EPI and creatinine-cystatin C-KDIGO equations.
Results
After adjustment of basic characteristics and comorbidities, linear regression showed that eGFR CKD-EPI was positively associated with FT3/FT4 (β = 23.31), and inversely correlated to PTFQI FT4 (β= −2.69) (both p < .001). When comparing the fourth versus the first quartile of PTFQI FT4, the odds ratio (OR) for a reduced renal function was 1.89 (95% CI 1.28–2.80), and the OR was 0.64 (95% CI 0.43–0.95) when comparing quartiles of FT3/FT4 (both p for trend< .05). In addition, for every 1SD increase in PTFQI FT4, the OR for a reduced renal function was 1.27 (95%CI 1.10–1.47). TSHI, TT4RI and TT3RI also showed a negative correlation to renal function. Similar results were obtained in SPINA-GD as in FT3/FT4.
Conclusions
In euthyroid individuals, decreased sensitivity to thyroid hormone is associated with reduced renal function. The composite PTFQIFT4 index correlates more strongly to renal function than TSH or T4 alone.
Decreased sensitivity to thyroid hormone is associated with reduced renal function in the euthyroid population.
The recently developed composite index PTFQIFT4 seems to correlate more strongly to renal function than individual TSH or FT4 parameters.
Innovative indices TT3RI and PTFQIFT3 based on the interaction between T3 and TSH may also reflect sensitivity to thyroid hormone.
KEY MESSAGES
Introduction
Impaired renal function and chronic kidney disease are global public health problems with growing prevalence and mortality, some of which can be attributed to hormonal and metabolic impact on renal function [Citation1,Citation2]. Thyroid hormones have been shown to affect glomerular filtration rate and renal tubular function [Citation3]. There has been a large number of studies on the correlation between thyroid disorders and reduced renal function, but the results have been inconsistent [Citation4–8]. Even in euthyroid population studies, no consensus has been reached on this topic. Some studies have shown that higher thyroid-stimulating hormone (TSH) levels within the reference range were associated with decreased estimated glomerular filtration rate (eGFR) or increased incidence of chronic kidney disease [Citation9–14]. However, others have only reported a positive correlation between free triiodothyronine (FT3) and eGFR [Citation15]. On the contrary, some studies have found that higher free thyroxine (FT4) was associated with decreased eGFR in the euthyroid population [Citation16,Citation17].
Classically, thyroid function is evaluated with simple serum FT3, FT4 and TSH levels. However, the complex interactions between FT3, FT4 and TSH can be assessed with sensitivity to thyroid hormone indices that can provide a new interpretation of thyroid status. In the central pituitary, thyroid hormones suppress TSH production by negative feedback, which can be assessed by TSH index (TSHI), thyrotroph T4 resistance index (TT4RI) and Parametric Thyroid Feedback Quantile-based Index (PTFQI) [Citation18–20]. In the peripheral tissues, FT3 to FT4 ratio (FT3/FT4) and the sum activity of peripheral deiodinases (SPINA-GD) are indices reflecting the conversion efficiency of FT4 to FT3, which can indirectly estimate the bioavailability of thyroid hormones [Citation21]. In the thyroid gland, its maximum secretory capacity can be calculated by SPINA-GT [Citation21]. Recently, some researchers have utilized sensitivity to thyroid hormone indices in euthyroid populations to associate with obesity, metabolic syndrome, and diabetes [Citation18,Citation22], which are underlying diseases that could also lead to impaired renal function [Citation23].
Whether sensitivity of thyroid hormone is associated with renal function remains unknown. Therefore, we investigated the relationship between thyroid and renal function from the perspective of sensitivity to thyroid hormone in the euthyroid population.
Materials and methods
Subjects
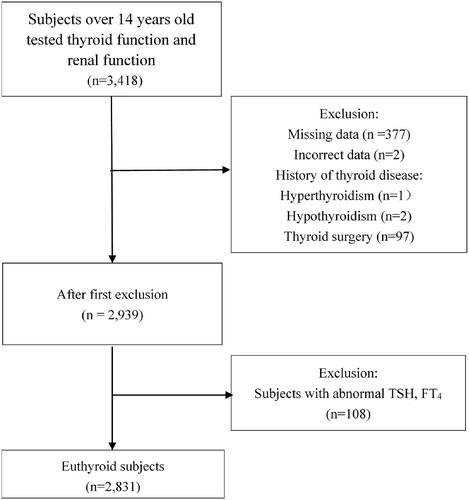
All subjects were enrolled in an annual physical examination between January 2017 and December 2018. Data were retrospectively collected from the Physical Examination Centre of the First Hospital of China Medical University. We included subjects over 14 years old who had been tested for thyroid function, renal function, and other metabolism-related indicators, including fasting plasma glucose, lipid profiles and uric acid (n = 3,418). The exclusion criteria included: (1) missing records of medical history or measurements of anti-thyroid peroxidase antibody, anti-thyroglobulin antibody and anthropometric parameters such as height, weight, and waist circumference (n = 377); (2) incorrect data due to measurement or recording error (n = 2); (3) having a history of thyroid surgery, thyroid disorder (hyperthyroidism or hypothyroidism), or pituitary disorder (n = 100); and (4) subjects with abnormal TSH and/or FT4 (n = 108) (). This retrospective study was approved by the Ethics Committee of the First Hospital of China Medical University. An informed consent waiver was obtained to use de-identified data [NO. (2019)241].
Body measurements and laboratory examinations
The basic information including gender, age, height, weight, waist circumference, blood pressure and heart rate were measured and recorded. And the medical history was obtained from the inquiry. Body mass index was calculated as weight (kg) divided by height (m) squared. Venous blood samples were collected after an overnight fast. The automatic biochemical analyzer (Hitachi, Japan) was used to measure biochemical parameters, including serum creatinine, urea nitrogen, uric acid, cystatin C, fasting plasma glucose, haemoglobin A1c and lipid profiles. The subjects with a previous diagnosis of diabetes or measuring fasting plasma glucose ≥ 7.0 mmol/L or haemoglobin A1c ≥ 6.5% were defined as diabetic. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or having a history of hypertension. Dyslipidemia was assessed by triglyceride ≥1.7 mmol/L or total cholesterol ≥5.2 mmol/L or high-density lipoprotein cholesterol ≤1.0 mmol/L or low-density lipoprotein cholesterol ≥3.4 mmol/L.
Serum levels of FT3, FT4, TSH, thyroid peroxidase antibody, and thyroglobulin antibody were measured by electrochemiluminescent immunoassays on Architect i2000SR (Abbott Laboratories, Chicago, IL, USA). Reference ranges for FT4, FT3, TSH, thyroid peroxidase antibody, and thyroglobulin antibody were 9.01–19.05 pmol/L, 2.63–5.70 pmol/L, 0.35–4.94 mIU/L, 0–5.61 IU/mL, 0–4.11 IU/mL, respectively. Euthyroid was defined as TSH and FT4 within reference ranges. Positive for thyroid peroxidase antibodies or thyroglobulin antibodies was defined as having a value above the upper limit.
Indices reflecting the sensitivity of thyroid hormone were calculated according to previous studies:
FT3/FT4 ratio = FT3 (pmol/L)/FT4 (pmol/L). Higher FT3/FT4 indicates higher peripheral thyroid hormone activity.
TSHI = ln TSH (mIU/L) + 0.1345 * FT4 (pmol/L) [Citation19].
TT4RI = FT4 (pmol/L) * TSH (mIU/L) [Citation20]. For TSHI and TT4RI, the higher the values, the lower the central sensitivity to thyroid hormones.
PTFQIFT4 is an approximation of the TFQI =
and can be adapted to different study populations. PTFQIFT4 shows the difference between the FT4 and reversed TSH quantiles. PTFQIFT4 was calculated with the Excel spreadsheet formula: NORM.DIST (FT4-cell, μ FT4, σFT4, TRUE) + NORM.DIST [ln (TSH-cell), μ ln TSH, σln TSH, TRUE]-1 [Citation18]. In our cohort, μ FT4, σFT4, μ ln TSH and σ ln TSH were 13.2678, 1.4871, 0.4757 and 0.4951, respectively. The value of PTFQI ranged from -1 to 1. The negative values indicated higher sensitivity to thyroid hormones in the pituitary; positive values indicated less sensitivity; the value of 0 indicated a normal sensitivity [Citation18].
Thyrotroph T3 resistance index (TT3RI) and PTFQIFT3: We innovated two new formulae based on the interaction between FT3 and TSH into the above formulae to obtain TT3RI and PTFQIFT3. The interpretation of TT3RI and PTFQIFT3 is similar to their respective original formula.
Thyroid secretory capacity SPINA-GT = βT (DT + [TSH]) (1 + K41[TBG] + K42[TBPA]) [FT4]/αT[TSH].
The sum activity of peripheral deiodinases SPINA-GD = β31 (KM1 + [FT4]) (1 + K30[TBG]) [FT3]/α31[FT4]. SPINA-GT and SPINA-GD parameters were calculated by the SPINA Thyr Software [Citation21].
Renal function was assessed by eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and creatinine-cystatin C-KDIGO (Cr–CysC) equation [Citation24,Citation25]. The number of patients in chronic kidney disease stages G3a to G5 was relatively small in the general physical examination population. Therefore, reduced renal function was defined as eGFR< 90 mL/min/1.73m2.
Statistical analysis
Continuous variables were presented as mean ± SD or medians (interquartile ranges) for those with normal or skewed distributions, respectively. Categorical variables were shown as numbers (proportions). The t-tests and non-parametric Mann–Whitney tests were used to compare quantitative variables between the reduced renal function and normal renal function groups. Comparison of categorical variables was conducted with chi-square tests. The values of TT4RI, TT3RI, SPINA-GT and SPINA-GD were normalized by natural log (ln) transformation for skewed distributions, then multiple linear regression was used to analyze the relationship between thyroid parameters and eGFR. Multivariable logistic regression analysis was used to examine the association of thyroid parameters in quartiles with reduced renal function. We selected confounders based on the clinical relevance or univariate relationship (p < 0.2) with renal function and sensitivity to thyroid hormone. Model 1 adjusted age and sex. Model 2 further adjusted for body mass index, waist circumference, heart rate, diabetes, hypertension, dyslipidemia, thyroid peroxidase antibody positivity and thyroglobulin antibody positivity. Analyses were also performed based on age (younger or older than 65 years) and renal function (normal or reduced renal function). In addition, we performed sensitivity analyses in non-diabetic and non-hypertensive subjects, respectively. All calculated p-values were two-sided, and a p-value< .05 was considered statistically significant. All analyses were conducted with the SPSS 22.0 software.
Results
Clinical characteristics of the participants
A total of 2831 euthyroid participants were included in this study, with a mean age of 51.08 years (SD 10.33 years, min 16 years, max 86 years); among them, 1717 (60.65%) were men. The clinical characteristics were shown in . The overall prevalence of reduced renal function based on eGFRCKD-EPI was 10.21% (289/2831). Participants who had lower eGFRCKD-EPI were older, more likely to be male, and have diabetes or hypertension (all p < .05). In addition, participants with lower eGFRCKD-EPI had higher body mass index, waist circumference, and serum uric acid levels (all p < .05). They also had lower FT3 levels, FT3/FT4 ratio and SPINA-GD (all p < .05). Although the indices reflecting central sensitivity of thyroid hormone were higher and the SPINA-GT was lower in patients with lower eGFR CKD-EPI, there was no statistically significant difference compared to the higher eGFRCKD-EPI group.
Table 1. Clinical characteristics of all participants.
Association of sensitivity of thyroid hormone indices with eGFR
Linear regression analysis was used to examine the relationship between thyroid parameters and eGFR. In Model 1, after adjustment of age and sex, eGFRCKD-EPI was positively associated with the level of FT3/FT4 (β = 20.60, p < .001) and negatively associated with PTFQIFT4 (β = −2.60, p < .001). The other indices reflecting central sensitivity of thyroid hormone (TSHI, Ln TT3RI, Ln TT4RI, except for PTFQIFT3) were also inversely correlated to eGFRCKD-EPI (β = −1.90 to −1.22, all p < .001). Ln SPINA-GT and Ln SPINA-GD were positively associated with eGFR CKD-EPI (β = 1.50 and 6.64, respectively, both p < .01). The above associations remained significant after further adjustment in Model 2. All correlations remained consistent when analyzed with eGFRCr-CysC, except for the association between Ln SPINA-GT and eGFRCr-CysC (, Supplementary Figure 1).
Table 2. Association of sensitivity of thyroid hormone indices with eGFR by linear regression.
Association of sensitivity of thyroid hormone indices with reduced renal function
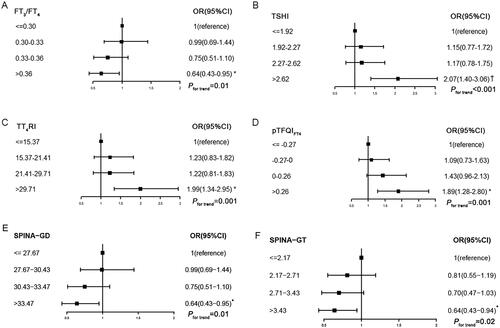
When thyroid parameters are categorized into quartiles, the odds ratio (OR) of the fourth versus the first quartile of PTFQIFT4 was 1.89 (95%CI 1.28–2.80, p for trend = .001) for reduced renal function (eGFRCKD-EPI <90 mL/min/1.73m2). The ORs of the fourth versus the first quartile of TSHI and TT4RI for reduced renal function were 2.07 (95%CI 1.40–3.06) and 1.99 (95%CI 1.34–2.95), respectively (both pfor trend < .05). The risk of reduced renal function in the highest quartile of FT3/FT4 was significantly lower than in the lowest quartile (OR = 0.64, 95%CI 0.43–0.95, pfor trend = .01). The results of SPINA-GT and SPINA-GD were similar to that of FT3/FT4 ().
Figure 2. Association of sensitivity of thyroid hormone indices quartiles with reduced renal function. Pfor trend calculated with sensitivity to thyroid hormone indices quartile ordinal as a continuous variable. Model 2: age, sex, body mass index, waist circumference, heart rate, diabetes, hypertension, dyslipidemia, TPOAb positive and TgAb positive were adjusted. FT3/FT4: free triiodothyronine to free thyroxine ratio; TSHI: thyroid-stimulating hormone index; TT4RI: thyrotroph T4 resistance index; PTFQIFT4: Parametric Thyroid Feedback Quantile-based Index calculated by FT4; SPINA-GT: the calculated secretory capacity of the thyroid gland; SPINA-GD: the sum activity of peripheral deiodinases; TPOAb: thyroid peroxidase antibody; TgAb: thyroglobulin antibody. *p<.05; †p<.001.
Based on Model 2 logistic regression, for every 1SD increase in FT3/FT4 or PTFQIFT4, the ORs for reduced renal function (eGFRCKD-EPI <90 mL/min/1.73m2) were 0.80 (95%CI 0.69–0.93) and 1.27 (95%CI 1.10–1.47), respectively. TSHI, TT4RI and TT3RI showed similar results as PTFQIFT4. The OR for reduced renal function with every 1SD increase in SPINA-GD was similar to FT3/FT4. However, there was no significant relationship between SPINA-GT and reduced renal function. The OR for a reduced renal function was 1.27 (95%CI 1.11–1.45) with every 1SD increase in TSH. PTFQIFT3 did not show a relationship with reduced renal function. These results remained consistent when renal function was assessed based on the eGFRCr-CysC equation. However, regression models with FT3 and FT4 were inconsistent when using different eGFR equations ().
Table 3. Association of sensitivity of thyroid hormone indices with reduced renal function by logistic regression.
We also performed stratified analyses in different age groups. The relationship between the sensitivity of thyroid hormone indices with eGFR and the effect of every 1SD increase in these indices on reduced renal function persisted in the younger group. However, we did not obtain significant results in the subjects aged over 65 years. The age strata only showed significant interactions in the relationships of TSHI, TT3RI and SPINA-GT with eGFRCKD-EPI decline (pfor interaction= 0.04, 0.03, and 0.01, respectively) (Supplementary Tables 1 and 2). Additionally, the indices reflecting sensitivity to thyroid hormone showed a significant association with eGFR in subjects with normal renal function, but there was no significance in subjects with reduced renal function (Supplementary Table 3). Furthermore, to examine the possible effects of diabetes or hypertension on renal function, sensitivity analyses were performed in non-diabetic and non-hypertensive subjects, respectively. The association between the sensitivity of thyroid hormone indices with eGFR and reduced renal function persisted within the non-diabetic population. The association in non-hypertensive subjects became non-significant for some indices, although the magnitudes for the ORs were close to the main sample (Supplementary Tables 4 and 5).
Discussion
In this population-based study, we found a relationship between indices measuring sensitivity to thyroid hormone and eGFR or the risk of reduced renal function in the euthyroid Chinese population. This correlation was most significant in subjects aged ≤65 years old, without diabetes or hypertension, or with normal renal function. In addition, we put forward the innovative TT3RI and PTFQIFT3 according to the established formulae and found that there was a relationship between TT3RI and renal function. These composite indices detected a stronger correlation between thyroid and renal dysfunction and provided a new thought for assessing thyroid function in the euthyroid population.
Given the effect of ageing, diabetes, and hypertension on renal function, we analyzed these specific subgroups. The relationships that we detected in the overall cohort were also observed in non-diabetic subjects and subjects ≤65 years old or with normal renal function. However, we failed to detect any significant correlation in the older group, which may be due to the insufficient sample size (n = 230) and the need for a lower threshold of eGFR to define a reduced renal function in the elderly. The relationship between these composite indices and reduced renal function in the subjects without hypertension was very close to the entire cohort, but the p-values were only marginally significant. The reduced renal function group was relatively small (n = 289 and 315 based on eGFRCKD-EPI and eGFRCr-CysC, respectively), which may have contributed to the lack of statistical significance.
The correlation between thyroid function and renal function remains a relevant topic as a consensus has not been reached despite much discussion in the literature from the past five years. Our results on the negative relationship between TSH and renal function were consistent with several previous findings [Citation9,Citation11,Citation14]. This observation is also supported by a study on urine albumin excretion that found higher TSH (even in the euthyroid range) was associated with a higher risk of microalbuminuria [Citation10]. Furthermore, in line with a Dutch study and a Mendelian randomization study, we did not find a significant relationship between FT4 and renal function [Citation13,Citation15]. Similar to the Dutch study, we are one of the few studies that have measured FT3, and both studies suggest a protective effect of FT3 on renal function in the euthyroid population [Citation15]. However, a Korean study reported that increased FT4 was associated with decreased eGFR, and the authors attributed it to functional hypothyroidism at the tissue level [Citation17]. In support of the Korean study is a Chinese study that did not find a significant relationship between FT3 and renal function in euthyroid participants, but observed that the risk of chronic kidney disease was 1.763-fold higher in the highest quartiles of FT4 compared with the lowest quartile [Citation16]. The race, age, and confounding factors adjustments in the Chinese cross-sectional study were similar to those in our study. All in all, these inconsistent results suggest that the dynamics of thyroid hormones with renal dysfunction in the euthyroid population require a new explanation.
The secretion of thyroid hormone is regulated by hypothalamic-pituitary-thyroid (HPT) axis. Thyrotropin-releasing hormone (TRH) from the hypothalamus promotes the synthesis and release of TSH from the anterior pituitary, which plays an important role in all stages related to the production and secretion of thyroid hormones from the thyroid gland. The levels of TRH and TSH are in turn modulated by the negative feedback of thyroid hormones. Thyroid hormones are mainly secreted in the form of T4, which is catalyzed by deiodinases to form the bioactive T3. Both will bind to carrier proteins in the circulation and enter cells via membrane transporters. T3 then further binds to the nuclear thyroid hormone receptors. Therefore, thyroid function is regulated by the HPT axis and other factors associated with thyroid hormone conversion and bioactivity [Citation26].
Given the complex interactions within the HPT axis, a single parameter may not reflect true thyroid status. Initial studies proposed TSHI and TT4RI to assess the central sensitivity of thyroid hormone [Citation19,Citation20]. The latest studies reported that obesity-related reduced sensitivity to thyroid hormone can be reversed after bariatric surgery-induced weight loss [Citation27]. Among type 2 diabetes patients, elevated TSHI and TT4RI were associated with an increased prevalence of kidney disorders [Citation28]. In 2019, Laclaustra et al. proposed a new index, PTFQI, that was thought to be more stable than TSHI and TT4RI. Their study showed the association of reduced sensitivity to thyroid hormone with obesity, metabolic syndrome, and diabetes even in the euthyroid population. They recommended that the new index can be used to identify reduced sensitivity to thyroid hormone [Citation18]. Recently, these indices were assessed in a Chinese euthyroid cohort study, which concluded that thyroid hormone sensitivity was associated with adipocyte fatty acid-binding proteins [Citation22].
Due to the inconsistencies in studies that employed simplified thyroid hormone measurements and the complex interactions in the HPT axis, we set out to explore the unsolved debate of the relationship between thyroid and renal function within the perspective of thyroid hormone sensitivity by using composite indices. Hence, in our study, the relationship between the thyroid and renal function was more stable when using composite sensitivity of thyroid hormone indices. Unlike the singular thyroid function parameters, we found consistent relationships between composite indices and reduced renal function, even when using different eGFR equations. In addition, these correlations were slightly stronger when using composite indices rather than using individual thyroid hormone parameters. For example, the OR for a reduced renal function was 1.30 versus 1.27 with every 1SD increase in TT4RI and TSH, respectively. Furthermore, we observed reduced values of FT3/FT4 and SPINA-GD in subjects with reduced renal function. These indices that contain FT3 can better reflect the peripheral action of thyroid hormones because the bioactive FT3 is converted from FT4 by deiodinases in the peripheral tissues and has a much higher affinity with thyroid receptors than T4. Therefore, reduced sensitivity to thyroid hormone at the level of deiodinases might be present in subjects with reduced renal function. Unfortunately, most of the previous studies lacked FT3 measurements, and hence the existing composite indices are all based on FT4. With the advantage of measuring FT3, we tried substituting FT3 into the existing formulae to obtain new indices, and excitingly, we obtained strong correlations. We hope that this pioneering attempt could prompt more detailed research in utilizing FT3.
In a previous study, a higher value of PTFQI indicated higher TSH than that expected for the actual FT4, indicating a lower sensitivity to FT4 [Citation18]. However, the reduced suppression of TSH may not only present a reduced sensitivity to thyroid hormone but also reflect an increased set-point of homeostasis, which is commonly observed in type 2 allostasis. Allostasis is defined as a dynamic response to maintain stability and consists of two types of allostatic load. Long-term type 2 allostatic load can contribute to obesity, hypertension, type 2 diabetes, and dyslipidemia [Citation29]. The metabolic disorders caused by type 2 allostatic load have a close connection with renal dysfunction. Therefore, reduced renal function may be due to type 2 allostasis, and metabolic diseases serve as intermediaries. Although we have adjusted for potential metabolic variables, we cannot completely rule out the lifestyle-related confounders. But we are assured of the results when the same conclusions were obtained in the subgroup analysis that excluded participants with diabetes or hypertension. Moreover, the production of TSH and thyroid hormones tend to be upregulated in type 2 allostasis [Citation29]. However, the subjects included in this study all had normal TSH and FT4 levels. Therefore, we believe that the elevated PTFQI in subjects with renal dysfunction mainly reflects reduced central sensitivity of thyroid hormone, but also, to a lesser extent, an increased set-point caused by type 2 allostatic load.
Past publications have evaluated in detail the potential mechanisms related to the connection between thyroid hormones and renal function. Thyroid hormones influence renal function by exerting effects on the following aspects: 1) system hemodynamics, including cardiac and vascular function, renin-angiotensin system and blood volume; 2) glomerular architecture, renal vasculature and the permeability of glomerular capillary; and 3) tubular function, for example, sodium and water homeostasis [Citation30]. The mechanisms underlying the association between sensitivity to thyroid hormone and renal function remain to be further explored. But the relative insufficiency of thyroid hormones caused by decreased thyroid hormone sensitivity might also affect renal function through the above mechanisms. Furthermore, TSH receptors are expressed in many tissues other than the thyroid, including the kidneys [Citation31]. This suggests that TSH may influence renal function by directly acting on renal cells or via indirect effects on systems other than the thyroid.
Renal dysfunction, in turn, interferes with the HPT axis and peripheral hormone metabolism. Previous studies have demonstrated that end-stage renal disease might alter TSH levels by dampening pituitary response to TRH, interfering with TSH diurnal rhythm and glycosylation, and reducing TSH clearance rate [Citation32]. Uraemic toxins and malnutrition also affect peripheral hormone metabolism by impairing the binding of T4 with carrier proteins, inhibiting cellular uptake of T4, and reducing the peripheral conversion from T4 to T3 [Citation32]. The previously detected positive relationship between creatinine clearance and SPINA-GT also points to the impairment of uraemic toxins on thyroid activity [Citation33]. And various conditions caused by kidney diseases (such as metabolic acidosis, trace element deficiencies, impaired clearance and retention of iodine, and vitamin D deficiency) and medications used in such patients are risk factors for thyroid dysfunction [Citation34,Citation35]. In addition, chronic kidney disease is a common pattern of non-thyroidal illness syndrome, which is characterized as decreased free or total T3 and impaired carrier protein binding to thyroid hormones. In severe cases, the set-point of the HPT axis is downregulated. TSH is reduced in this condition, even though the level of T4 is normal or decreased [Citation29,Citation36]. Others have also detected that a decreased blood urea after a period of low protein diet could improve the low T3 syndrome in renal dysfunction patients [Citation33]. This finding also suggests a negative effect of azotaemia on thyroid function.
Though this study is novel and has obtained consistently significant results between different indices and formulae, there are also some limitations. The first is the lack of data on medication and 2-hour post-load serum glucose levels in this study. Thus, the influence of residual confounding factors must be considered. In addition, reduced renal function outcome was based on measurements at a single time point. Therefore, we used both the eGFRCKD-EPI equation and the eGFRCr-CysC equation to mitigate this limitation and have obtained consistent results with either equation. Both of these equations have been previously confirmed to be suitable for the evaluation of the glomerular filtration rate in the Chinese population [Citation24]. Moreover, we defined reduced renal function as eGFR <90mL/min/1.73m2, which is useful for early detection and management of renal dysfunction. Lastly, due to the limitations of a cross-sectional study design, we failed to obtain causative association. Hence, more large longitudinal studies are needed to clarify the causal relationship.
In conclusion, we have found that reduced renal function was associated with decreased central sensitivity and reduced peripheral activity of thyroid hormones in the euthyroid population, particularly in those aged 65 years or under, without diabetes, or with normal renal function. Compared with individual parameters such as TSH or FT4, composite indices such as TFQI provided a more systemic and comprehensive way to evaluate thyroid homeostasis. And it was possible to create composite indices, such as TT3RI and PTFQIFT3, based on the interactions between FT3 and TSH. Future large prospective studies are needed to confirm these findings and more attention should be paid to the epidemiologic and mechanistic research in thyroid hormone sensitivity.
Author contributions
HG and WW contributed to the conception and design of the study. SY, SL, ZW and AL collected, analyzed, and interpreted the data. SY and SL drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Additional information
Funding
References
- Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733.
- Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–1304.
- Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):407–415.
- Huang X, Ding L, Peng K, et al. Thyroid hormones associate with risk of incident chronic kidney disease and rapid decline in renal function: a prospective investigation. J Transl Med. 2016;14(1):336.
- Chang YC, Chang CH, Yeh YC, et al. Subclinical and overt hypothyroidism is associated with reduced glomerular filtration rate and proteinuria: a large cross-sectional population study. Sci Rep. 2018;8(1):2031.
- Han Q, Zhang J, Wang Y, et al. Thyroid hormones and diabetic nephropathy: an essential relationship to recognize. Nephrology. 2019;24(2):160–169.
- Rhee CM, Kalantar-Zadeh K, Ravel V, et al. Thyroid status and death risk in US veterans with chronic kidney disease. Mayo Clin Proc. 2018;93(5):573–585.
- You AS, Sim JJ, Kovesdy CP, et al. Association of thyroid status prior to transition to end-stage renal disease with early dialysis mortality. Nephrol Dial Transplant. 2019;34(12):2095–2104.
- Peixoto de Miranda EJF, Bittencourt MS, Goulart AC, et al. Thyrotropin levels are associated with chronic kidney disease among healthy subjects in cross-sectional analysis of the Brazilian longitudinal study of adult health (ELSA-Brasil). Clin Exp Nephrol. 2017;21(6):1035–1043.
- Das G, Taylor PN, Abusahmin H, et al. Relationship between serum thyrotropin and urine albumin excretion in euthyroid subjects with diabetes. Ann Clin Biochem. 2019;56(1):155–162.
- Tanaka Y, Furusyo N, Kato Y, et al. Correlation between thyroid stimulating hormone and renal function in euthyroid residents of Japan: Results from the kyushu and Okinawa population study (KOPS). J Atheroscler Thromb. 2018;25(4):335–343.
- Toda A, Hara S, Kato M, et al. Association of thyrotropin concentration with chronic kidney disease in a Japanese general population cohort. Nephron. 2019;142(2):91–97.
- Ellervik C, Mora S, Ridker P, et al. Hypothyroidism and kidney function – a Mendelian randomization study. Thyroid. 2020;30(3):365–379.
- Schairer B, Jungreithmayr V, Schuster M, et al. Effect of thyroid hormones on kidney function in patients after kidney transplantation. Sci Rep. 2020;10(1):2156.
- Anderson JLC, Gruppen EG, van Tienhoven-Wind L, et al. Glomerular filtration rate is associated with free triiodothyronine in euthyroid subjects: comparison between various equations to estimate renal function and creatinine clearance. Eur J Intern Med. 2018;48:94–99.
- Wang K, Xie K, Gu L, et al. Association of thyroid function with the estimated glomerular filtration rate in a large chinese euthyroid population. Kidney Blood Press Res. 2018;43(4):1075–1083.
- Kim SH, Min HK, Lee SW. Relationship between thyroid and kidney function: analysis from the Korea National Health and Nutrition Examination Survey between 2013 and 2015. Kidney Blood Press Res. 2020;45(3):442–454.
- Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. 2019;42(2):303–310.
- Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH index. Clin Endocrinol. 2009;71(4):529–534.
- Yagi HP, Hayashi Y. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors b, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3*-triiodothyroinine binding affinity. J Clin Endocrinol Metabol. 1997;82(5):1608–1614.
- Dietrich JW, Landgrafe-Mende G, Wiora E, et al. Calculated parameters of thyroid homeostasis: emerging tools for differential diagnosis and clinical research. Front Endocrinol. 2016;7:57.
- Nie X, Ma X, Xu Y, et al. Increased serum adipocyte fatty acid-binding protein levels are associated with decreased sensitivity to thyroid hormones in the euthyroid population. Thyroid. 2020;30(12):1718–1723.
- Romagnani P, Remuzzi G, Glassock R, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088.
- Hu J, Xu X, Zhang K, et al. Comparison of estimated glomerular filtration rates in Chinese patients with chronic kidney disease among serum creatinine-, cystatin-C- and creatinine-cystatin-C-based equations: a retrospective cross-sectional study. Clin Chim Acta. 2020;505:34–42.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, et al. Hypothalamus-pituitary-thyroid axis. Compr Physiol. 2016;6(3):1387–1428.
- Juiz-Valiña P, Cordido M, Outeiriño-Blanco E, et al. Central resistance to thyroid hormones in morbidly obese subjects is reversed after bariatric surgery-induced weight loss. J Clin Med. 2020;9(2):359.
- Chen Y, Zhang W, Wang N. Thyroid parameters and kidney disorder in type 2 diabetes: results from the METAL study. J Diabetes Res. 2020;2020:4798947.
- Chatzitomaris A, Hoermann R, Midgley JE, et al. Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain. Front Endocrinol. 2017;8(163):163.
- Iglesias P, Bajo MA, Selgas R, et al. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord. 2017;18(1):131–144.
- de Lloyd A, Bursell J, Gregory JW, et al. TSH receptor activation and body composition. J Endocrinol. 2010;204(1):13–20.
- Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev. 1996;17(1):45–63.
- Rosolowska-Huszcz D, Kozlowska L, Rydzewski A. Influence of low protein diet on nonthyroidal illness syndrome in chronic renal failure. Endocrine. 2005;27(3):283–288.
- Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with hashimoto's thyroiditis and normal vitamin D status. Exp Clin Endocrinol Diabetes. 2017;125(4):229–233.
- Rhee CM. Thyroid disease in end-stage renal disease. Curr Opin Nephrol Hypertens. 2019;28(6):621–630.
- Fliers E, Boelen A. An update on non-thyroidal illness syndrome. J Endocrinol Invest. 2021;44(8):1597–1607.