Abstract
Objective
To develop a Fear of Cancer Scale (FOCS) for non-cancer populations.
Methods
FOCS was developed by classical measurement theory. A total of 15 college students were invited to conduct semi-structured interviews. Seven experts were invited for expert consultation. A total of 2012 Chinese college students who had completed the electronic questionnaire on WJX.cn platform was included. The reliability and validity of FOCS were verified. Multiple linear regression analysis was adopted to explore the influencing factors of cancer fear among college students and further verify the validity of FOCS.
Results
There were 17 items in the FOCS, including two subscales – direct fear (8 items), and indirect fear (9 items). FOCS had good validity and reliability. Multiple linear regression showed that GAD-7 score, CSDS score, negative coping score, positive coping score, guardian’s highest education, gender, life satisfaction, nationality and major were the influencing factors of cancer fear (p < .05).
Conclusions
The 17-item FOCS was a reliable and valid measure to examine the level of cancer fear in non-cancer populations.
1. Introduction
As one of the most important public health issues in the world today, and one of the most feared diseases, cancer is often a source of fear for both cancer patients and non-cancer populations (such as family members/caregivers, and other healthy people). Previous research mostly focussed on the fear of cancer recurrence (FCR) or progression in cancer patients or survivors, known as FCR. High levels of FCR can be found in newly diagnosed cancer patients, adult cancer survivors and haematologic tumour survivors receiving haematopoietic stem cell transplantation (HSCT), etc.. Anxiety and depression symptoms, post-traumatic stress symptoms and use of psychotropic drugs are significantly positively associated with FCR levels [Citation1–3]. A poor patient–physician relationship (PPR) was also identified as a contributing factor to high levels of FCR [Citation4]. FCR is also present in relatives of cancer patients and is negatively associated with quality of life [Citation5]. A lack of awareness of cancer may arouse fear of cancer symptoms, changes of the disease, social, therapeutic and psychological impacts caused by cancer in the relatives [Citation6]. Reducing FCR in relatives is helpful to improve the patient’s mental state [Citation7].
Presently, there are few reports on cancer fear in people without a history of cancer, but cancer fear does exist in healthy people. About 54.0% of the Mexican female participants had high levels of fear of breast cancer, and their lower fear scores were associated with better health, older age, being born in the USA and having a regular physician [Citation8]. Lung cancer was the biggest fear for healthy South Korean men and stomach cancer for women. The most feared side effects of cancer treatment were pain, psychological problems, general weakness, digestive dysfunction, fatigue and changes in appearance [Citation9].
Champion’s Breast Cancer Fear Scale (CBCFS) is developed to evaluate the fear of breast cancer in healthy women, whose Cronbach’s α is 0.91, and the predictive factors include threat, benefits, self-efficacy and fatalism [Citation10]. This scale has shown good validity good in studies Iran, Turkey, Saudi Arabia and other countries [Citation11–13]. Colorectal Cancer Fear Scale (CRCFS), adapted from CBCFS by Hong Kong scholars, is used to measure colorectal cancer fear in elderly community people, showing Cronbach’s α of 0.95, and retesting reliability of 0.52 [Citation14]. The authors believe that colorectal cancer fear is positively associated with susceptibility, severity and psychological disorders, and reducing colorectal cancer fear is beneficial to improving colorectal cancer screening rate [14]. Cancer Worry Scale (CWS) is applicable to cancer patients/survivors. With Cronbach’s α of 0.87 in patients with general gynaecologic diseases, CWS can also be used to evaluate the fear of cancer in patients with gynaecologic diseases [Citation15]. All the above scales have good reliability and validity, but their scopes of application are limited (applicable to a single target, a single target type of cancer or not specifically used for people without a history of cancer). We believe that cancer fear is widespread in general public and could be about any type of cancer. There is an urgent need for a systematic, comprehensive and universal tool to measure the level of cancer fear among the public. In order to obtain more objective evaluation data, we chose Chinese college students without cancer histories as participants and developed Fear of Cancer Scale (FOCS).
2. Materials and methods
2.1. Development of the Fear of Cancer Scale (FOCS)
Searching “Fear of disease,” we reviewed relevant literatures and achieved a descriptive definition of “Fear of Cancer.” College students were invited to conduct semi-structured interviews. The sample size was determined by the “information saturation method.” The interview was terminated when no new and effective information could be obtained from more interviewees. Qualitative research method was used to extract information from the interview records and construct the item pool.
The semi-structured interview questions included:
①Do you fear cancer?
②Why would you fear cancer?
③What are your fears of cancer?
④What adverse effects of cancer do you fear?
⑤What cancer-related things do you fear?
⑥What else do you think about cancer?
Experts in clinical medicine, nursing, public health and social psychology were invited to revise the item pool using Delphi expert consultation, and to score the content validity for each item. With a scoring range from 0 to 10 points and a scoring precision to 0.1-point, higher score meant better match with the scale. The scorer reliability was measured by Kendall Concordance Coefficient, and the I-CVI value of each item was calculated. A small sample of college students were invited to conduct at least two rounds of cognitive interviews, to conduct a pre-survey on the scale items. Further revision of the items was made and a complete FOCS was finally formed.
2.2. Survey tools
The questionnaire was a self-evaluation questionnaire designed and compiled by our team, which included six parts: ① a table for the collection of social demographic data of the college students; ② a form for health information collection; ③ Chinese version of FOCS; ④ Chinese version of Generalised Anxiety Disorder-7 (GAD-7); ⑤ Chinese version of Cancer Symptom Discrimination Scale (CSDS) [Citation16]; and ⑥ Chinese version of Trait Coping Style questionnaire (TCSQ) [Citation17].
2.3. Data collection
Samples were selected by convenience sampling combined with snowball sampling. The electronic questionnaire was compiled on WJX.cn platform, and questionnaire link or QR code poster were forwarded to college students through instant messaging softwares, WeMedia platforms and college teachers. Participants clicked the link or scanned the QR code then logged on the home page. This study was conducted on voluntary, anonymous, confidential basis without commercial interests attached.
Inclusion criteria for the participants were one was: ① a Chinese college student; ② voluntary and consent to participate; and ③ without past history of malignant tumour. Participants would read an informed consent form on the front page of the questionnaire. Statements on agreeing to participate or NOT, having a history of malignant tumour or NOT, and being a college student or NOT were required from the participants so as to verify whether they met the inclusion criteria. If they met, the body of the questionnaire would be loaded; If NOT, the programme would exit automatically. To avoid data missing, every item was required be completed when setting. Participants could quit at any time. This study was approved and supervised by the Ethics Committee of the Sixth Affiliated Hospital of Kunming Medical University.
This cross-sectional study was set for 30 d. The valid sample size should be 5–10 times larger than the number of FOCS scale items, to be suitable for exploratory factor analysis, confirmatory factor analysis and multiple linear regression analysis [Citation18].
2.4. Statistical analysis
Office Excel 2016 software was used to export data from WJX.cn, and a database was established. SPSS version 19.0 (SPSS Inc., Chicago, IL) and AMOS version 22.0 were used for data processing and statistical analysis. According to the design of the scale, the structural equation model was constructed, and the structural validity test of FOCS was carried out by using confirmatory factor analysis to calculate the model fitting degree. The content validity of the I-CVI scale was evaluated by Pearson correlation analysis and expert consultation. Factor analysis was used for dimensionality reduction of FOCS, and two factors were extracted to calculate the convergent validity and discriminatory validity. Using CAD-7 as the standard, the criterion validity of FOCS was tested by Pearson correlation analysis. Cronbach α and Spearman–Brown Coefficient were used to evaluate the internal consistency of FOCS. Intraclass correlation coefficient (ICC) was used to evaluate test-retest reliability. Multiple linear regression analysis stepwise regression method was adopted to explore the influencing factors of cancer fear among college students and further verify the validity of FOCS, in which FOCS score was taken as the dependent variable while sociodemographic data, health information, GAD-7 score, CSDS score, negative coping score and positive coping score as the independent variable.
3. Results
3.1. Development of the FOCS
We invited 15 college students to conduct semi-structured interviews, among which there were 7 males and 8 females, 4 were within the age bracket of 16–19 years old, and 11 were 20 years old or above. There were 5 medical students and 10 non-medical students. Then, we extracted and established a 17-itemed pool, which was divided into two dimensions.
Seven experts were invited for expert consultation. In the first round of expert consultation, the wording of seven items was revised without item addition or deletion. In the second round of expert consultation, the same panel of experts were invited to rate the content validity and calculate the I-CVI value of each item. Then, we invited another 14 college students to conduct cognitive interviews who did not propose any modifications to the items. Finally, 17 items of FOCS were identified for subsequent studies (see ).
Table 1. The factor loading of FOCS.
3.2. Sample size
From 8 November 2020 to 8 December 2020, a total of 2338 people approached this study and clicked on the questionnaire home page. Among them, 326 people were excluded because they stated refusal to participate, or NOT college students, or claimed to have a history of malignant tumour. Therefore, a total of 2012 effective samples were included, with an effective rate of 86.06%. Since there are 17 items in the FOCS scale, the minimum sample size of this study was set to > 170. The samples had outnumbered the minimum size [18].
Among the respondents, 1317 (65.46%) were female and, 695 (34.54%) were male. There were 1181 (58.70%) were within the age bracket of 16–19, and 831 (41.30%) were 20 or above. A total of 783 respondents (38.92%) were studying for their vocational college degrees, and 1229 (61.08%) were studying for their bachelor degrees or above. A total of 1524 (75.75%) were Han people, and 488 (24.25%) were ethnic minorities. The status of education of the guardians of the interviewees were listed here:473 (23.51%) had an education under primary school, 838 (41.65%) finished middle school, 390 (19.38%) finished high school/vocational high school, 125 (6.21%) finished vocational college, and 186 (9.24%) finished college or above. There were 878 (43.64%) medical students and 1,134 (56.36%) non-medical students. When assessing their life satisfaction, 1239 (61.58%) rated “so so” and 773 (38.42%) rated “perfect.” A total of 363 (18.04%) reported having a cancer patient relative/friend, 188 (9.34%) reported having cared for a cancer patient, and 1145 (56.91%) reported having no cancer-related knowledge.
3.3. Structural validity
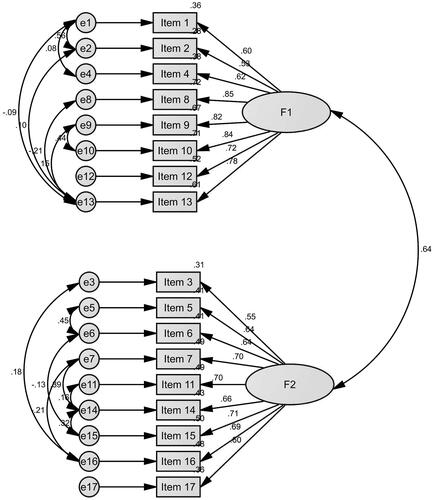
A two-factor structural equation model was constructed according to the two-dimension design of FOCS. Confirmatory factor analysis results showed a good fitting degree, thus demonstrating a superiority of the modified two-factor model to the unmodified model and good structural validity of FOCS (see ). The good model-fitting degree of modified two-factor model included goodness of fit index (GFI = 0.913), adjusted GFI (AGFI = 0.872), comparative fit index (CFI = 0.924), normed fit index (NFI = 0.919), Tucker–Lewis index (TLI= 0.900), incremental fit index (IFI = 0.924) and root mean squared error of approximation (RMSEA =0.085).
F1 was named “Direct fear” (8 items), mainly describing an individual’s fear that s/he or her/his relatives and friends may suffer from cancer, as well as the negative impacts of cancer on life, finance, mental health, family relations and other aspects. This dimension directly reflects the individual's fear and behaviour of cancer. F2 was named “Indirect fear” (9 items), describing the psychological fear and behaviours–such as speculation, avoidance and rejection – caused by certain connections between an individual and cancer patients or cancer-related issues, which were the intermediaries of those individual cancer fear and behaviours in F2.
3.4. Content validity
The Kendall Concordance coefficient for the content validity of the items was 0.542 (p < .001) from the 7 experts in the Delphi consultation, suggesting good Scorer reliability, and the I-CVI of FOCS was between 0.807 and 0.990, suggesting good content validity of the FOCS items. Pearson correlation analysis showed that the correlation coefficients of F1 score, F2 score and total score of FOCS were 0.874 and 0.896, respectively (p< .001). The correlation coefficient ranged from 0.538 to 0.737 (p < .001) between the score of a single item and the total score of FOCS. The correlation coefficient between the items and F1 dimension ranged from 0.688 to 0.849 (p < .001), and ranged from 0.657 to 0.766 (p < .001) between the items and F2 dimension, indicating good content validity of FOCS, the subscales and the items.
3.5. Convergent validity and discriminatory validity
Kaiser–Meyer–Olkin (KMO)=0.918. The chi-square value of Bartlett’s sphericity test was 19769.706 (p<.001), and the scale data were suitable for factor analysis. Principal component analysis and maximum variance method were used to extract two common factors, and the cumulative variance contribution rate was 56.66%. Composite reliability (CR) of F1 was 0.903, and average variance extracted (AVE) =0.543, while CR was 0.887 and AVE = 0.470 for F2, suggesting good polymerization validity of the two factors of FOCS. The factor load of each item in its dimension was greater than its cross load in the other dimension, suggesting good discriminatory validity of the two factors of FOCS (see ).
3.6. Criterion validity
In this study, Cronbach’s α of GAD-7 was 0.935, showing good reliability, therefore, was used as a reference frame to test the criterion validity of FOCS. Pearson correlation analysis showed that the correlation coefficient γ = 0.322 (p < .001) between the total score of FOCS and the total score of GAD-7. The samples were divided into low group (≤9 points, N = 1685) and high group (>9 points, N = 327) with a cut-off value of 9 in GAD-7 score, and the total score of FOCS between the two groups was statistically significant (35.28 ± 14.11 vs. 42.37 ± 13.15, p < .001).
3.7. Internal consistency
The Cronbach’s α of FOCS was 0.916. The Corrected Item-Total Correlation (CITC) ranged from 0.472 to 0.690. The item alphas (Cronbach’s alpha if item deleted) ranged from 0.908 to 0.914. Cronbach’s α of F1 and F2 were 0.902 and 0.877. The results showed good internal consistency reliability between FOCS and its two subscales.
The split-half reliability of the scale was verified by dividing the items into two halves. Spearman-Brown Coefficient of FOCS, F1 and F2 were 0.887, 0.859 and 0.843, respectively, demonstrating good split-half reliability of FOCS and the subscales.
3.8. Test–retest reliability
Three months after the first survey, 40 participants took part in the second test for test–retest reliability. The ICC of the FOCS, F1 and F2 were 0.696, 0.670 and 0.731, respectively.
3.9. Influencing factors of cancer fear among college students
The multiple linear regression equation established with FOCS score as the dependent variable was statistically significant (F = 50.222, p < .001), and the model fitting degree R = 0.429, R2 =0.184. A total of nine variables were included in the multiple linear regression equation, and the collinearity diagnostics showed that the variance inflation factor (VIF) was between 1.006 and 1.407. GAD-7 score, CSDS score, negative coping score, positive coping score, guardian’s highest education, gender, life satisfaction, nationality and major were the influencing factors of cancer fear (p < .05) (see ).
Table 2. Multiple linear regression analyses of the FOCS scores.
4. Discussion
A 17-itemed FOCS was successfully developed using the classical measurement theory, which was a set of specialized scales for measuring the level of cancer fear in people without a history of cancer. After careful statistical analysis, we believe that FOCS has good reliability and validity. Structural equation model verified the direct fear and indirect fear dimension of FOCS. The good discriminatory validity and convergent validity also proved the actual differences in the connotation between the two dimensions, while the connotation of the items in the same dimension was consistent. In the content validity test, the I-CVI of each item was >0.8, indicating consistency in the item and scale design and also reasonable distribution, which was further confirmed by the correlation analysis between the item score and the score in the subscale or total scale. Through reliability test, good consistency, stability and reliability of the FOCS were also confirmed.
A total of nine variables were included into the multiple linear regression equation. The VIF was close to 1, and there was no collinearity among the independent variables. We found that the higher the scores of generalized anxieties, cancer symptom discrimination, positive coping and negative coping were, the higher the scores of predicted cancer fear were. These results amazed us. There may be a clear association between disease-related fear and generalised anxiety, and the two may also have overlapping predictors [Citation19]. For people with significant levels of cancer fear, it is necessary to identify whether there is combined anxiety symptoms or anxiety disorders, and anti-anxiety treatment may be effective for this group of people. Our previous research has predicted that public discrimination against cancer symptoms may come from their fear of cancer. This study verifies this hypothesis: College students’ discrimination and fear of cancer symptoms are indeed positively associated. Therefore, one of the tasks of eliminating cancer discrimination among the public is to effectively reduce the level of fear of cancer among the public. Neurophysiological studies suggest that individual coping styles are associated its fear conditioning [Citation20], and our study shows that both positive coping and negative coping styles are positively associated with the level of cancer fear in college students, suggesting that cancer fear may directly increase all individual coping behaviours, regardless of whether the coping styles are positive or negative [Citation21].
This study has found that the lower education the guardian had, the higher the cancer fear score was in the participants; also, participants with only an average life satisfaction, who was a female, an ethnic minority, or a non-medical college student had a higher cancer fear score compared with their counterparts (with higher life satisfaction, males, Han people or medical college students). The negative association between the education level of the guardian and the fear of cancer suggests that negative psychological events such as fear of diseases may be associated to the factors such as the family education background and the mental health literacy of the guardian [Citation22]. Life satisfaction is an important predictor of mental health issues such as anxiety and depression, and is also strongly associated with COVID-19 fear levels [Citation23,Citation24]. Results from this study further suggest that life satisfaction may also be a negative predictor of cancer fear. Women have more fear of cancer than men, which may be associated to the increasing incidences of female malignant tumours, such as breast cancer, uterine cancer, and cervical cancer [Citation25]. Other studies have found that cancer fear and fatalism are more common among ethnic minority women than among white women in the UK, which may affect cancer prevention and early screening in women [Citation26]. In this study, ethnic minorities and non-medical college students have more obvious fear of cancer, which may be associated to low awareness of cancer knowledge. Understandably, medical college students receive more systematic, comprehensive, and authoritative professional knowledge of medicine and associated disciplines than non-medical college students, and have better health literacy [Citation27]. Other study also found that awareness of cancer-related knowledge – cervical cancer, breast cancer and other cancers – and cancer screening among ethnic minority women in some regions of China is relatively inadequate. Therefore, the cancer health education and publicity among ethnic minority people in these regions should be strengthened to improve the cancer screening rate, which may also be helpful to reduce the level of cancer fear [Citation28,Citation29].
However, some limitations should be considered. Impacted by the COVID-19 epidemic, most universities in China are still in strict epidemic prevention and control, which makes it not convenient for us to carry out random sampling in schools. Convenience sampling combined with snowball sampling adopted in this study caused imbalance in sample category ratios to some extent (for example, the ratio of male to female =1:1.89). This study issued electronic questionnaires, therefore, the three inclusion criteria on the first page of the questionnaire were completely dependent on the honesty of the participants. Therefore, there could be data included that should had been excluded. College students are a highly intellectual group with the most active thoughts and the most rapid acceptance for new things. Their health literacy, psychological activities and behaviour patterns have profound impacts on their future self-growth and development. Therefore, it is conducive to the healthy growth of college students if we pay more attention to their fear of diseases, disease anxiety and other psychological issues. However, this study only analysed the reliability and validity of FOCS and the influencing factors of cancer fear among college students. The reliability and validity of FOCS in other groups (such as community residents, teachers, women, etc.), as well as the influencing factors of cancer fear in these groups, need to be further explored in subsequent studies.
It should be noted that the FOCS questionnaire was in Chinese and administered in Chinese to the participants, all of whom were Chinese. This article showed the results of the reliability and validity tests of the FOCS in Chinese language. We encourage researchers to translate Chinese FOCS into different language to test its reliability and validity and widely apply it to people in different countries and regions. With the corresponding author’s the permission and with no commercial interests involved, researchers can use FOCS for free in their own research.
5. Conclusion
In conclusion, FOCS contains 17 items and is divided into direct fear and indirect fear dimensions. It has good reliability and validity. It is a psychological scale specially used to measure the level of cancer fear in people without cancer history, and can be used for cancer fear screening. It is of help to reduce cancer fear and psychological distress in general public (especially in close contacts of cancer patients, people at high risk of cancer, patients with benign tumours, and patients with precancerous lesions), increase early cancer screening rate, promote patients' psychological rehabilitation and social function recovery, and to improve social supports for the patients. Generalized anxiety, cancer symptom discrimination, positive coping style, negative coping style, female, ethnic minority, and non-medical majors may be positive predictors of cancer fear among college students. Life satisfaction and guardian’s educational background may be negative predictors of cancer fear among college students. We will continue to apply FOCS to cancer fear research in different groups, to provide theoretical basis for study of high-risk groups of cancer fear.
Ethical approval
This is an exploratory, cross-sectional study conducted in China which was approved by the Ethics Committee of the Sixth Affiliated Hospital of Kunming Medical University.
Author contributions
LF, XW and ZD contributed to the writing and statistical analyses of this article, including putting forward this study and carrying out the study. LF and ZD were listed as co-first authors. LF and XW were listed as co-corresponding authors. QL, QY, FY, QW and YZ contributed to performing the investigation and collecting all data. They had contributed equally to the manuscript. RY, CT, LY and WZ contributed to performing the investigation and collecting a part of data.
Acknowledgements
We would like to extend our heartfelt gratitude to all interviewees for their participation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used and analysed during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Luo X, Li W, Yang Y, et al. High fear of cancer recurrence in Chinese newly diagnosed cancer patients. Front Psychol. 2020;11:1287.
- Vandraas KF, Reinertsen KV, Kiserud CE, et al. Fear of cancer recurrence among young adult cancer survivors-exploring long-term contributing factors in a large, population-based cohort. J Cancer Surviv. 2021;15(4):497–508.
- Brice L, McErlean G, Donovan C, et al. Fear of cancer recurrence following allogeneic haematopoietic stem cell transplantation (HSCT) for haematological malignancy: a cross-sectional study. Eur J Oncol Nurs. 2020;49:101845.
- Alkan A, Yaşar A, Güç ZG, et al. Worse patient-physician relationship is associated with more fear of cancer recurrence (Deimos Study): a study of the Palliative Care Working Committee of the Turkish Oncology Group (TOG). Eur J Cancer Care. 2020;29(6):e13296.
- Peikert ML, Inhestern L, Krauth KA, et al. Fear of progression in parents of childhood cancer survivors: a dyadic data analysis. Psychooncology. 2020;29(10):1678–1685.
- Morowatisharifabad MA, Gerayllo S, Jouybari L, et al. Concerns and fear of esophageal cancer in relatives of patients with cancer: a qualitative study. J Gastrointest Cancer. 2020;51(3):957–964.
- Hu X, Wang W, Wang Y, et al. Fear of cancer recurrence in patients with multiple myeloma: prevalence and predictors based on a family model analysis. Psychooncology. 2020;30:176–184.
- Flores-Luevano S, Shokar NK, Dwivedi AK, et al. Breast cancer fear among Mexican American women in the United States. Breast Cancer (Auckl). 2020;14:1178223420952745.
- Park K, Kim Y, Yang HK, et al. The fear of cancer from the standpoint of oneself, the opposite sex and the fear of side effects of cancer treatment. Cancer Res Treat. 2020;52(4):993–1001.
- Champion VL, Skinner CS, Menon U, et al. A breast cancer fear scale: psychometric development. J Health Psychol. 2004;9(6):753–762.
- Moshki M, Shahgheibi S, Taymoori P, et al. Psychometric properties of the mammography self-efficacy and fear of breast cancer scales in Iranian women. BMC Public Health. 2017;17(1):534.
- Secginli S. Mammography self-efficacy scale and breast cancer fear scale: psychometric testing of the Turkish versions. Cancer Nurs. 2012;35(5):365–373.
- Alyami M, Al-Sharef A, Al-Aseri M, et al. Mammography self-efficacy scale and breast cancer fear scale: psychometric properties of the Arabic versions among Saudi women. Cancer Nurs. 2021;44(2):163–170.
- Leung DY, Wong EM, Chan CW. Adapting champion’s breast cancer fear scale to colorectal cancer: psychometric testing in a sample of older Chinese adults. Eur J Oncol Nurs. 2014;18(3):281–285.
- Uner FO, Korukcu O. A prevalence and psychometric study on fear of cancer in women with abnormal cervical cytology undergoing colposcopy. Psycho-Oncology. 2020;29(11):1850–1855.
- Feng LS, Dong ZJ, Yan RY, et al. Development and validation of the cancer symptoms discrimination scale: a cross-sectional survey of students in Yunnan, China. BMC Palliat Care. 2020;19(1):156.
- Liu X, Tein JY, Zhao Z. Coping strategies and behavioral/emotional problems among Chinese adolescents. Psychiatry Res. 2004;126(3):275–285.
- MacCallum RC, Widaman KF, Zhang S, et al. Sample size in factor analysis. Psychol Methods. 1999;4(1):84–99.
- Schweda A, Weismüller B, Bäuerle A, et al. Phenotyping mental health: age, community size, and depression differently modulate COVID-19-related fear and generalized anxiety. Compr Psychiatry. 2021;104:152218.
- Klucken T, Kruse O, Schweckendiek J, et al. Increased skin conductance responses and neural activity during fear conditioning are associated with a repressive coping style. Front Behav Neurosci. 2015;9:132.
- Wakashima K, Asai K, Kobayashi D, et al. The Japanese version of the fear of COVID-19 scale: reliability, validity, and relation to coping behavior. PLoS One. 2020;15(11):e0241958.
- Cormier E, Park H, Schluck G. eMental health literacy and knowledge of common child mental health disorders among parents of preschoolers. Issues Ment Health Nurs. 2020;41(6):540–551.
- Gawrych M, Cichoń E, Kiejna A. COVID-19 pandemic fear, life satisfaction and mental health at the initial stage of the pandemic in the largest cities in Poland. Psychol Health Med. 2021;26(1):107–113.
- Satici B, Gocet-Tekin E, Deniz ME, et al. Adaptation of the fear of COVID-19 scale: its association with psychological distress and life satisfaction in Turkey. Int J Ment Health Addict. 2020;19:1980–1988.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33.
- Vrinten C, Wardle J, Marlow LA. Cancer fear and fatalism among ethnic minority women in the United Kingdom. Br J Cancer. 2016;114(5):597–604.
- Miles R, Rabin L, Krishnan A, et al. Mental health literacy in a diverse sample of undergraduate students: demographic, psychological, and academic correlates. BMC Public Health. 2020;20(1):1699.
- Abulizi G, Abulimiti T, Li H, et al. Knowledge of cervical cancer and pap smear among Uyghur women from Xinjiang, China. BMC Womens Health. 2018;18(1):21.
- Wu E, Tiggelaar SM, Jiang T, et al. Cervical cancer prevention-related knowledge and attitudes among female undergraduate students from different ethnic groups within China, a survey-based study. Women Health. 2018;58(6):661–684.