Abstract
Background
Breast cancer survivors may be at risk of experiencing rotator cuff disease after treatment. Biomechanical alterations following surgery potentially predispose survivors to develop this disorder.
Objective
To examine scapular kinematics in breast cancer survivors with and without impingement pain during an overhead reach task.
Design
A cross-sectional study.
Methods
Three surgery groups were included: non-cancer controls, mastectomy-only survivors and post-reconstruction survivors. Breast cancer survivor groups were also categorized by the presence of impingement pain. Scapular motion was tracked during an overhead reach task, performed separately by both arms. Maximum scapular internal rotation, upward rotation and tilt were calculated. Two-way analyses of variance with interactions (p < .05) were used to test the effects of group (control, mastectomy-only, reconstruction) and impingement pain (pain, no pain) on each variable within a (left/right) side.
Results
Scapular kinematics varied with the group by pain interaction. On the right side, the mastectomy-pain group had reduced upward rotation, while the reconstruction-pain group had higher upward rotation (mastectomy-only: 22.9° vs. reconstruction: 31.2°). On the left side, the mastectomy-pain group had higher internal rotation, while the reconstruction-pain group had reduced internal rotation (mastectomy-only: 45.1° vs. reconstruction: 39.3°). However, time since surgery was longer in the mastectomy-pain group than reconstruction-pain group, suggesting there may be a temporal component to kinematic compensations.
Conclusions
There are kinematic alterations in breast cancer survivors that may promote future development of rotator cuff disease. Compensations may begin as protective and progress to more harmful alterations with time.
Scapular kinematics varied with surgery and pain interaction: upward rotation was lower and internal rotation higher in mastectomy-pain group, while upward rotation was higher and internal rotation lower in reconstruction-pain group.
Kinematics alterations may also be associated with time since surgery, as the mastectomy-pain group had longer time since surgery than the reconstruction-pain group.
Kinematic alterations may transition from protective to harmful over time.
In-depth analyses by reconstruction type are needed to determine surgery-specific effects on kinematics and their potential impact on the development of rotator cuff disease.
KEY MESSAGES
Introduction
Upper limb morbidities are common after breast cancer treatment. Limitations such as reduced range of motion, reduced strength, swelling or loss of sensation are commonly reported for several years after treatment [Citation1,Citation2]. Additionally, breast cancer survivors may be more likely to have secondary upper limb conditions [Citation3–5], possibly as a result of the side effects of treatment or secondary to the above-mentioned limitations. One secondary morbidity that breast cancer survivors may experience is rotator cuff disease [Citation3,Citation6]. Rotator cuff disease, which for the purpose of this paper will encompass all tendinitis, tendinopathy and tears of the rotator cuff, is associated with upper limb disability [Citation7]. For breast cancer survivors who may already experience physical limitations after cancer treatment, pain and reduced range of motion associated with rotator cuff disease [Citation8] could lead to additional limitations that interfere with activities of daily living and occupational tasks if left untreated. Although this relationship is posited, there is actually very little research specifically on rotator cuff pathology among breast cancer survivors.
Rotator cuff disease is most often a repetitive strain injury, with onset due to overuse and cumulative damage to the rotator cuff tendons [Citation9]. Shoulder biomechanics are considered to play an important role in the development rotator cuff disease, with the injury often initially appearing as supraspinatus impingement [Citation10]. Both humeral and scapular kinematics may contribute to impingement, with the accepted notion that altered kinematics may reduce the subacromial space, causing repeated damage to the bursal side of the tendon [Citation9]. Humeral kinematic changes are often a result of fatigue and lack of endurance of the rotator cuff muscles causing the humeral head to migrate upwards [Citation11], while scapular kinematic changes, often termed “dyskinesis”, can manifest in altered scapular motion in all scapular angles. Reduced upward rotation and increased scapular internal rotation are the common alterations observed in persons with rotator cuff disease [Citation12,Citation13]. However, results are inconsistent [Citation12,Citation14,Citation15] and it is not clear if the scapular kinematic alterations are the cause or result of the injury and pain.
Post-treatment kinematic compensations may contribute to rotator cuff disease in breast cancer survivors. Recent investigations of scapular kinematics in breast cancer survivors are sparse, and there is study heterogeneity in terms of motions evaluated, treatment types and timing of measurements. However, in aggregate, the results suggest that the observed kinematic alterations are harmful and are consistent with those associated with rotator cuff disease [Citation16–19]. In particular, a recent study by our research group showed upper limb kinematics in mastectomy-only breast cancer survivors were markedly influenced by the presence of impingement-related pain: scapular upward rotation in overhead movements was reduced only in the pain group [Citation17]. It was hypothesized that the reduced upward rotation contributed to the pain and may lead to further injury and disability, but with a small, cross-sectional sample including only one surgery type, the applications of results are limited.
While the combination of mastectomy and impingement pain appears to be associated with potentially harmful kinematics, there are few investigations of breast cancer survivor biomechanics after mastectomy plus reconstruction (termed “reconstruction” in this paper) [Citation18,Citation20,Citation21]. Findings from our research group suggest that reconstruction breast cancer survivors have reduced humeral internal rotation and anterior tilt during functional tasks [Citation20], but that study did not consider impingement-related pain. Therefore, the purpose of this study was to measure scapular kinematics of reconstruction breast cancer survivors, with and without impingement pain, during an overhead reaching task and compare them to mastectomy-only survivors, with and without impingement pain and non-cancer controls. It was hypothesized that the breast cancer survivors with pain would have decreased upward rotation [Citation17].
Methods
This study is a continuation of a previously published report on shoulder kinematics in mastectomy-only breast cancer survivors [Citation17]. In the current manuscript, the same mastectomy-only breast cancer survivors, divided by impingement pain and controls are compared to a newly assessed group: reconstruction breast cancer survivors, divided by impingement pain. Findings regarding reconstruction kinematics, without consideration of impingement pain, have been reported in another publication [Citation20].
Participants
Breast cancer survivors and non-cancer controls were recruited from the community via posters in medical and community centres, advertisements on social media and word of mouth from clinicians and support groups. All breast cancer survivors were required to have undergone mastectomy, and women with one of the three types of post-mastectomy reconstructions were also included (subpectoral implants (implants), deep inferior epigastric perforator (DIEP) and latissimus dorsi flap (LD)). Breast cancer survivors were at least six months post their latest surgery. Members of the control group were free from any upper limb pain and all participants were women between the ages of 35 and 65. Exclusion criteria included breast conserving therapy, inability to raise arms overhead and allergies to adhesives. Our goal was to recruit 25 participants for each group (control, mastectomy, implants, DIEP and latissimus dorsi); however, as data collection had to be suspended indefinitely due to the COVID-19 pandemic, the 45 breast cancer survivors with reconstructions were grouped together. The sample size was originally derived from our previous study [Citation17] that focussed on mastectomy-only breast cancer survivors. The study protocol was approved by the university ethics board.
Procedures
Upon arrival, participants provided informed consent and completed a disability questionnaire (QuickDASH). Participants also provided written consent for inclusion of anonymized photos in research materials. Treatment information was provided from participant recall. Each participant was then evaluated with three impingement provocation tests: Neer’s sign, Hawkins-Kennedy and empty can [Citation22,Citation23]. A positive result (meaning presence of pain) on any test warranted exclusion from the control group or placement in the pain group for the breast cancer survivors. Motion of the torso, scapulae and humeri were tracked via reflective markers affixed to the skin, where possible, or to fitted clothing (torso cluster) based on ISB standards [Citation24], with 8 MX 20 and 2 Vantage Vicon cameras (Vicon Motion Systems, Oxford, UK) that were placed around the collection space to maximize tracking of movement in all planes of motion. The scapulae were tracked with acromial marker clusters using a double calibration; the accuracy of this method has been found to be in the accepted error range (4 to 10° depending on the scapular angle and level of humeral elevation) [Citation25].
Participants then performed an overhead reaching task. This required participants to reach to a shelf set to 1.5 m above the ground, centred in front of their body, while seated [Citation17,Citation26]. The reach was performed separately by the right and left arms, both unloaded () and with a 1 kg load. Each combination (right/left, loaded/unloaded) was performed three times, for a total of 12 reaches per participant.
Analysis
Kinematic data were processed with a custom MATLAB® code. All raw kinematic data were filtered with a low pass zero-lag fourth order Butterworth filter with a 6 Hz cut-off [Citation27]. Scapular rotations were calculated with the joint coordinate system method using a Y-X’-Z” rotation sequence to acquire clinically relevant angles: internal/external rotation, upward/downward rotation and anterior/posterior tilt, again conforming to International Society of Biomechanics standards [Citation24]. Upward rotation of the right scapula and internal rotation of the left scapula were adjusted to be positive values. Each repetition of the overhead reach was one cycle. Maximum scapular angles were calculated for each cycle and all repetitions were averaged within participants.
All breast cancer survivors with any type of reconstruction were grouped into one reconstruction group (n = 45: 19 implant, 16 DIEP and 10 LD). Affected shoulders were coded per side, and breast cancer survivor shoulders that did not have any type of surgery were excluded from the analysis. For example, if a participant had a reconstruction on the right side but no surgery on the left side, data from their right side were included in the reconstruction group and compared to right sides of other groups. Data from their left side were not included in any comparisons. Forty-three survivors (33 reconstruction, 10 mastectomy-only) had bilateral procedures. If a participant had bilateral procedures, data from both sides were included and compared to respective sides of other groups, but not within a person. Sides were analysed separately based on results of a previous mastectomy-only comparison showing that only right-side scapular kinematics were affected by presence of impingement pain [Citation17] and to circumvent within-person dominance effects. All but two participants were right-hand dominant (one control participant and one mastectomy-only participant). The mastectomy-only and reconstruction groups were then divided by the presence of impingement pain (positive pain result on at least one test) to create five groups: control, mastectomy-no pain, mastectomy-pain, reconstruction-no pain and reconstruction-pain. There were no differences in kinematics between loaded and unloaded conditions, so all right reaches were grouped together and all left reaches were grouped together.
Statistical analysis was performed with SPSS Statistics for Windows, version 27 (SPSS Inc, IMB, Chicago, IL, USA). Two-way analyses of variance (ANOVAs) (group × pain) were used to determine main and interaction effects on each variable for each side. Post hoc Tukey honestly significant differences were used to identify differences between groups after a significant result from the ANOVAs. Effect sizes were calculated for significantly different groups using Cohen’s d: where 0.2 is considered a small effect size, 0.5 considered medium and 0.8 or above is considered large and clinically meaningful [Citation28].
Results
Impingement pain and breast cancer treatment group interacted to influence scapular kinematics in the overhead reach, but differentially bilaterally. On the right side, scapular upward rotation was affected: the mastectomy-pain group had significantly lower peak upward rotation (max difference = 8.3°, F = 13.1, p <.001, d = 1.3) compared to controls, the mastectomy-no pain, and the reconstruction-pain (). On the left side, internal rotation was affected: the reconstruction-pain group had lower peak scapular internal rotation (max difference = 5.8°, F = 13.4, p = .010, d = 0.9) compared to the reconstruction-no pain and mastectomy-pain groups (). Overall, the survivors with impingement pain in the mastectomy group had lower upward rotation and higher internal rotation than those without pain, while the opposite pattern characterized the reconstruction group (). While these left scapular internal rotation results demonstrated p values below .05, the homogeneity of variance and normality assumptions were violated for the ANOVAs. These findings were included, still using this analysis, due to the exploratory nature of this study and the pattern of differences that corresponded with the right upward rotation results. Further explanation of the statistical assumptions and violations is included in Supplementary material. There were no significant interaction or main effects for scapular anterior/posterior tilt on either side. Although the three different types of reconstructions were grouped for statistical analysis, right upward rotation and left internal rotation results are also presented with the three types separated to demonstrate the trends across reconstruction types ().
Figure 2. Interaction plot of scapular kinematics for the pain and no pain groups. Upward rotation values are from the right side, while internal rotation values are from the left side. For the mastectomy group, those with pain had lower upward rotation and higher internal rotation. The opposite pattern was found in the reconstruction group.
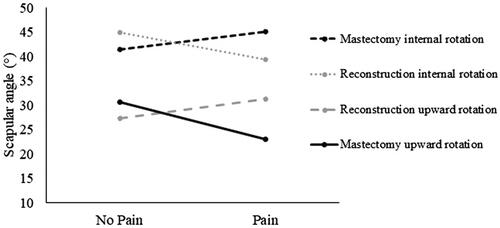
Figure 3. Peak right upward rotation (top) and left internal rotation (bottom) presented with reconstruction groups split by surgery types and pain status. Reconstruction types were grouped together for analysis due to small sample sizes for each type. Error bars represent standard deviation. Mastectomy: mastectomy-only; implant: subpectoral implant; DIEP: deep inferior epigastric perforator; LD: latissimus dorsi flap.
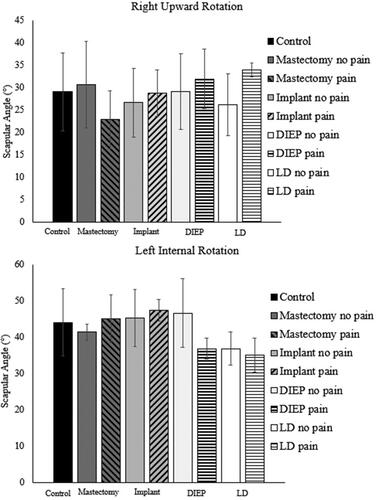
Table 1. Participant characteristics [mean (95% CI)] for all five combinations of breast cancer treatment and presence of pain.
Time since surgery was also different for the different surgery and pain combinations (). The mastectomy-pain group had the longest time since surgery, while the reconstruction-pain group had the shortest. Finally, there was also an interaction effect of impingement pain and breast cancer surgery on self-reported disability (QuickDASH) (F = 24.6, p < .001, d = 1.4), which was highest in the survivors with impingement pain in both the mastectomy and reconstruction groups ().
Discussion
This study of impingement pain in breast cancer survivors provides unprecedented insight into treatment and biomechanical factors that may be related to injury development. The mastectomy-only and reconstruction groups exhibited opposing kinematic compensations, with the mastectomy group demonstrating lower scapular upward rotation and higher internal rotation on the right and left side, respectively, and reconstruction group displaying the opposite pattern. Along with surgery type, there is a possibility that time since surgery plays an important role in shoulder kinematic changes in breast cancer survivors.
Breast reconstruction, while invasive and intensive procedures that can cause significant shoulder impairment [Citation20,Citation21,Citation29], may help to prevent rotator cuff disease. Findings from our recent work looking at survivors after reconstruction suggest that, when not divided by impingement pain, kinematic alterations after breast reconstruction might reduce risk of rotator cuff disease. Specifically, humeral internal rotation and scapular anterior tilt are both consistently reduced in the reconstruction group [Citation20]. The hypothesis is that the detrimental effects to the humeral internal rotators (pectoralis major and latissimus dorsi) and strength deficits after surgery could contribute to the kinematic compensations favourable for rotator cuff disease prevention. As a result, it is possible that the protective kinematics present in the reconstruction-pain group occur partially as a result of the reconstruction surgeries. When considering the descriptive results of pain by reconstruction type, findings suggest that implants may result in the more harmful kinematic strategies (less upward rotation and more internal rotation), regardless of pain status, while DIEP and LD groups are skewed to the more protective strategies. However, a higher proportion of both DIEP and LD reconstruction survivors displayed impingement-related pain: the removal of the pectoralis major sternocostal muscle fibres during implant surgery could reduce superior translation of the humerus during arm elevation, reducing compression of the supraspinatus tendon in the implant reconstructions. Taken together, the findings further highlight need for kinematic evaluations with homogenous breast reconstruction groups.
Time since surgery was notably different between the groups and may contribute to observed differences. The mastectomy-pain group had their surgery approximately three years earlier than the reconstruction-pain group. The difference between these groups could be a result of 2 main factors in this sample: first, the reconstruction-pain group was, on average, eight years younger than the mastectomy-pain group, and second, some reconstruction procedures were delayed as opposed to immediate (the average delay was just under three years). Time since last surgery may mediate rotator cuff disease related compensations. For both sides, the mastectomy-pain group demonstrated kinematics considered “high risk” or harmful, with respect to rotator cuff disease development [Citation9,Citation12,Citation13], while the reconstruction-pain group exhibited kinematics that may be protective against rotator cuff disease. Higher upward rotation and lower internal rotation of the scapula, as occurred in the reconstruction-pain group, generally increase the size of the subacromial space [Citation9,Citation30], which would prevent or reduce impingement of the rotator cuff tendons. Because the reconstruction-pain group were evaluated at shorter time since surgery, their movement and muscle patterns may still be attempting to prevent further pain and damage, while the mastectomy-pain group may be beyond this point. It is possible that recruitment of a future reconstruction group with a higher average age and evaluated at a greater point in time post surgery might yield kinematics approximating those of the current mastectomy-pain group.
The differing results by side suggest that dominant (right) and non-dominant (left) arms are influenced differently by impingement pain. Scapular kinematics differ between sides in healthy individuals [Citation31–33], so dominance may also affect how motion is affected by disorder. However, the breast cancer survivors with pain on the right side may also have more severe rotator cuff disease development; the number of positive results per participant on impingement tests was greater on the right side than the left side (1.8 positives on the right, 1.4 on the left).
Based on these results and findings from previous literature, we hypothesise that rotator cuff disease in breast cancer survivors could be influenced by aberrations in shoulder kinematics. It is well established that pectoralis tightness commonly occurs after all types of breast cancer treatment [Citation5]. This tightness may then lead to, or at least be associated with, thoracic kyphosis and/or increased scapular internal rotation in the early phase of recovery [Citation16,Citation34]. After prolonged time in kyphotic position, the anterior muscles may tighten further, the posterior muscles may lengthen, and most importantly, the serratus anterior may shorten and weaken [Citation35,Citation36]. As a result, the upper trapezius muscle may attempt to compensate by increasing activity to maintain upward rotation and external rotation of the scapula [Citation37,Citation38], a kinematic alteration displayed in the reconstruction-pain group of this study. Prolonged upper trapezius over-activation and tightness could lead to a muscular imbalance of the upper trapezius-serratus anterior couple [Citation37,Citation39]. This imbalance would result in decreased upward rotation [Citation17], and a possible return to internal rotation, as exhibited by the mastectomy-pain group of the current study, contributing to the development of rotator cuff disease. This hypothesis suggests that different compensations of the mastectomy-pain and reconstruction-pain groups could be mediated by the time since surgery as well as surgery type. The reconstruction-pain group may still be implementing protective compensations. Regardless, taken together, the findings from this study and previous literature suggest that kinematic changes are related to rotator cuff disease and may cause further alterations that lead to more severe rotator cuff injury. However, there are other biomechanical phenomena, including joint stability and tissue response, and individual or pre-operative factors that are not included here that may play an important role in secondary dysfunction after breast cancer treatment.
While these findings indicate an interesting possibility, at this time we are unable to conclusively state that shoulder kinematic alterations after breast cancer surgery lead to rotator cuff disease: work remains to test this relationship comprehensively. The current evidence suggests that mastectomy-only, time since surgery and participant age, might influence kinematic changes indicative of provoking rotator cuff disease in breast cancer survivors. The influence of reconstruction type on impingement pain, rotator cuff disease and related kinematic alterations remains elusive. Similarly, radiation and lymph treatments varied in the study cohort; neither factor related significantly to kinematics, but an analysis focussed on these treatment factors could elucidate their importance. An in-depth analysis by reconstruction type, and including delayed versus immediate reconstruction as a factor, is clearly needed to fully understand the influence of surgery and time on rotator cuff disease related kinematic alterations.
Study limitations
While this novel research provides some insight into a potential connection of biomechanics to rotator cuff disease in breast cancer survivors, limitations to this work exist. Namely, while the differences in kinematics and treatment factors combine to provide insight into a potential timeline of pain and injury, this is still a case-control study. Only preliminary inferences can be made based on these data, and the cause-and-effect of pain and kinematic injuries remains unclear. Somewhat small differences between groups, especially on the left side, may complicate interpretation. Further, more deliberate sampling based on surgery type, radiation, lymph node treatment and impingement pain would allow for more specific conclusions. Inclusion of a non-cancer rotator cuff disease group would also delineate any additional morbidity caused by breast cancer surgery, which is a goal for future research for the authors. An analysis using the same procedures as this paper, but including this non-cancer rotator cuff disease group would also address any potential statistical bias present in the current analysis due to unbalanced data. Due to the COVID-19 pandemic, participant recruitment ended early, causing the three types of breast reconstructions to be grouped together in the current analysis despite differences in surgical procedures. Other potential sources of the kinematics differences, such as thoracic postural variation, were not considered, which could modulate interpretations of the findings. Also, in some cases, the torso marker cluster was affixed to the participant’s clothing. While the clothing was fitted to the body to limit movement of the cluster, this placement may have limited the accuracy of marker tracking and subsequent results. Finally, these results are specific to the forward reaching movement. While this is a functional movement that may be performed repetitively, other types of movement may not show compensatory kinematics that coincide with potential rotator cuff disorder development.
Future research
This initial exploration of post-mastectomy and post-reconstruction biomechanical decrements gives rise to a number of related recommendations for future study: in-depth analysis and comparison of upper limb kinematics by reconstruction type; deliberate study of delayed versus immediate breast reconstruction; effects of adjuvant treatment (radiation, chemotherapy, lymph node removal) on function and kinematics; comparison to a non-cancer rotator cuff injury group; and finally, a controlled trial to assess kinematic changes over time in breast cancer survivors.
Conclusions
This study aimed to define kinematic compensations present in breast cancer survivors that may relate to the development of rotator cuff disorders. The findings outline preliminary kinematic alteration outcomes in two groups of breast cancer survivors that differed by surgery type (mastectomy-only versus breast reconstruction) and presence of pain. Kinematic alterations in reconstruction survivors may be protective, while mastectomy-only alterations may be harmful (reduced upward rotation and increased internal rotation). Differences in time since surgery in groups suggest a temporal component to biomechanical changes but more research is needed to confirm these differences. Finally, further investigation of homogenous subgroups of breast cancer survivors with different reconstruction types is required.
Author contributions
All authors were involved in the conception and design of this research. Drs. Lang and Kim were primarily responsible for the analysis and interpretation of the data and drafting the paper. All authors contributed to revising and approving the final version for publication.
Supplemental Material
Download MS Word (46 KB)Acknowledgements
The authors would like to thank Alyssa Moore and Nick Ryan for assistance with data collection and Dr. John Barden for sharing his research space at the University of Regina.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [SK], upon reasonable request.
Additional information
Funding
References
- Lang AE, Murphy M, Dickerson CR, et al. Shoulder dysfunction in breast cancer survivors: can treatment type or musculoskeletal factors identify those at higher risk? Rehabil Oncol. 2021;39(3):1–9.
- Kootstra JJ, Dijkstra PU, Rietman H, et al. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat. 2013;139(1):125–134.
- Yang EJ, Park W-BB, Seo KS, et al. Longitudinal change of treatment-related upper limb dysfunction and its impact on late dysfunction in breast cancer survivors: a prospective cohort study. J Surg Oncol. 2010;101(1):84–91.
- Stubblefield MD, Keole N. Upper body pain and functional disorders in patients with breast cancer. Pm R. 2014;6(2):170–183.
- Lee CH, Chung SY, Kim WY, et al. Effect of breast cancer surgery on chest tightness and upper limb dysfunction. Medicine. 2019;98(19):e15524.
- Ebaugh D, Spinelli B, Schmitz KH. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Med Hypotheses. 2011;77(4):481–487.
- Piitulainen K, Ylinen J, Kautiainen H, et al. The relationship between functional disability and health-related quality of life in patients with a rotator cuff tear. Disabil Rehabil. 2012;34(24):2071–2075.
- Mccabe RA, Nicholas SJ, Montgomery KD, et al. The effect of rotator cuff tear size on shoulder strength and range of motion; 2005 [Accessed 2020 Nov 19]. Available from: www.jospt.org.
- Seitz AL, Mcclure PW, Finucane S, et al. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech. 2011;26(1):1–12.
- Neer CS. Impingement lesions. Clin Orthop Relat Res. 1983;173:70–77.
- Chopp JN, O'Neill JM, Hurley K, et al. Superior humeral head migration occurs after a protocol designed to fatigue the rotator cuff: a radiographic analysis. J Shoulder Elbow Surg. 2010;19(8):1137–1144.
- Ludewig PM, Reynolds JF. The association of scapular kinematics and glenohumeral joint pathologies. J Orthop Sports Phys Ther. 2009;39(2):90–104.
- Keshavarz R, Bashardoust Tajali S, Mir SM, et al. The role of scapular kinematics in patients with different shoulder musculoskeletal disorders: a systematic review approach. J Bodyw Mov Ther. 2017;21(2):386–400.
- Lukasiewicz AC, McClure P, Michener L, et al. Comparison of 3-dimensional scapular position and orientation between subjects with and without shoulder impingement . J Orthop Sports Phys Ther. 1999;29(10):574–586.
- McClure PW, Michener LA, Karduna AR. Shoulder function and 3-dimensional scapular kinematics in people with and without shoulder impingement syndrome. Phys Ther. 2006;86(8):1075–1090.
- Borstad JD, Szucs KA. Three-dimensional scapula kinematics and shoulder function examined before and after surgical treatment for breast cancer. Hum Mov Sci. 2012;31(2):408–418.
- Lang AE, Dickerson CR, Kim SY, et al. Impingement pain affects kinematics of breast cancer survivors in work- related functional tasks. Clin Biomech. 2019;70:223–230.
- Spinelli BA, Silfies S, Jacobs LA, et al. Scapulothoracic and glenohumeral motions during functional reaching tasks in women with a history of breast cancer and healthy age-matched controls. Rehabil Oncol. 2016;34(4):127–136.
- Ribeiro ILL, Camargo PRR, Alburquerque-Sendín F, et al. Three-dimensional scapular kinematics, shoulder outcome measures and quality of life following treatment for breast cancer – a case control study. Musculoskelet Sci Pract. 2019;40:72–79.
- Lang AE, Card A, Barden J, et al. The effect of breast reconstruction on kinematics and performance during a range of upper limb functional tasks. Plast Reconstr Surg. 2022; in press.
- Leonardis JM, Diefenbach BJ, Lyons DA, et al. The influence of reconstruction choice and inclusion of radiation therapy on functional shoulder biomechanics in women undergoing mastectomy for breast cancer. Breast Cancer Res Treat. 2019;173(2):447–453.
- Moen MH, de Vos R-J, Ellenbecker TS, et al. Clinical tests in shoulder examination: how to perform them. Br J Sports Med. 2010;44(5):370–375.
- Calis M. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis. 2000;59(1):44–47.
- Wu G, Van Der Helm FCT, Veeger HEJ, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion-Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981–992.
- Lang AE, Kim SY, Milosavljevic S, et al. The utility of the acromion marker cluster (AMC) in a clinical population. J Electromyogr Kinesiol. 2022;62:102298.
- Brookham RL, Cudlip AC, Dickerson CR. Examining upper limb kinematics and dysfunction of breast cancer survivors in functional dynamic tasks. Clin Biomech. 2018;55:86–93.
- Winter DA. Biomechanics and motor control of human movement. 4th ed. New Jersey: John Wiley & Sons; 2009.
- Cohen J. Statistical power analysis for the social sciences. 2nd ed. New Jersey: Laurence Erlbaum; 1988.
- Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg. 2014;134(2):303–314.
- Lawrence RL, Braman JP, Staker JL, et al. Comparison of 3-Dimensional shoulder complex kinematics in individuals with and without shoulder pain, part 1: sternoclavicular, acromioclavicular, and scapulothoracic joints. J Orthop Sports Phys Ther. 2014;44(9):636–644.
- Morais NV, Pascoal A. Scapular positioning assessment: is side-to-side comparison clinically acceptable? Man Ther. 2013;18(1):46–53.
- Matsuki K, Matsuki KO, Mu S, et al. In vivo 3-dimensional analysis of scapular kinematics: comparison of dominant and nondominant shoulders. J Shoulder Elb Surg. 2011;20(4):659–665.
- Schwartz C, Croisier J-L, Rigaux E, et al. Dominance effect on scapula 3-dimensional posture and kinematics in healthy male and female populations. J Shoulder Elbow Surg. 2014;23(6):873–881.
- Malicka I, Barczyk K, Hanuszkiewicz J, et al. Body posture of women after breast cancer treatment. Ortop Traumatol Rehabil. 2010;12(4):353–361.
- Thigpen CA, Padua DA, Michener LA, et al. Head and shoulder posture affect scapular mechanics and muscle activity in overhead tasks. J Electromyogr Kinesiol. 2010;20(4):701–709.
- Weon J-H, Oh J-S, Cynn H-S, et al. Influence of forward head posture on scapular upward rotators during isometric shoulder flexion. J Bodyw Mov Ther. 2010;14(4):367–374.
- Contemori S, Panichi R, Biscarini A. Effects of scapular retraction/protraction position and scapular elevation on shoulder girdle muscle activity during glenohumeral abduction. Hum Mov Sci. 2019;64:55–66.
- Umehara J, Kusano K, Nakamura M, et al. Scapular kinematic and shoulder muscle activity alterations after serratus anterior muscle fatigue. J Shoulder Elbow Surg. 2018;27(7):1205–1213.
- Paine RM, Voight M. The role of the scapula. Int J Sports Phys Ther. 2013;8(5):617–629.