Abstract
Background
Primary Sjögren’s syndrome (pSS) is an autoimmune disease with increased risk of infections. Here, we assessed whether pSS patients were at higher risk of hospitalization for community and opportunistic infections.
Methods
We selected newly hospitalized pSS patients between 2011 and 2018, through a nationwide population-based retrospective study using the French Health insurance database. We compared the incidence of hospitalization for several types of infections (according to International Classification for Disease codes, ICD-10) between pSS patients and an age- and sex-matched (1:10) hospitalized control group. We calculated adjusted Hazard Ratios (aHR, 95% CI) adjusted on socio-economic status, past cardiovascular or lung diseases and blood malignancies factors.
Results
We compared 25 661 pSS patients with 252 543 matched patients. The incidence of hospitalizations for a first community infection was increased in pSS patients [aHR of 1.29 (1.22–1.31), p < .001]. The incidence of hospitalization for bronchopulmonary infections was increased in pSS patients [aHR of 1.50 (1.34–1.69), p < .001, for pneumonia]. Hospitalizations for pyelonephritis and intestinal infections were increased [aHR of 1.55 (1.29–1.87), p < .001 and 1.18 (1.08–1.29), p < .001, respectively]. Among opportunistic infections, only zoster, and mycobacteria infections (tuberculosis and non-tuberculous) were at increased risk of hospitalization [aHR of 3.32 (1.78–6.18), p < .001; 4.35 (1.41–13.5), p = .011 and 2.54 (1.27–5.06), p = .008, respectively].
Conclusions
pSS patients are at higher risk of hospitalization for infections. The increased risk of hospitalization for mycobacterial infections illustrates the potential bilateral relationship between the two conditions. Vaccination against respiratory pathogens and herpes zoster virus may help prevent some hospitalizations in pSS patients.
Primary Sjögren’s syndrome (pSS) increases hospitalization risk for community infections: bronchopulmonary, skin, dental, ear–nose–throat, intestinal infections and pyelonephritis.
Hospitalizations for zoster and mycobacterial infections are also increased in this population.
Dedicated preventive measures and vaccination campaigns could decrease the burden of infections in pSS patients.
KEY MESSAGES
Introduction
Sjögren’s syndrome (SS) is an autoimmune disease with a wide clinico-pathologic spectrum expanding from an autoimmune exocrinopathy, to systemic disease, and can evolve to B-lymphocyte malignancy [Citation1]. Leading causes of mortality are blood malignancies [Citation1], cardiovascular diseases [Citation2] and infections [Citation3–5], especially bronchopulmonary infections. Infections were responsible for 24% of deaths in one series of 1045 SS patients [Citation1].
The impact of infections on the prognosis and mortality of autoimmune diseases has been widely described in systemic lupus erythematosus (SLE) [Citation6], systemic sclerosis [Citation7] and rheumatoid arthritis [Citation8]. SS differs from these diseases due to the reduced amount of steroids or immunosuppressive treatments used [Citation9] and a pathophysiology potentially involving microbial triggers such as Epstein Barr Virus [Citation10] or mycobacteria [Citation11].
Among all causes of hospitalizations of pSS patients, infections make up around 30% of cases [Citation5], including in intensive care units [Citation12]. However, little is known about the epidemiology of severe infections (i.e. requiring hospitalization) in large cohorts of pSS patients [Citation13]. A better description of the risks of specific infections in pSS patients could help to tailor preventive strategies. We aimed to describe the comparative incidence of hospitalization for community and opportunistic infections between pSS hospitalized patients and matched controls.
Methods
Database
We performed a historical paired exposed–unexposed cohort study by analysing data from the French National Hospital discharge database (‘programme de médicalisation des systèmes d‘information’), which covers 99% of hospitalized patients. Available data were: age, sex, entry and discharge dates, discharge diagnoses’ codes according to international classification of diseases (ICD-10), death in hospitalization, and socioeconomic status (specific insurance coverage for low-income people). Information on clinical (systemic involvement of the disease), biological (including autoantibody positivity), pathological data, procedures or treatments (including steroids or immunosuppressive treatments) was not available. We studied a 10-year period between 1 January 2009 and 31 December 2018.
Study population
We included all patients with a first hospitalization for pSS from 2011. First, we selected all hospitalized patients with at least one ICD-10 code of SS (M350). The date of the first hospitalization was defined as the index date. We excluded all patients with a suspected secondary SS (sSS, i.e. SLE, systemic sclerosis, rheumatoid arthritis, vasculitis, hepatitis C virus etc. Supplementary Table S1), identified through their corresponding ICD-10 codes over the entire study period. Patients with a first code of SS in 2009 or 2010 were excluded, to have at least 2 years of available medical history of hospitalization prior to the index date. This cohort was described in previous studies [Citation14]. We randomly constituted a control group (1:10) of hospitalized patients matched on age, sex and index date within the same database (excluding pSS subjects). We excluded patients who died in hospitalization within the 90 days after the index date in both groups, to avoid selection of patients with acute severe and critical illnesses at the index date. For each studied infection, all subjects with prior hospital admission for the condition before the incidence study period, and their matched patients, were excluded.
Table 1. Characteristics of hospitalized primary Sjögren’s syndrome patients and their matched controls, and adjustment covariates.
Outcomes
We recorded the first occurrence of hospitalization for all pooled community infections, and all pooled opportunistic infections, in pSS and controls. We then recorded the first hospitalization for each infection within these groups. Conditions were identified according to their ICD-10 codes (Supplementary Table S2). Community infections of interest were: bronchopulmonary infections (pneumonia, bronchitis, flu); urinary tract infections, pyelonephritis, prostatitis; meningitis; skin infections (erysipelas, dermo-hypodermitis and skin abscesses); endocarditis and other sepsis (staphylococcus, Hemophilus influenzae, anaerobes, anaerobes or unspecified sepsis); phlegmon; dental and Ear–Nose–Throat (ENT) infections; abdominal infections (anorectal abscess, intestinal abscess or fistula, peritonitis, bacterial intestinal infections, cholecystitis, diverticulitis); pelvic infections (salpingitis and oophoritis, acute parametritis and pelvic cellulitis, diseases of Bartholin’s gland, vulvo-vaginitis); arthritis and bone infections (osteomyelitis, infection and inflammatory reaction due internal orthopaedic prosthetic devices, implants and grafts, infective spondylopathies and discitis).
Table 2. Incidence of first hospitalization for community infections in hospitalized primary Sjögren’s syndrome patients and controls.
For opportunistic infections, we recorded: herpes, zoster, chicken pox, cytomegalovirus, non-tuberculous mycobacteria (NTM), tuberculosis, listeria, salmonella non-typhii, pneumocystis, toxoplasmosis, histoplasmosis, candidiasis and aspergillosis.
We also studied the comparative mortality incidence in hospital for the following infections: pneumonia, pyelonephritis, intestinal infections, and dental and ENT infections.
Covariates
We searched for existing conditions potentially influencing infection risks prior to the 90th day after the index date: low socioeconomic status (LSS), annual rate of hospitalization before the index date, hypertension, diabetes, obesity, cardiovascular diseases, dialysis, neuropsychiatric disorders (dementia, depression or anxiety), chronic obstructive pulmonary disease (COPD), interstitial pneumonitis and lung fibrosis (interstitial lung disease, ILD), and blood malignancies (lymphoma, Waldenström macroglobulinaemia, multiple myeloma, and leukemia, Supplementary Table S1).
Statistical analysis
Conditions were compared between cases and controls using χ2, Fisher exact test, or Mann–Whitney rank-sum test when appropriate. The incidences of overall community and opportunistic infections, and the incidences of each infection, were calculated in each exposure group, with their 95% confidence intervals (CI), after excluding patients who were already hospitalized for the studied condition before the 90th day after the index date. A survival analysis by Cox proportional hazard models, with stratification on the matched subjects, was then performed to compare incidence between exposure groups. Follow-up time was calculated as the interval between the index date and the date of the first event detected, or until December 2018, whichever occurred first. Follow-up of patients who died in hospital before December 2018, without any event, were censored at the date of death to take into account competitive risks. Analysis was made on the available data (no imputation of missing data).
For the study of the incidence of infections, we first built a crude statistical model. Then, to take into account potential confounding factors [Citation3] influencing the incidence of infections, we built an adjusted model including LSS, annual hospitalization rate, history of hypertension, diabetes, obesity, dialysis, cardiovascular diseases, blood malignancies and neuropsychiatric disorders. For the study of hospitalizations for bronchopulmonary infections (pneumonia, bronchitis, flu, tuberculosis, non-tuberculous infections, pneumocystis and aspergillosis), we also included in our adjustment factors history of COPD and ILD. To study whether the association between bronchopulmonary infections and pSS was mediated by the onset of ILD, we performed an additional model including this parameter as a time-dependent covariate.
Statistical analyses were performed at the conventional two-tailed α level of 0.05 using Statistical Analysis Systems Enterprise Guide 7.1 (SAS Institute, Cary, NC).
Ethics
French National Hospital discharge database is an anonymized national health insurance database and studies on anonymized national health insurance database are authorized by the National Commission of Information Technology and Liberty (CNIL). No specific consent or ethical approval was thus needed for this study (https://www.cnil.fr/fr/declaration/mr-005-etudes-necessitant-lacces-aux-donnees-du-pmsi-etou-des-rpu-par-les-etablissements).
Results
Study population
The characteristics of the study population are shown in . We identified 25,661 hospitalized patients with pSS and 252,543 age- and sex- matched hospitalized controls from the French National Health insurance database (Supplementary Figure S1). The population consisted of 87.7% of female patients, with a mean age of 60.0 (±16.3) years and a median follow-up time of 3.96 years. We observed a higher proportion of blood hypertension, diabetes, obesity, cardiovascular diseases, dialysis, blood malignancies and neuropsychiatric conditions in pSS than controls. Moreover, there was a higher proportion of lung diseases (ILD: 2.71% versus 0.06%, and COPD: 1.72% versus 0.48%) among pSS patients.
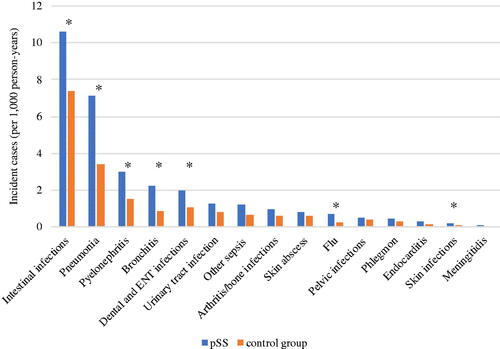
Figure 1. Comparative incidence of hospitalization for community infections in primary Sjögren’s syndrome patients versus matched control. pSS: primary Sjögren’s syndrome patients; ENT: ear-nose-throat. The asterisk * marks the infections with statistically different incidences between pSS patients and matched controls.
Infections reported during hospitalization before the incidence study period are listed in Supplementary Table S3.
Table 3. Incidence of first hospitalization for opportunistic infections in hospitalized primary Sjögren’s syndrome patients and controls.
Incidence of community infections
The 2254 pSS patients (8.78%) experienced at least one hospitalization for a community infection within the study period (). After adjustment, the incidence of hospitalizations for a first community infection was increased in pSS patients compared with matched controls [aHR = 1.29, 95% CI (1.22–1.31), p < .001]. The four leading causes of incident hospitalizations for infections in pSS patients were: (i) intestinal infections; (ii) bronchopulmonary infections; (iii) pyelonephritis and (iv) dental and ENT infections. shows the comparative incidence of hospitalization for specific subgroups of community infections.
The incidence of hospitalization was increased for intestinal infections in pSS patients aHR = 1.18 [95% CI (1.08–1.29), p < .001]. Similarly, pSS patients had a significantly higher incidence rate of hospitalization for bronchopulmonary infections compared with matched controls: aHR were 1.50 [95% CI (1.34–1.69), p < .001] for pneumonia, 1.70 [95% CI (1.36–2.11), p < .001] for bronchitis and 1.98 [95% CI (1.32–2.97), p < .001] for flu. When integrating ILD as a time-dependent covariate in our bronchopulmonary infections survival model, the association between pSS and pneumonia, bronchitis and flu remained similar aHR = 1.48 [95% CI (1.32–1.68), p < .001]; aHR = 1.70 [95% CI (1.36–2.11), p < .001]; aHR = 1.97 [95% CI (1.32–2.96), p < .001], respectively). Incident hospitalization rates for pyelonephritis, skin infections and dental and ENT infections were also increased in pSS patients: aHR 1.55 [95% CI (1.29–1.87), p < .001]; 3.54 [95% CI (1.54–8.18), p = .003] and 1.27 [95% CI (1.04–1.56), p = .021], respectively.
Incidence of opportunistic infections
The 104 pSS patients (0.04%) experienced at least one opportunistic infection over the study period. The incidence of hospitalizations for a first opportunistic infection was increased in pSS patients compared with matched controls [aHR = 1.98, 95% CI (1.46–2.69), p < .001]. Hospitalizations for zoster were increased in the pSS patients [aHR = 3.32, 95% CI: (1.78–6.18), p < .001]. Incident hospitalizations for mycobacteria were increased in pSS patients [NTM: aHR = 4.35, 95% CI: (1.41–13.5), p = .011; and tuberculosis: aHR = 2.54, 95% CI: (1.27–5.06), p = .008, ]. After integrating ILD as a time-dependent covariate in our model, the comparative incidence of NTM and tuberculosis did not change [aHR = 4.37, 95% CI: (1.41–13.5), p = .011]; aHR = 2.55 [95% CI: (1.28–5.09), p = .008]. Aspergillosis incident rate of hospitalization was higher in pSS patients [aHR = 3.94, 95% CI: (2.34–6.64), p < .001], but this association disappeared when integrating ILD as an adjustment factor [aHR = 1.68, 95% CI: (0.62–4.57), p = .307]. Notably, the incident hospitalization rate for ILD was increased in pSS [aHR = 9.04, 95%CI: (6.94–11.80), p < .001]. There was no incident toxoplasmosis in both groups, and 1 histoplasmosis in control group.
Mortality
The incidence of in-hospital mortality of pSS patients was not different from that of controls for each group of main infection (pneumonia, pyelonephritis, intestinal infections or dental and ENT infections), regardless of the adjustment factors included.
Discussion
Our nationwide database study highlights the burden of infections on the hospitalizations of pSS patients in comparison with matched hospitalized patients. We showed an increased risk for hospitalization for community infections, especially bronchopulmonary, intestinal, pyelonephritis, and dental and ENT infections, and for some opportunistic infections (zoster and mycobacteria). Our results are in line with the literature. Among 69,239 hospitalizations of pSS US patients, leading causes of infections were pneumonia, sepsis, soft tissue, skin and subcutaneous infections, and urinary tract infections, while opportunistic infections only involved 3% of subjects [Citation3]. However, to our knowledge, the increased incidence of intestinal infections has never been reported and needs further confirmation.
Factors associated with community infections
Sicca syndrome may be partially responsible for the higher incidence of dental and ENT infection, skin infections, pyelonephritis (as a consequence of vaginal dryness), and bronchopulmonary infections. The higher rates of intestinal infections may arise from functional digestive disorders and gastroparesia [Citation15], and gut microbial overgrowth (exacerbated by sicca syndrome) [Citation16].
Opportunistic infections
Although absolute numbers of recorded infections were relatively low, we found increased hospitalization incidence in pSS patients for zoster and mycobacteria. A Taiwanese study including 18 000 pSS patients showed that pSS patients had the lowest incidence of opportunistic infections compared with other auto-immune diseases (dermato-myositis, scleroderma or rheumatoid arthritis [Citation9]). Even for herpes and zoster infection, our incidence rate was lower than previously published in pSS and auto-immune disease populations [Citation9, Citation17]. This is likely due to only considering inpatients, and to the lower burden of immunosuppressants (especially glucocorticoids) in pSS [Citation9].
We found an increased risk of hospitalization for tuberculous and NTM infections in pSS patients. Chang et al. [Citation18] also found an increased risk of tuberculosis infection in 4822 Taiwanese pSS patients, and Chao et al. [Citation19] observed an increased risk of NTM in 6554 incident Taiwanese SS patients after adjusting for a comorbidity index, glucocorticoids and immunosuppressants use. In a nationwide study involving 5751 pSS patients, an association between a history of NTM and incident pSS was found [Citation20]. The role of sex hormone deprivation on mycobacteria and pSS onset has been proposed [Citation21, Citation22]. An alternative immunological hypothesis based on a long-lasting immune reaction against mycobacteria has been suggested in pSS patients, through a higher prevalence of anti-hsp65 antibodies (targeting mycobacterium antigen) [Citation23]. The role of TNFAIP3 (TNF-alpha-induced Protein 3, modulating NF-kB activation) has also been suggested in both pSS-related lymphomas [Citation24] and mycobacterial susceptibility [Citation25].
Interestingly, we found a higher incidence of hospitalization for ILD in pSS patients, as previously reported [Citation26, Citation27], which may have modulated the infectious respiratory risks in pSS patients. In our study, the over-risks of pneumonia, bronchitis, flu, NTM and tuberculosis in pSS patients were not modified when including ILD as a time-dependent covariate. Conversely, the relation between SS and aspergillosis may be partly mediated by the increased risk of ILD in pSS patients. Several pathophysiological mechanisms could be involved: (i) ILD may lead to bronchopulmonary damage, facilitating aspergillosis colonization and survival; (ii) local mucosal-immune surveillance may be impaired and (iii) immunosuppressant treatments of ILD, including glucocorticoids, may further weaken immune defences [Citation27].
We did not find any over-risk of hospitalization for pneumocystis in pSS patients. This contrasts with the excess incidence of pneumocystis of 6.68 (4.03–11.07) in 23 048 pSS patients, in the study by Hsu et al. [Citation28]. These discrepancies may partially be explained by the potential confounding factors involved in the models, such as glucocorticoids and immunosuppressants use, or ILD [Citation28].
Prevention of higher infection risks
Maintaining good oral health is essential in pSS patients to prevent dental and ENT infections. Ensuring proper skin moisturizing and a good vaginal trophicity may also prevent urinary tract infections and pyelonephritis, especially among postmenopausal patients.
Bronchopulmonary infections and ENT infections could also be reduced through appropriate vaccination, such as annual flu and anti-pneumococcal vaccines. In a previous study, Morel et al. [Citation29] described that only 31.5% of their series of 111 pSS patients were vaccinated against flu, and 11.7% against pneumococcus. Vaccination is probably efficient within pSS population, as shown by the post-vaccination immune response of anti-influenza IgG levels in pSS patients [Citation30], and the anti-pneumococcal response in 15 patients with pSS [Citation31].
Strengths and limitations
Our methodological approach presents some limits. We only collected events in hospitalized patients as we wanted to focus on the most severe cases. Deaths occurring outside hospitalization were not recorded. Since the diagnoses were based on ICD-10 codes, some patients could have been misclassified for pSS diagnosis or for infections. We could not integrate all the potential cofounding factors for infection risk such as tobacco and alcohol use, and glucocorticoids or immunosuppressants exposure. Indeed, the burden of immunosuppressants in pSS could affect infectious risk, especially in some subgroups of patients with active disease (such as ILD) [Citation9, Citation28]. However, considering a recent study involving 134 French pSS patients, this impact may be limited since only 17% were treated by steroids and 14% by immunosuppressive drug [Citation32]. Thus, we included the available comorbid conditions (e.g. obesity, cardio-vascular diseases, lung conditions) in our statistical models, since these comorbidities could alter the onset of infections in both groups. Finally, we selected controls who were hospitalized for another reason than pSS. It is therefore probable that controls were less ‘healthy’ than the general population, weakening the strength of identified associations and reinforcing our conclusions about the increased risk of some infections in pSS patients.
Conclusions
In this large nationwide study, hospitalized pSS patients showed a higher risk of hospitalization for community infections, especially bronchopulmonary infections and intestinal infections, compared with matched controls. Likewise, incidence of opportunistic infections was increased, and a potential bilateral relationship between SS and mycobacteria is suggested. A targeted vaccination campaign against respiratory pathogens and strict management of sicca syndrome could be beneficial in reducing this risk.
Author contributions
All authors made substantial contributions to this work. Conceptualization, R.G., D.N., P.G. and T.M.; Data curation, R.G.; Formal analysis, R.G., A.M., J.M., P.W.-D.-V., K.H., N.N., P.L., C.R., D.M., P.G. and T.M.; Investigation, R.G., P.G. and T.M.; Methodology, R.G., A.M., P.G. and T.M.; Software, R.G. and T.M.; Supervision, P.G. and T.M.; Validation, P.G. and T.M.; Visualization, R.G., A.M., J.M., D.N., C.R., D.M., K.H., P.G. and T.M.; Writing—original draft, R.G., A.M., J.M., P.G. and T.M.; Writing—review and editing, R.G., A.M., J.M., P.W.-D.-V., N.N., P.L., C.R., D.N., D.M., K.H., T.M., P.G.
Supplemental Material
Download MS Word (89.4 KB)Acknowledgements
We sincerely thank Sarah Kabani for editing assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
- Brito-Zerón P, Kostov B, Solans R, et al. Systemic activity and mortality in primary Sjögren syndrome: predicting survival using the EULAR-SS disease activity index (ESSDAI) in 1045 patients. Ann Rheum Dis. 2016;75(2):348–355.
- Beltai A, Barnetche T, Daien C, et al. Cardiovascular morbidity and mortality in primary Sjögren’s syndrome: a systematic review and meta-analysis. Arthritis Care Res. 2020;72(1):131–139.
- Singh JA, Cleveland JD. Serious infections in Sjögren’s syndrome patients: a national U.S. study. Clin Exp Rheumatol. 2020;38 Suppl 126(4):47–52.
- Consani Fernández SA, Díaz Cuña CL, Fernández Rey L, et al. Infections in systemic autoimmune diseases. Reumatol Clin. 2021;17:582–587.
- Atisha-Fregoso Y, Rivera-Vicencio Y, Baños-Pelaez M, et al. Main causes and risk factors for hospitalisation in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol. 2015;33:721–725.
- Moghaddam B, Marozoff S, Li L, et al. All-cause and cause-specific mortality in systemic lupus erythematosus: a population-based study. Rheumatology 2021;61(1):367–376.
- Kouchit Y, Morand L, Martis N. Mortality and its risk factors in critically ill patients with connective tissue diseases: a meta-analysis. Eur J Intern Med. 2022;98:83–92.
- Bechman K, Halai K, Norton S, et al.; British Society for Rheumatology Biologics Register for Rheumatoid Arthritis Contributors Group. Non-serious infections in patients with rheumatoid arthritis; results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73(Supplement_2):1800–1809.
- Hsu C-Y, Ko C-H, Wang J-L, et al. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther. 2019;21(1):211.
- Barcelos F, Martins C, Monteiro R, et al. Association between EBV serological patterns and lymphocytic profile of SjS patients support a virally triggered autoimmune epithelitis. Sci Rep. 2021;11(1):4082.
- Dow CT, Chan ED. What is the evidence that mycobacteria are associated with the pathogenesis of Sjogren’s syndrome? J Transl Autoimmun. 2021;4:100085.
- Ruiz-Ordoñez I, Aragón CC, Padilla-Guzmán A, et al. Sjögren syndrome in the intensive care unit: an observational study. J Clin Rheumatol. 2020;26(7S):S174–S179.
- Pego-Reigosa JM, Restrepo Vélez J, Baldini C, et al. Comorbidities (excluding lymphoma) in Sjögren’s syndrome. Rheumatology 2021;60:2075–2084.
- Goulabchand R, Malafaye N, Jacot W, et al. Cancer incidence in primary Sjögren’s syndrome: data from the French hospitalization database. Autoimmun Rev. 2021;20(12):102987.
- Parreau S, Jacques J, Dumonteil S, et al. Abdominal symptoms during Sjogren’s syndrome: a pilot study. Adv Rheumatol. 2021;61(1):5.
- Cano-Ortiz A, Laborda-Illanes A, Plaza-Andrades I, et al. Connection between the gut microbiome, systemic inflammation, gut permeability and FOXP3 expression in patients with primary Sjögren’s syndrome. Int J Mol Sci. 2020;21:E8733.
- Chen J-Y, Wang L-K, Feng P-H, et al. Risk of shingles in adults with primary Sjogren’s syndrome and treatments: a nationwide population-based cohort study. PLoS One 2015;10(8):e0134930.
- Chang Y-S, Liu C-J, Ou S-M, et al. Tuberculosis infection in primary Sjögren’s syndrome: a nationwide population-based study. Clin Rheumatol. 2014;33(3):377–383.
- Chao W-C, Lin C-H, Liao T-L, et al. The risk of nontuberculous mycobacterial infection in patients with Sjögren’s syndrome: a nationwide, population-based cohort study. BMC Infect Dis. 2017;17(1):796.
- Chao W-C, Lin C-H, Liao T-L, et al. Association between a history of mycobacterial infection and the risk of newly diagnosed Sjögren’s syndrome: a nationwide, population-based case-control study. PLoS One 2017;12(5):e0176549.
- Uwamino Y, Nishimura T, Sato Y, et al. Low serum estradiol levels are related to Mycobacterium avium complex lung disease: a cross-sectional study. BMC Infect Dis. 2019;19(1):1055.
- Danley J, Kwait R, Peterson DD, et al. Normal estrogen, but low dehydroepiandrosterone levels, in women with pulmonary Mycobacterium avium complex. A preliminary study. Ann Am Thorac Soc. 2014;11(6):908–914.
- Zhang P, Minardi LM, Kuenstner JT, et al. Serological testing for mycobacterial heat shock protein Hsp65 antibody in health and diseases. Microorganisms 2019;8(1):47.
- Nocturne G, Tarn J, Boudaoud S, et al. Germline variation of TNFAIP3 in primary Sjögren’s syndrome-associated lymphoma. Ann Rheum Dis. 2016;75(4):780–783.
- Hanson EP, Monaco-Shawver L, Solt LA, et al. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122(6):1169–1177.e16.
- Ng K-H, Chen D-Y, Lin C-H, et al. Risk of interstitial lung disease in patients with newly diagnosed systemic autoimmune rheumatic disease: a nationwide, population-based cohort study. Semin Arthritis Rheum. 2020;50(5):840–845.
- Luppi F, Sebastiani M, Silva M, et al. Interstitial lung disease in Sjögren’s syndrome: a clinical review. Clin Exp Rheumatol. 2020;38 Suppl 126(4):291–300.
- Hsu H-C, Chang Y-S, Hou T-Y, et al. Pneumocystis jirovecii pneumonia in autoimmune rheumatic diseases: a nationwide population-based study. Clin Rheumatol. 2021;40(9):3755–3763.
- Morel J, Letaief H, Guilpain P, et al. Pneumococcal and Diphtheria–Tetanus–Poliomyelitis vaccine coverage in patients with primary Sjögren’s syndrome: a cross-sectional study. Vaccines 2019;8(1):3.
- Brauner S, Folkersen L, Kvarnström M, et al. H1N1 vaccination in Sjögren’s syndrome triggers polyclonal B cell activation and promotes autoantibody production. Ann Rheum Dis. 2017;76(10):1755–1763.
- Nived P, Saxne T, Geborek P, et al. Antibody response to 13-valent pneumococcal conjugate vaccine is not impaired in patients with rheumatoid arthritis or primary Sjögren’s syndrome without disease modifying treatment. BMC Rheumatol. 2018;2:12.
- Dubost J-J, Couderc M, Pereira B, et al. Concomitant fibromyalgia in primary Sjögren’s syndrome in the French ASSESS cohort: comparison of the ACR 1990 and ACR 2016 criteria, FiRST questionnaire and physician’s opinion. Clin Exp Rheumatol. 2021;39(6):140–145.
