Abstract
Purpose
Diabetes mellitus (DM) increases the risk of morbidity and mortality after liver resection. Albuminuria is associated with a higher risk for all-cause and cardiovascular mortality. This study evaluated albuminuria as a predictor of the outcome of living donor liver transplantation (LDLT) in patients with pre-existing DM.
Methods
This retrospective study involved 103 type II diabetic patients with end-stage liver disease who received LDLT. Preoperative spot urine albumin: creatinine ratio was used to determine the degree of albuminuria. The primary outcome measure was the impact of urinary albumin excretion on the 3-year mortality rate after LDLT in this diabetic cohort.
Results
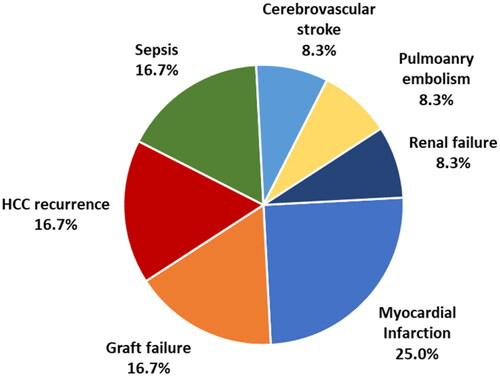
Hepatitis C virus infection was the main cause of cirrhosis. Albuminuria was detected in 41 patients (39.8%); 15 had macroalbuminuria, while 26 had microalbuminuria. Patients with microalbuminuria were significantly older than those with macroalbuminuria and normal albumin in urine. After 3 years, twenty-four patients (23.3%) died within 3 years after LT. Myocardial infarction was the leading cause of death (25%). Albuminuria was an independent factor affecting 3-year mortality with an odds ratio of 5.17 (95% CI: 1.86–14.35).
Conclusion
Preoperative albuminuria is an independent factor affecting mortality within 3 years after LDLT in type II diabetic patients. Myocardial infarction was the leading cause of death in 25% of cases, followed by hepatocellular carcinoma recurrence, sepsis, and graft failure.
Diabetes mellitus (DM) increases the risk of morbidity and mortality after liver resection.
Albuminuria is associated with a higher risk for all-cause and cardiovascular mortality.
Preoperative albuminuria is a significant predictor of mortality within 3 years after LDLT in diabetic patients.
KEY MESSAGES
Keywords:
Introduction
In Egypt, 13 centres perform living donor liver transplantation (LDLT). By June 2014, 2,406 procedures were done. The main indication was cirrhosis post-hepatitis-C viral infection [Citation1]. Deceased donor liver transplant has not yet been authorised due to religious and cultural factors [Citation2]. Patient survival after liver transplantation (LT) has markedly improved thanks to the modifications in surgical techniques and postoperative care [Citation3]. However, the impact of associated morbidities on the outcome of LT varies widely [Citation4].
Type II diabetes mellitus (T2DM) is frequently detected in patients with severe liver diseases, including cirrhosis and hepatocellular carcinoma (HCC) [Citation5,Citation6] that may require LT in the terminal stage. It was found that T2DM increases the risk of morbidity and mortality after liver resection for HCC and liver metastasis [Citation7]. It was reported that at the time of LT, 33–50% of the patients had renal dysfunction, and 6.3% were receiving dialysis [Citation8,Citation9]. Albuminuria is widely recognised as a marker for kidney disease in healthy subjects and patients with diabetes, hypertension, and obesity [Citation10]. Higher albuminuria degrees are associated with a higher risk for all-cause and cardiovascular mortality and chronic kidney disease evolution [Citation11].
This study aimed to evaluate albuminuria as an early marker for renal dysfunction in patients with pre-existing T2DM for predicting the outcome of LDLT.
Patients and methods
This retrospective analysis was performed from May 2005 to June 2012. All type II diabetic patients with end-stage liver disease who received LDLT were enrolled. Patients <18 years of age, having a previous liver transplant, with non-diabetic causes of albuminuria, and those with elevated serum creatinine above 1.2 and 1.4 mg for females and males, respectively, and/or end-stage renal disease were excluded. The study protocol adhered to the ethical guidelines of the 1975 Declaration of Helsinki. The local ethical committee of Cairo University Hospitals approved the protocol and patients’ consent was waived due to the retrospective nature of the study.
All patients’ medical records were reviewed to extract the following data: demographic information, aetiology of primary liver disease, clinical and laboratory parameters, graft–recipient weight ratio, donor age and degree of steatosis, duration of hospital and ICU stay, and outcome within 3 years.
The severity of liver disease was assessed by Child-Pugh points [Citation12] and model for end-stage liver disease (MELD) score [Citation13]. T2DM was diagnosed according to the American diabetes association criteria based on fasting plasma glucose (FPG) level. Clinical diabetes mellitus (DM) was defined as FPG 126 mg/dL or HbA1c 6.5%. This was confirmed by repeated blood sampling unless the patient has clinical symptoms or a glucose level of 200 mg/dL, previous history of DM, and/or consumption of anti-diabetes medications [Citation14].
Determination of micro- and macro-albuminuria was based on preoperative spot urine albumin creatinine ratio (uACR) measurements. Data from early morning urine samples were used. Normoalbuminuria was defined as uACR ≤2.5 mg/mmol for males and ≤3.5 mg/mmol for females. Microalbuminuria was defined as uACR >2.5–30 mg/mmol for males and >3.5–30 mg/mmol for females. Macroalbuminuria was defined as uACR >30 mg/mmol [Citation15]. eGFR was computed using the modified Modification of Diet in Renal Disease formula [Citation16].
The primary outcome measure was the impact of urinary albumin excretion on the 3-year mortality rate after LDLT in this type II diabetic cohort.
Statistical analysis
Statistical analysis was done using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test was used to examine the relation between qualitative variables. For quantitative data, comparison between two groups was made using independent sample t-test or Mann–Whitney test. Comparison between three groups was made using ANOVA test, or Kruskal–Wallis test followed by the appropriate post hoc test. Odds ratio (OR) with its 95% confidence interval (CI) were used for risk estimation. A p-value <.05 was considered significant.
Results
A total of 103 patients were enrolled in the study. According to the level of uACR, albuminuria was detected in 41 patients (39.8%); 15 had macroalbuminuria, while 26 had microalbuminuria. presents a comparison between the three groups; macroalbuminuria, microalbuminuria, and no albuminuria. Patients with macroalbuminuria were significantly older than those without albuminuria (p = .014) and had comparable age with the microalbuminuria group (p = .234). Microalbuminuria and no albuminuria groups were comparable in recipient age (p = .443). Otherwise, there was no significant difference between the three groups in all demographic, clinical, and laboratory characteristics of the patients ().
Table 1. Comparison between patients with macroalbuminuria, microalbuminuria, and no albuminuria regarding demographic, clinical, and laboratory characteristics.
At 3 years, 24 patients (23.3%) died, and myocardial infarction was the leading cause of death (25%) (). Albuminuria, whether micro- or macroalbuminuria, was associated with significantly higher 3-year mortality (). Three-year mortality was also higher in patients with cardiomyopathy (p = .039). On multivariate analysis, albuminuria was the only independent factor affecting 3-year mortality ().
Table 2. Factors associated with 3-year mortality in the studied group.
Table 3. Multivariate logistic regression model for factors affecting 3-year mortality.
compares patients with any degree of albuminuria and non-albuminuric patients. Patients with albuminuria were significantly older (p = .021) and had lower BMI (p = .019) and shorter duration of DM (p = .028). Seventeen patients (70.8%) of those who died within 3 years had albuminuria. Albuminuria was an independent factor affecting 3-year mortality with an OR of 5.17 (95% CI: 1.86–14.35).
Table 4. Comparison between patients with albuminuria and those with no albuminuria regarding demographic, clinical, and laboratory characteristics.
shows causes of death in patients with macroalbuminuria, microalbuminuria, and no albuminuria.
Table 5. Causes of death in patients with macroalbuminuria, microalbuminuria, and no albuminuria.
Discussion
This study found that preoperative detection of albuminuria was significantly associated with mortality within 3 years after LDLT in type II diabetic patients. Myocardial infarction was the leading cause of death in 25% of cases, followed by HCC recurrence, sepsis, and graft failure.
In Egypt, T2DM is a rapidly growing health problem affecting about 15.6% of adults. It is the primary cause of end-stage renal disease in Egypt [Citation17]. In this retrospective study, we investigated the predictive value of urinary albumin excretion in diabetic patients with liver cirrhosis subjected to LDLT for mortality within 3 years after surgery. In 70% of the current series, cirrhosis was due to viral hepatitis infection, whether hepatitis C virus (HCV) or HBV. Liver cirrhosis is commonly associated with DM. It is estimated that DM is present in 12.3–57.0% of patients with cirrhosis [Citation18]. Also, patients with HCV were found to be more prone to develop T2DM [Citation19]. The coexistence of DM and chronic liver diseases are associated with a higher risk of hepatic decompensation, HCC, and mortality [Citation20].
In patients with end-stage liver disease, DM positively correlates with disease severity [Citation21,Citation22]. Many studies reported lower survival in decompensated liver disease in patients with DM [Citation23]. The presence of diabetes was associated with about a 50% greater risk of mortality in patients with CLD [Citation24]. Pre-existing DM was shown as an independent factor for developing ascites, bacterial infections, and renal dysfunction in patients with post-HCV cirrhosis [Citation25].
In terms of LT, pre-transplant DM is an important predictor of post-transplant DM and metabolic syndrome [Citation26,Citation27]. The presence of post-transplant metabolic syndrome increases the incidence of cardiovascular events [Citation28].
The effect of pre-existing DM on survival after LT has been previously investigated with controversial results. Some studies did not find an association between DM before LT and survival after LT [Citation29–31]. On the other hand, John and Thuluvath reported significant post-LT morbidity in patients with pre-existing DM [Citation32]. The Scientific Registry of Transplant Recipients (SRTR) database demonstrated that pre-existing DM increased post‐transplant mortality risk by 20% [Citation33]. Reduced survival was mainly found in patients requiring insulin or having underlying chronic hepatitis C infection [Citation34,Citation35].
Albuminuria was demonstrated as a predictor of mortality in diabetic patients of Asian and Caucasian populations [Citation36,Citation37]. It was found to be associated with in-hospital deaths of diabetic patients with foot complications [Citation38]. It was also associated with a higher risk of all-cause and cardiovascular mortality than those without albuminuria after long-term follow-up [Citation39]. Another meta-analysis of 148,350 cases confirmed these findings in diabetic patients with microalbuminuria and macroalbuminuria [Citation40].
Moreover, even with low concentrations, albuminuria has been recognized as a critical cardio-renal risk marker [Citation41]. Albumin excretion in urine indicates glomerular and tubular damage resulting in functional renal impairment [Citation42,Citation43]. On the other hand, the link between renal and cardiovascular damage is explained by the fact that endothelial damage indicated by albuminuria leads to increased systemic vascular permeability. Endothelial damage causes increased cardiovascular risk [Citation44]. Diabetic patients with albuminuria are more prone to developing myocardial infarction and stroke compared with the general population [Citation45]. Moreover, a previous study had demonstrated that preoperative proteinuria may anticipate the occurrence of renal injury and that can affect mortality in those undergoing cardiac surgery [Citation46]. In addition, it was reported that proteinuria was linked to postoperative acute kidney injury and 30-day unplanned readmission independent of preoperative eGFR [Citation47].
The current work found that preoperative albuminuria was significantly associated with mortality post LDLT. Previous studies have tried to investigate the effect of albuminuria on LT. Pan and colleagues found that the presence of proteinuria ahead of LT is a negative predictor for in-hospital survival. In addition, they reported that the existence of proteinuria after LT was an early prognostic factor of the short-term outcome. Based on these findings, repeated surveying of proteinuria in the preoperative and postoperative periods was advocated [Citation48].
Moreover, Amygdalos and his colleagues studied 390 LT recipients and demonstrated that preoperative proteinuria is a reliable tool to predict mortality in LT recipients [Citation49].
The present study has some limitations. The retrospective nature of the study was the main limitation of the present study. However, the relatively large number of the study sample and the reasonably acceptable follow-up period are points of strength that can support the findings. Another limitation was the heterogenous causes of liver disease that indicated LT. However, to our knowledge, only a few studies addressed the impact of urinary albumin excretion in diabetic patients, especially after LDLT. Therefore, this article adds momentum to the literature regarding this unique topic. Furthermore, it is sensible to say that this work may point to nongraft potential contributing factors that may affect prognosis after LT and may provide novel horizons in terms of this aspect. This can make it an attractive goal for therapeutic approaches to focus on detecting albuminuria to improve the outcome after LT. Most importantly, given the statistical results, the study underlines that urinary albumin excretion has a substantial impact on mortality after LT.
In summary, pre-existing albuminuria in diabetic patients subjected to LDLT is a significant predictor of mortality within 3 years. In this series, myocardial infarction was the leading cause of death in 25% of cases, followed by HCC recurrence, sepsis, and graft failure. Although albuminuria is an early marker of renal disease, it is—more notably—a significant predictor of all-cause and cardiovascular mortality and LT.
Author contributions
Ahmed Salman takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process, and general idea of work. Mohamed Salman was involved the drafting of the paper, and revising it critically for intellectual content. Mostafa Said was accountable for general framing of work, and making modifications as appropriate as the work progresses. Hesham Elkassar was accountable for specifying other investigator roles, and final approval of the version to be published. Mohammad El Sherbiny was accountable for gathering some of the database, drafting the work, and putting broad lines of the paper. Ahmed Youssef was accountable for data collection, supervising other data collection, and interpretation of data for the work. Mohammed Elbaz was accountable for putting steps the work, making modifications as appropriate as the work progresses. Mohamed Badr Hassan was accountable for revision of data critically, general design of work with gathering of preliminary important information. Ahmed M. Elmeligui was accountable for final approval of the version to be published. Mahmoud Gouda Omar was accountable for revising paper critically for important intellectual content. Hussien Samir was accountable for arrangement of all aspects of the work and ensuring that questions related to any part of the work are appropriately investigated and resolved. Mohamed Abdelkader Morad was accountable for supervising the work, statistical analysis of the data, and collection of some data. Hossam El-Din Shaaban was accountable for interpretation of data for the work, and collection of patients. Mohamed Youssef was accountable for making modifications as appropriate as the work progresses, and putting broad lines of the work. Ahmed Moustafa was accountable for supervising the work, and helping in data base gathering. Mohamed Sabry Tourky was accountable for developing of the hypothesis of the study and participated in data collection. Ahmed Elewa was involved in the conception and design, analysis and interpretation of the data. Sadaf Khalid was accountable for making modifications as appropriate as the work progresses in addition to gathering some data of the work. Khaled Monazea has provided the initial idea of the manuscript, and arranged the roles for each author. Mohamed Shawkat was involved in gathering of preliminary important information and final approval for publication. All authors agree to be accountable for all aspects of the work.
Acknowledgment
No funding was received.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Salman Ahmed, upon reasonable request.
References
- Amer KE, Marwan I. Living donor liver transplantation in Egypt. Hepatobiliary Surg Nutr. 2016;5(2):98–106.
- Abd Elbaset HS, Sultan AM, Montasser IF, Scientific Committee of Ministry of Health (MOH) National Project of Waiting Lists, Egypt, et al. Egyptian protocol for living donor liver transplantation (LDLT) during SARS-CoV-2 pandemic. Egypt Liver J. 2021;11(1):14.
- Durand F. How to improve long-term outcome after liver transplantation? Liver Int. 2018;38:134–138.
- Tovikkai C, Charman SC, Praseedom RK, et al. Time-varying impact of comorbidities on mortality after liver transplantation: a national cohort study using linked clinical and administrative dataBMJ. Open. 2015;5(5):e006971.
- El-serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468.
- Huo T-I, Wu J-C, Lui W-Y, et al. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99(8):1479–1487.
- Little SA, Jarnagin WR, DeMatteo RP, et al. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6(1):88–94.
- Sharma P, Welch K, Eikstadt R, et al. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15(9):1142–1148.
- Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35(5):1179–1185.
- Lezaic V. Albuminuria as a biomarker of the renal disease. In: Patel VB, editor. Biomarkers in kidney disease. Dordrecht: Springer Netherlands; 2015. p. 1–18.
- van der Velde M, Matsushita K, Coresh J, Chronic Kidney Disease Prognosis Consortium, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352.
- Tsoris A, Marlar CA, StatPearls. Use of the child Pugh score in liver disease. Treasure Island, FL: StatPearls Publishing; 2020.
- Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- Buse JB, Caprio S, Cefalu WT, et al. How do We define cure of diabetes? Diabetes Care. 2009;32:2133–2135.
- Bakker SJL. Chronic kidney disease: defining clinical cut-offs for albumin: creatinine ratio. Nat Rev Nephrol. 2013;9(12):710–712.
- Vervoort G, Willems HL, Wetzels JFM. Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation. Nephrol Dial Transplant. 2002;17(11):1909–1913.
- Hegazi R, El-Gamal M, Abdel-Hady N, et al. Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann Glob Health. 2015;81(6):814–820.
- Pazhanivel M, Jayanthi V. Diabetes mellitus and cirrhosis liver. Minerva Gastroenterol Dietol. 2010;56:7–11.
- Chehadeh W, Abdella N, Ben-Nakhi A, et al. Risk factors for the development of diabetes mellitus in chronic hepatitis C virus genotype 4 infection. J Gastroenterol Hepatol. 2009;24(1):42–48.
- Chung W, Promrat K, Wands J. Clinical implications, diagnosis, and management of diabetes in patients with chronic liver diseases. World J Hepatol. 2020;12:533–557.
- Blanco CDV, Gentile S, Marmo R, et al. Alterations of glucose metabolism in chronic liver disease. Diabetes Res Clin Pract. 1990;8:29–36.
- Grancini V, Trombetta M, Lunati ME, et al. Contribution of β-cell dysfunction and insulin resistance to cirrhosis-associated diabetes: role of severity of liver disease. J Hepatol. 2015;63(6):1484–1490.
- Moreau R, Delègue P, Pessione F, et al. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int. 2004;24(5):457–464.
- Stepanova M, Clement S, Wong R, et al. Patients with diabetes and chronic liver disease are at increased risk for overall mortality: a population study from the United States. Clin Diabetes. 2017;35(2):79–83.
- Elkrief L, Chouinard P, Bendersky N, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60(3):823–831.
- Bigam DL, Pennington JJ, Carpentier A, et al. Hepatitis C–related cirrhosis: a predictor of diabetes after liver transplantation. Hepatology. 2000;32(1):87–90.
- Pagadala M, Dasarathy S, Eghtesad B, et al. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15(12):1662–1670.
- Laryea M, Watt KD, Molinari M, et al. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13(8):1109–1114.
- Zein NN, Abdulkarim AS, Wiesner RH, et al. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000;32(2):209–217.
- Menon KVN, Nyberg SL, Harmsen WS, et al. MELD and other factors associated with survival after liver transplantation. Am J Transplant. 2004;4(5):819–825.
- Blanco JJ, Herrero JI, Quiroga J, et al. Liver transplantation in cirrhotic patients with diabetes mellitus: midterm results, survival, and adverse events. Liver Transpl. 2001;7(3):226–233.
- John PR, Thuluvath PJ. Outcome of liver transplantation in patients with diabetes mellitus: a case-control study. Hepatology. 2001;34(5):889–895.
- Younossi ZM, Stepanova M, Saab S, et al. The impact of type 2 diabetes and obesity on the long-term outcomes of more than 85 000 liver transplant recipients in the US. Aliment Pharmacol Ther. 2014;40(6):686–694.
- Yoo HY, Thuluvath PJ. The effect of insulin-dependent diabetes mellitus on outcome of liver transplantation. Transplantation. 2002;74:1007–1012.
- Velidedeoglu E, Mange KC, Frank A, et al. Factors differentially correlated with the outcome of liver transplantation in HCV + and HCV- recipients. Transplantation. 2004;77:1834–1842.
- Wada T, Haneda M, Furuichi K, Research Group of Diabetic Nephropathy, Ministry of Health, Labour, and Welfare of Japan, et al. Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol. 2014;18(4):613–620.
- Svensson MK, Cederholm J, Eliasson B, Swedish National Diabetes Register, et al. Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with type 2 diabetes: a nationwide observational study from the Swedish National Diabetes Register. Diab Vasc Dis Res. 2013;10(6):520–529.
- Aragón-Sánchez J, Lázaro-Martínez JL, García-Álvarez Y, et al. Albuminuria is a predictive factor of in-hospital mortality in patients with diabetes admitted for foot disease. Diabetes Res Clin Pract. 2014;104(1):e23-25–e25.
- Hsieh Y-M, Lee W-J, Sheu WH-H, et al. Inpatient screening for albuminuria and retinopathy to predict long-term mortality in type 2 diabetic patients: a retrospective cohort study. Diabetol Metab Syndr. 2017;9(1):29.
- Toyama T, Furuichi K, Ninomiya T, et al. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all-cause mortality, and renal events in diabetic patients: meta-analysis. PLOS One. 2013;8(8):e71810.
- Pafundi PC, Garofalo C, Galiero R, et al. Role of albuminuria in detecting cardio-renal risk and outcome in diabetic subjects. Diagnostics (Basel). 2021;11(2):290.
- El Nahas M. Cardio-kidney-damage: a unifying concept. Kidney Int. 2010;78(1):14–18.
- Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25.
- Oomen PH, Jager J, Hoogenberg K, et al. Capillary permeability is increased in normo- and microalbuminuric type 1 diabetic patients: amelioration by ACE-inhibition. Eur J Clin Invest. 1999;29(12):1035–1040.
- Klimontov VV, Korbut AI. Albuminuric and non-albuminuric patterns of chronic kidney disease in type 2 diabetes. Diabetes Metab Syndr. 2019;13(1):474–479.
- Coca SG, Jammalamadaka D, Sint K, et al. Preoperative proteinuria predicts acute kidney injury in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2012;143(2):495–502.
- Wahl ST, Graham LA, Morris MS, et al. Association between preoperative proteinuria and postoperative acute kidney injury and readmission. JAMA Surg. 2018;153(9):e182009.
- Pan HC, Chen YJ, Lin JP, et al. Proteinuria can predict prognosis after liver transplantation. BMC Surg. 2016;16(1):63.
- Amygdalos L, Bednarsch J, Meister FA, et al. Clinical value and limitations of the preoperative C-reactive-protein-to-albumin ratio in predicting post-operative morbidity and mortality after deceased-donor liver transplantation: a retrospective single-Centre study. Transpl Int. 2021;34(8):1468–1480