Abstract
Introduction
Febrile neutropenia (FN) is one of the major complications with high mortality rates in cancer patients undergoing chemotherapy. The Multinational Association for Supportive Care in Cancer (MASCC) risk-index score has limited applicability for routine use in the emergency department (ED). This study aimed to develop simplified new nomograms that can predict 28-day mortality and the development of serious medical complications in patients with FN by using a combination of complete blood count (CBC) parameters with quick Sequential Organ Failure Assessment (qSOFA).
Methods
In this retrospective observational study, various models comprising qSOFA score and individual CBC parameters (red cell distribution width, delta neutrophil index, mean platelet volume (MPV)) were evaluated for association with outcomes by a multivariate logistic analysis. Subsequently, nomograms were developed for outcome prediction. The primary outcome was mortality at 28 days from ED presentation; the secondary outcome was the development of serious medical complications.
Results
A total of 378 patients were included. Among the CBC parameters, only MPV was significantly associated with 28-day mortality and serious medical complications in patients with FN. The nomogram developed to predict 28-day mortality and serious medical complications showed good discrimination with area under the receiver-operating characteristic curve (AUC) values of 0.729 and 0.862 (95% CI, 0.780–0.943), respectively, which were not different from those of the MASCC score (0.814, 95% CI, 0.705–0.922; p = .07 and 0.921, 95% CI, 0.863–0.979; p = .11, respectively) in the validation set. The calibration of both nomograms demonstrated good agreement in the validation set.
Conclusion
In this study, a novel prognostic nomogram using qSOFA score and MPV to identify cancer patients with FN with high risk of 28-day mortality and serious medical complications was verified and validated. Prompt management of fatal complications of FN can be possible through early prediction of poor outcomes with these new nomograms.
Among the evaluated CBC parameters, only mean platelet volume was associated with 28-day mortality and serious medical complications in cancer patients with febrile neutropenia.
A novel and rapid prognostic nomogram was developed using quick Sequential Organ Failure Assessment score and mean platelet volume to identify cancer patients with febrile neutropenia having high risk of 28-day mortality and serious medical complications.
The nomogram developed to predict 28-day mortality and serious medical complications in patients with febrile neutropenia showed good discrimination and provides rapid patient evaluation that is especially applicable in the emergency department.
KEY MESSAGES
Introduction
Febrile neutropenia (FN) is the major complications in cancer patients undergoing chemotherapy. Despite significant progress in the prevention and treatment of FN, the mortality rate in inpatients with FN ranges up to 20% [Citation1–2]. Early prediction of poor outcomes and application of broad-spectrum antibiotics are crucial for cancer patients with immune-compromised conditions, especially in the emergency department (ED), to manage fatal complications and achieve maximum benefit from the prescribed chemotherapy [Citation3–6].
Klastersky et al. developed the Multinational Association for Supportive Care in Cancer (MASCC) risk-index score in 2000 to recognise cancer patients with FN with a low risk of medical complications and who are therefore potentially suitable for outpatient clinic-based treatment [Citation7]. An MASCC risk-index score ≥21, which was based on clinical characteristics, was recommended as the threshold for low risk. This index has been validated by various studies and used as a basis for several guidelines [Citation8–10]. However, the MASCC risk-index score has limited utility for routine application in the ED, where patients with a high risk of complications and mortality should be identified [Citation11]. Moreover, its subjectivity could be an issue because the ‘burden of illness’ was evaluated by attending physicians using visual analogue scales that measure symptom severity and physiologic reserve [Citation7].
In 2016, the Sepsis-3 task force introduced the quick Sequential Organ Failure Assessment (qSOFA) score as a new method to identify among patients outside the intensive care unit (ICU) with suspected infection, those with a higher risk of poor outcomes. The qSOFA score can be easily obtained at the bedside to screen patients with infection who are likely to have a poor prognosis [Citation12–13]. Kim et al. reported the predictive role of qSOFA for patients with FN who were admitted to the ICU. They showed that the qSOFA score was an independent predictive factor to identify sepsis and ICU admission in multivariate analysis [Citation3]. However, the area under the receiver-operating characteristic curve (AUC) values of the qSOFA score for predicting sepsis, 28-day mortality, and ICU admission were 0.678, 0.651, and 0.715, respectively, which were inferior to those of the MASCC risk-index score (0.831, 0.856, and 0.835, respectively). Therefore, qSOFA alone may not be enough if it is not supported by other elements that increase the discriminative ability.
Furthermore, in the ED, promptly predicting deterioration of patients with FN using laboratory results such as the complete blood count (CBC) is difficult. Kim et al. introduced a scoring system to predict mortality in patients with sepsis and septic shock using red cell distribution width (RDW), delta neutrophil index (DNI), and platelet count [Citation14]. According to the study, the AUC of this scoring system was 0.785, whereas those of lactate and SOFA score were 0.724 and 0.738, respectively. Furthermore, mean platelet volume (MPV) is known as a marker of platelet function and activation, related to inflammation and disease activity of chronic inflammatory disorders [Citation15]. Another study showed that an increase in MPV during the first 72 h of admission was significantly associated with 28-day mortality in patients with sepsis and septic shock [Citation16]. However, no studies have been performed using these laboratory tests in patients with FN.
Thus, the objective of this study was to develop simplified nomograms that can predict the prognosis of patients with FN who underwent chemotherapy by using a combination of CBC parameters along with qSOFA. The nomogram is a useful method since its statistical predictions can be represented visually; furthermore, this is a more exact tool than the conventional prediction model using odds ratios [Citation17]. External validation of the nomogram was also performed.
Materials and methods
Study design and population
This study was a retrospective observational analysis of cancer patients aged over 18 years with FN who visited the ED in Severance Hospital, Yonsei University College of Medicine. FN was defined as a body temperature of over 38.0 °C with an absolute neutrophil count (ANC) of <500 cells/mm3 or a confirmed decrease in ANC from <1000 cells/mm3 to <500 cells/mm3 within 48 h [Citation18]. Patients who signed a ‘Do not attempt resuscitation’ order and refused to be treated in an ICU were excluded. We also excluded patients without outcome data. This study was approved by the Institutional Review Board of Yonsei University College of Medicine, Severance Hospital (no. 4-2020-0095). The requirement for individual consent from patients for using their data was waived because of the retrospective study design.
Data collection
The data of cancer patients admitted to the ED between May 2017 and August 2018 were retrieved retrospectively from the electronic medical record (EMR). The following data were obtained: past medical history, physical examination, vital signs at the ED visit, laboratory tests including CBC parameters, such as RDW, MPV, and DNI, possible infection sources, use of mechanical ventilation, use of vasopressors, application of continuous renal replacement therapy, ICU admission, and mortality. The MASCC and qSOFA scores were calculated using the available clinical information at the time of the ED visit. The primary outcome was mortality at 28 days from ED presentation. The secondary outcome was the development of serious medical complications, such as hypotension, respiratory failure, ICU admission, disseminated intravascular coagulation, confusion or altered mental state, congestive cardiac failure, bleeding severe enough to require transfusion, arrhythmia or ECG changes requiring treatment, or renal failure requiring investigation and/or treatment [Citation7]. The final outcome of each febrile neutropenic episode was considered as the development of serious medical complications [Citation18].
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R software version 3.4.4 for Windows (R foundation for statistical computing, Vienna, Austria). The results were presented as mean ± standard deviation (SD) or median (interquartile range) for continuous variables and frequencies (%) for categorical variables. For comparisons of continuous variables, we used independent two-sample t-tests if the data were normally distributed or the Mann–Whitney U test if the data were not normally distributed. The chi-squared test was used for categorical variables.
The study population was randomly allocated to either a training set or a validation set at a 7:3 ratio. In the training set, we created various types of combinations composed of qSOFA and individual CBC parameters such as MPV and then selected the models independently associated with primary or secondary outcomes by a multivariate logistic analysis method. Finally, the nomograms were developed for outcome prediction with the training set using variables included in the models. The performances of the nomograms were evaluated with respect to discrimination and calibration [Citation19]. The predictive accuracy (discrimination) of the models was assessed by using the AUC values with their 95% confidence intervals (CIs), which quantifies the level of concordance between the predicted probabilities and the actual chance of the event of interest occurring. The DeLong method was used to compare the AUC for the predictive value of the new nomogram with those of the qSOFA and MASCC risk-index scores regarding the 28-day mortality and development of serious medical complications in patients with FN. Calibration of the nomograms determines how far the predicted probabilities are from the observed outcome frequencies using graphic representations (calibration curve). A plot along the 45-degree line would indicate a perfect calibration model in which the predicted probabilities are identical to the actual outcomes. The calibration curves are presented as calibration plots using the bootstrapping methods with 200 re-samples. Thereafter, the predictive accuracy and calibration of the new nomograms generated with the training set were tested in the validation set. All reported p-values were 2-sided, and results were considered statistically significant at p < .05.
Results
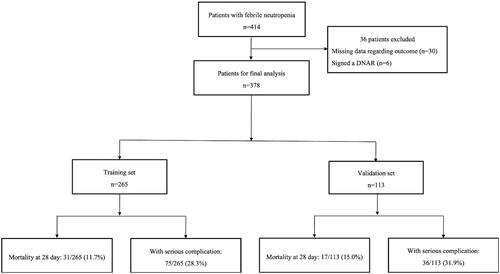
A total of 414 patient data, which showed FN after chemotherapy, were reviewed. Thirty patients whose outcome data were missing and six patients who signed a ‘Do not attempt resuscitation’ order were excluded. We included 378 patients in the final analysis, of whom 265 were randomly allocated to the training set and 113 to the validation set (). All variables except ANC values were not significantly different between the training and validation set.
28-Day mortality
In the training set, 31 out of 265 (11.7%) patients died within 28 days after the ED visit. The baseline characteristics are summarised in . Among the three CBC parameters, the MPV values of those who died were higher than those of survivors (10.0 ± 1.7 vs. 8.8 ± 1.4; p < .001), while DNI and RDW values showed no differences between the groups. Further, the qSOFA and MASCC scores were significantly different between the groups (1.0 ± 0.9 vs. 0.3 ± 0.5; p < .001 and 14.3 ± 5.3 vs. 20.0 ± 4.4; p < .001, respectively). All CBC parameters were considered while generating models to predict 28-day mortality in patients with FN. Each CBC parameter was entered individually into the multivariate logistic regression analysis along with qSOFA among the patients in the training set (models 1–3). summarises the results of the multivariate logistic regression analysis that revealed that only MPV was associated with 28-day mortality in patients with FN (p < .001).
Table 1. Patient demographics and clinical characteristics according to survival outcome at 28 days.
Table 2. Multivariate analysis predicting 28-day mortality and serious medical complications in patients with febrile neutropenia from the training set.
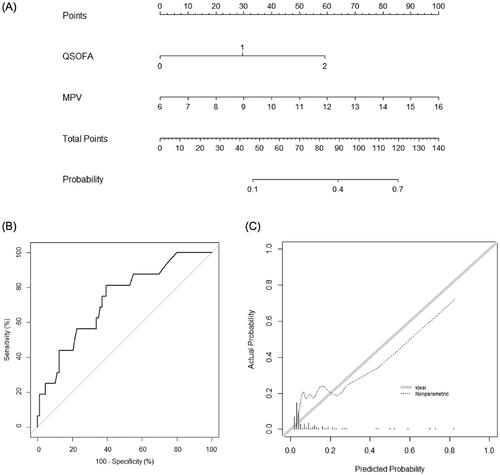
A new nomogram incorporating MPV and qSOFA score was established (); it illustrated that MPV was the largest contributor to prognosis in patients with FN. By calculating the total number of points and locating it on the total point scale, we were easily able to draw a straight line down to estimate the predicted probability of 28-day mortality. The AUC of the new nomogram was 0.834 (95% CI, 0.762–0.905), which was higher than that of the qSOFA score (0.718, 95% CI, 0.613–0.824; p = .002) and similar to that of the MASCC score (0.808, 95% CI, 0.718–0.898; p = .55). In the validation set, discrimination was good with an AUC value of 0.729 (95% CI, 0.598–0.861), which was not different to those of the qSOFA or MASCC scores (0.710, 95% CI, 0.580–0.841; p = .63 and 0.814, 95% CI, 0.705–0.922; p = .07, respectively) (). The calibration plot of the nomogram demonstrated a good agreement between the predicted and observed probabilities of survival discharge in the validation set ().
Figure 2. Nomogram for predicting 28-day mortality. (A) The novel nomogram for predicting 28-day mortality, developed using the training data set, is shown. (B) The discriminative ability of the nomogram is good, with an area under the receiver-operating characteristic curve of 0.729 (95% confidence interval: 0.598–0.861). (C) The calibration plot of the prediction model indicates good agreement between the predicted and observed probabilities of survival discharge. qSOFA: quick Sequential Organ Failure Assessment; MPV: mean platelet volume.
Serious medical complications
In the training set, 75 out of 265 patients (28.3%) experienced serious medical complications. The baseline characteristics and variables found to be associated with the development of serious medical complications are summarised in . Among the three CBC parameters, MPV and RDW, but not DNI, showed statistically significant differences between patients with and without serious medical complications (p < .001 and p = .01, respectively). The qSOFA and MASCC scores were also significantly different between the groups (1.0 ± 0.7 vs. 0.1 ± 0.3; p < .001, and 14.3 ± 4.5 vs. 21.3 ± 3.4; p < .001, respectively). Using multivariate logistic regression based on the combination of individual CBC parameters and the qSOFA scores, we found that MPV was associated with the occurrence of serious medical complications (p = .01), while RDW was not (p = .06) (). Similarly, we developed a new nomogram for predicting the development of serious medical complications (), wherein MPV was found to be the most contributing factor (). The AUC value of the nomogram was 0.894 (95% CI, 0.846–0.942). Further, in the validation set, discrimination was good with an AUC value of 0.862 (95% CI, 0.780–0.943), which was higher than that of the qSOFA score (0.814, 95% CI, 0.729–0.899; p = .03) and similar to that of the MASCC score (0.921, 95% CI, 0.863–0.979; p = .11) (). The calibration plot of the nomogram demonstrated good agreement between the predicted and observed probabilities of the development of serious medical complications in the validation set ().
Figure 3. Nomogram for predicting the development of serious medical complications. (A) The novel nomogram for predicting the development of serious medical complications, developed using the training data set, is shown. (B) The discriminative ability of the nomogram is good, with an area under the receiver-operating characteristic curve of 0.862 (95% confidence interval: 0.780–0.943). (C) The calibration plot of the prediction model indicates good agreement between the predicted and observed probabilities of the development of serious medical complications. qSOFA: quick Sequential Organ Failure Assessment; MPV: mean platelet volume.
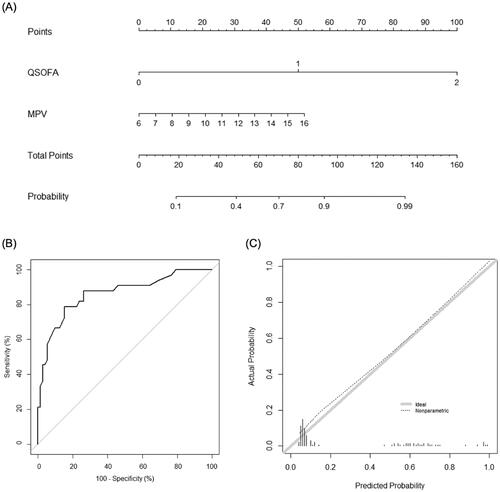
Table 3. Patient demographics and clinical characteristics associated with the development of serious medical complications.
Discussion
This study investigated the predictive performance of a new prognostic model developed using a combination of CBC parameters and the qSOFA score in FN to predict 28-day mortality and serious medical complications in cancer patients presenting at the ED. Although the MASCC risk-index score has been validated under various conditions [Citation20–22], it has limited utility especially in the ED due to its subjective approach in evaluating the ‘burden of illness’ and its nature of identifying mainly low-risk patients suitable for outpatient management [Citation23]. Thus, a rapid and objective risk stratification tool should be developed and applied for use in the ED. This study revealed that when MPV was added to the qSOFA score, contrary to other CBC parameters such as DNI and RDW, the predictive performance was better than that of the qSOFA score and similar to that of the MASCC score. The qSOFA scoring system, developed by the Sepsis-3 task force, is a simple bedside measure used to identify patients outside the ICU with suspected infection who may have poor outcomes. It contains three different variables: altered mental status, respiratory rate (RR) ≥22/min, and SBP ≤100 mmHg. Patients showing at least two of these criteria were considered more likely to have sepsis [Citation24]. It showed a discriminative ability superior to that of the systemic inflammatory response syndrome (SIRS) criteria for predicting mortality in patients with sepsis [Citation13]. Kim et al. revealed that the qSOFA score showed superior discriminative ability to SIRS criteria, but inferior to the MASCC score in predicting sepsis, 28-day mortality, and ICU admission in patients with FN [Citation3]. Therefore, an additional variable is needed to improve the performance of qSOFA in predicting poor outcomes of FN.
Furthermore, although several studies highlighted the utility of laboratory results as a predictive factor [Citation25–26], no studies have used CBC parameters to predict the prognosis of patients with FN. A new prognostic model using CBC parameters, which are rapidly and cost-effectively measured, is reasonable for use in the EDs in general. MPV are not only an indicator of haemostasis and thrombosis but are also thought to play a critical role in inflammatory responses [Citation27–29]. Platelets are known to change their structure and function from inactive to active platelets owing to physiologic signals [Citation30]. As MPV reflects platelet size and activity, which are associated with inflammatory and thrombotic processes, it can be a marker of platelet activation [Citation31]. Several studies suggest that elevated MPV is associated with an increased risk of mortality in critically ill patients [Citation32–33]. Our study, conducted in the EDs, showed that a new prognostic model that included MPV with qSOFA revealed a poor prognosis of patients with FN.
Cancer patients receive platelet concentrate transfusion to manage disease course for several reasons since low platelet counts frequently lead to bleeding complications. A platelet concentrate transfusion might affect CBC parameter and platelet indices. In this study, 77/265 patients in the training set and 28/113 patients in the validation set received platelet concentrate transfusion within 1 week of FN encounter. MPV were 9.751 ± 1.717 and 8.590 ± 1.194 in patients who received platelet concentrates transfusion and who did not, respectively, in the training set (p = <.001). In the validation set, MPV were 9.748 ± 2.508 and 8.567 ± 1.142 in patients who received platelet concentrate transfusion and who did not, respectively (p = .025). To identify the difference in the predictive performance of the nomogram to predict 28-days mortality depending on the administration of platelet concentrate transfusion, we performed AUC subgroup comparison. The AUC of the nomogram for the subgroup with platelet concentrate transfusion was 0.795 (98% CI 0.664–0.927), while for the subgroup without transfusion was 0.844 (95% CI 0.753–0.934). There was no statistically significant difference between the two subgroups (p = .55), and no significant difference was observed between the entire cohorts and each subgroup with and without transfusion (p = .57, 0.83, respectively). Likewise, for AUC comparing the predictive performance of the nomogram of platelet concentrate transfusion administration on serious medical complications, the nomogram for the platelet concentrate transfusion subgroup was 0.837 (95% CI 0.734–0.941), while the subgroup without transfusion was 0.913 (95% 0.856–0.970). There was no statistically significant difference in the two subgroups (p = .21), and no significant difference was shown between the entire cohorts and each subgroup with and without transfusion (p = .28, 0.51, respectively). Therefore, the performance of nomogram to predict adverse outcome of FN was not associated with platelet concentrates transfusion, though MPV might be affected by platelet concentrates transfusion.
Elevated MPV was related to decreased renal function in a previous study, although the mechanism of MPV change in patients with decreased renal function is not fully understood. Ucar et al. suggested that the shortened lifespan of platelets in uraemic conditions could have stimulated platelet production [Citation34]. Thus, the correlation between the estimated glomerular filtration rate (eGFR) and MPV was analysed, and the results showed a negligible correlation between eGFR and MPV (for eGFR (MDRD); R = −0.224, for eGFR (CKD-EPI); R = −0.257, all p < .001) [Citation35]. Therefore, the difference in patients’ eGFR in this study was not considered to affect MPV value significantly.
Moreover, cancer types can be important in predicting prognosis of FN when MPV is used as a predictive factor. Previous studies identified the role of MPV in predicting disease course in various types of malignancy. MPV has prognostic value in many types of solid tumours and haematologic malignancies [Citation36–39]. However, it is difficult to identify the different MPV impacts and mechanisms of action on the prognosis for each cancer type. In this study, for patients with solid tumours, the AUC of the nomogram to predict 28-days mortality was 0.862 (95% CI 0.787–0.936), which was not statistically different from the nomogram for the entire cohort [0.834 (95% CI 0.762–0.905), p = .46]. Likewise, for patients with haematologic malignancies, the AUC of the monogram was 0.759 (95% CI 0.614–0.905) and was not statistically different from the entire cohort’s nomogram (p = .32). For predicting serious medical complications development, the AUC of the monogram was 0.927 (95% CI 0.876–0.979) in the subgroup with solid tumours, which was not significantly different from the nomogram derived from the entire cohort [0.894 (0.846–0.942), p = .20]. Moreover, for patients with haematologic malignancies, the AUC of the monogram was 0.817(0.703–0.930), which was not different from the nomogram for entire cohort (p = .18). Although the nomogram developed in this study seemed to show a slightly better performance in predicting adverse outcome of FN in patients with solid tumours, the prognostic performance of the nomogram for the solid tumour’s subgroups and haematologic malignancies was not statistically different (28-days mortality; p = .09, serious medical complication; p = .16). In most solid tumours, the association between platelet sizes and prognosis is explained by activated platelets releasing chemokines, proteolytic factors, and growth factors for growth and tumour invasion. In addition, platelets are involved in tumour cells aggregation, which protects them from the immunologic system [Citation40, Citation41]. Conversely, in haematological malignancy, MPV is more likely to associate with the extent of inflammation [Citation42]. Cornillie et al. suggested that the release of platelets with small sizes from the bone marrow with haematologic malignancy increases the secretion of pro-inflammatory cytokines, which hinders megakaryopoiesis activity and accelerates activated platelets consumption [Citation43]. Thus, one explanation for the different model performances is that several platelet mechanisms play a slightly different role in each cancer type.
The major advantage of the prognostic nomogram developed in our study compared to the MASCC or other models from previous study is the easier accessibility owing to both simple laboratory results and bedside patient evaluation. Previous studies revealed that several biomarkers could be a prognostic factor to predict outcomes of FN. According to Combariza et al., C-reactive protein (CRP) added to the MASCC model was useful in predicting mortality of FN in haematological malignancies [Citation44]. Moreover, Ahn et al. introduced a new prognostic model including procalcitonin as prognostic factor of FN, which classifies patients for poor outcomes and bacteraemia. The model showed higher specificity and negative predictive values than the MASCC model [Citation23]. In this study, the CRP of those who died within 28 days was higher than survivors (210.6 ± 113.8 vs. 96.6 ± 95.5; p < .001), and procalcitonin of those who died was also higher than survivors (22.4 ± 34.8 vs. 3.9 ± 13.5; p = .02). Moreover, CRP and procalcitonin also varied significantly between patients with and without serious medical complications (159.6 ± 123.1 vs. 90.3 ± 88.8; p < .001, and 14.2 ± 28.0 vs. 1.5 ± 6.3; p < .001, respectively). In this study, we developed a simple model for rapid prediction of poor outcome of FN at ED presentation. CBC is rapid, easily accessible, cost-effective, and repeatable. CBC parameters such as MPV as a prognostic factor would allow a quick assessment of the patient at the time of diagnosis at the ED since CBC parameters can be obtained without any additional laboratory test in most hospitals. Besides, qSOFA was used as the baseline tool for evaluating FN patients since qSOFA is one of the easiest methods at the bedside. The nomogram provides rapid patient evaluation that is especially applicable in the ED, and its discriminative ability was shown to be accurate enough to be approximate to the MASCC risk-index score statistically.
This study has some limitations. First, as this study is retrospective in nature, selection bias may have distorted the results. Second, generalisability of the study may be restricted due to the enrolment of patients in a single centre. Third, we did not analyse the effect of different regimens and the intensity of chemotherapy on the outcome, and these conditions could be possible confounders. Thus, further research is needed to identify the association between these factors and the prognostic performance of the nomogram.
In this study, we have verified and validated a novel and rapid prognostic nomogram to identify cancer patients with FN who have a high risk of 28-day mortality and serious medical complications. Early and accurate prediction enables prompt management of fatal complications of chemotherapy through close monitoring and rapid application of proper treatments such as antibiotics and source management. Furthermore, the accurate disposition of patients can be possible, particularly in ED. External validation and prospective multicentre studies are required to confirm the performance of our nomogram for future research.
Author contribution
AC, MJK, and YSP conceived and designed the study. MJK and YSP supervised conduction of the study and data collection. AC, IP, and JC managed the data, including quality control. HSL provided statistical advice on the study design and analysed the data. AC and YSP drafted the manuscript, and all authors contributed substantially to its revision. MJK and YSP share equal overall responsibility for the study and the manuscript.
| Abbreviations | ||
| ANC | = | absolute neutrophil count |
| AUC | = | receiver-operating characteristic curve |
| BT | = | body temperature |
| CBC | = | complete blood count |
| CI | = | confidence interval |
| CRP | = | C-reactive protein |
| DNAR | = | Do not attempt resuscitation |
| DNI | = | delta neutrophil index |
| ED | = | emergency department |
| eGFR | = | estimated glomerular filtration rate |
| EMR | = | electronic medical record |
| FN | = | febrile neutropenia |
| ICU | = | intensive care unit |
| MASCC | = | the Multinational Association for Supportive Care in Cancer |
| MPV | = | mean platelet volume |
| OR | = | odds ratio |
| PR | = | pulse rate |
| qSOFA | = | quick Sequential Organ Failure Assessment |
| RDW | = | red cell distribution width |
| RR | = | respiratory rate |
| SBP | = | systolic blood pressure |
| SD | = | standard deviation |
| WBC | = | white blood cell |
Data availability statement
The data acquisition of the present study was approved by the Ethics Committee of Severance Hospital. Datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
The authors report no conflicts of interest.
References
- Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266.
- Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2008;6(2):122–174.
- Kim M, Ahn S, Kim WY, et al. Predictive performance of the quick sequential organ failure assessment score as a screening tool for sepsis, mortality, and intensive care unit admission in patients with febrile neutropenia. Support Care Cancer. 2017;25(5):1557–1562.
- Schilling MB, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exp Ther Med. 2011;2(5):859–866.
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–237.
- Taj M, Nadeem M, Maqsood S, et al. Validation of MASCC score for risk stratification in patients of hematological disorders with febrile neutropenia. Indian J Hematol Blood Transfus. 2017;33(3):355–360.
- Klastersky J, Paesmans M, Rubenstein EB, et al. The multinational association for supportive care in cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18(16):3038–3051.
- Worth LJ, Lingaratnam S, Taylor A, Australian Consensus Guidelines 2011 Steering Committee, et al. Use of risk stratification to guide ambulatory management of neutropenic fever. Australian consensus guidelines 2011 steering committee. Intern Med J. 2011;41(1b):82–89.
- de Naurois J, Novitzky-Basso I, Gill MJ, ESMO Guidelines Working Group, et al. Management of febrile neutropenia: ESMO clinical practice guidelines. Ann Oncol. 2010;21 Suppl 5:v252–6.
- Freifeld AG, Bow EJ, Sepkowitz KA, Infectious Diseases Society of America, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–e93.
- Klastersky J, Paesmans M. The multinational association for supportive care in cancer (MASCC) risk index score: 10 years of use for identifying low-risk febrile neutropenic cancer patients. Support Care Cancer. 2013;21(5):1487–1495.
- Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014;90(3):190–199.
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). Jama. 2016;315(8):762–774.
- Kim YC, Song JE, Kim EJ, et al. A simple scoring system using the red blood cell distribution width, Delta neutrophil index, and platelet count to predict mortality in patients with severe sepsis and septic shock. J Intensive Care Med. 2019;34(2):133–139.
- Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44(8):805–816.
- Kim CH, Kim SJ, Lee MJ, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One. 2015;10(3):e0119437.
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370.
- Baskaran ND, Gan GG, Adeeba K. Applying the multinational association for supportive care in cancer risk scoring in predicting outcome of febrile neutropenia patients in a cohort of patients. Ann Hematol. 2008;87(7):563–569.
- Yoon JH, Lee HS, Kim E, et al. A nomogram for predicting malignancy in thyroid nodules diagnosed as atypia of undetermined significance/follicular lesions of undetermined significance on fine needle aspiration. Surgery. 2014;155(6):1006–1013.
- Uys A, Rapoport BL, Anderson R. Febrile neutropenia: a prospective study to validate the multinational association of supportive care of cancer (MASCC) risk-index score. Support Care Cancer. 2004;12(8):555–560.
- Uys A, Rapoport BL, Fickl H, et al. Prediction of outcome in cancer patients with febrile neutropenia: comparison of the multinational association of supportive care in cancer risk-index score with procalcitonin, C-reactive protein, serum amyloid A, and interleukins-1beta, -6, -8 and -10. Eur J Cancer Care. 2007;16(6):475–483.
- Klastersky J, Paesmans M, Georgala A, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol. 2006;24(25):4129–4134.
- Ahn S, Lee YS, Lee JL, et al. A new prognostic model for chemotherapy-induced febrile neutropenia. Int J Clin Oncol. 2016;21(1):46–52.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama. 2016;315(8):801–810.
- Liu X, Wang DF, Fang Y, et al. Initial procalcitonin level predicts infection and its outcome in patients with non-Hodgkin lymphoma with febrile neutropenia. Leuk Lymphoma. 2015;56(1):85–91.
- Shaikh AJ, Bawany SA, Masood N, et al. Incidence and impact of baseline electrolyte abnormalities in patients admitted with chemotherapy induced febrile neutropenia. J Cancer. 2011;2:62–66.
- Sezgi C, Taylan M, Kaya H, et al. Alterations in platelet count and mean platelet volume as predictors of patient outcome in the respiratory intensive care unit. Clin Respir J. 2015;9(4):403–408.
- Semple JW, Italiano JE, Jr., Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274.
- Vieira-de-Abreu A, Campbell RA, Weyrich AS, et al. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34(1):5–30.
- Shah B, Valdes V, Nardi MA, et al. Mean platelet volume reproducibility and association with platelet activity and anti-platelet therapy. Platelets. 2014;25(3):188–192.
- Thompson CB, Eaton KA, Princiotta SM, et al. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50(3):509–519.
- Oh GH, Chung SP, Park YS, et al. Mean platelet volume to platelet count ratio as a promising predictor of early mortality in severe sepsis. Shock. 2017;47(3):323–330.
- Taskesen T, Sekhon H, Wroblewski I, et al. Usefulness of mean platelet volume to predict significant coronary artery disease in patients with Non-ST-Elevation acute coronary syndromes. Am J Cardiol. 2017;119(2):192–196.
- Ucar H, Gur M, Koyunsever NY, et al. Mean platelet volume is independently associated with renal dysfunction in stable coronary artery disease. Platelets. 2014;25(4):274–278.
- Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71.
- Kılınçalp S, Ekiz F, Başar O, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25(8):592–594.
- Sakin A, Secmeler S, Arici S, et al. Prognostic significance of mean platelet volume on local advanced Non-Small cell lung cancer managed with chemoradiotherapy. Sci Rep. 2019;9(1):3959.
- Baldane S, Ipekci SH, Sozen M, et al. Mean platelet volume could be a possible biomarker for papillary thyroid carcinomas. Asian Pac J Cancer Prev. 2015;16(7):2671–2674.
- Masternak M, Puła B, Knap J, et al. Mean platelet volume has prognostic value in chronic lymphocytic leukemia. Cancer Manag Res. 2020;12:9977–9985.
- Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–3436.
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590.
- Ghia P, Chiorazzi N, Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Intern Med. 2008;264(6):549–562.
- Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63(11):1721–1727.
- Combariza JF, Lombana M, Pino LE, et al. C-reactive protein and the MASCC risk index identify high-risk patients with febrile neutropenia and hematologic neoplasms. Support Care Cancer. 2015;23(4):1009–1013.