Abstract
Introduction: Phytochemicals have garnered much attention because they are useful in managing several human diseases. Yohimbine is one such phytochemical with significant pharmacological potential and could be exploited for research by medicinal chemists. It is an indole alkaloid obtained from various natural/synthetic sources.
Aims and Results: The research on yohimbine started early, and its use as a stimulant and aphrodisiac by humans has been reported for a long time. The pharmacological activity of yohimbine is mediated by the combined action of the central and peripheral nervous systems. It selectively blocks the pre and postsynaptic α2-adrenergic receptors and has a moderate affinity for α1 and α2 subtypes. Yohimbine also binds to other behaviourally relevant monoaminergic receptors in the following order: α-2 NE > 5HT-1A>, 5HT-1B > 1-D > D3 > D2 receptors.
Conclusion: The current review highlights some significant findings that contribute to developing yohimbine-based drugs. It also highlights the therapeutic potential of yohimbine against selected human diseases. However, further research is recommended on the pharmacokinetics, molecular mechanisms, and drug safety requirements using well-designed randomized clinical trials to produce yohimbine as a pharmaceutical agent for human use.
Yohimbine is a natural indole alkaloid with significant pharmacological potential.
Humans have used it as a stimulant and aphrodisiac from a relatively early time.
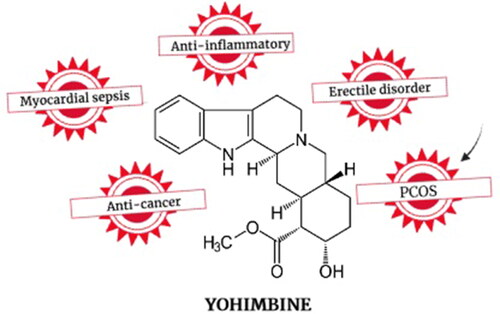
It blocks the pre- and postsynaptic α2-adrenergic receptors that could be exploited for managing erectile dysfunction, myocardial dysfunction, inflammatory disorders, and cancer.
Key Messages
Introduction
Yohimbine is a monoterpenoid, indole alkaloid with potent pharmacological effects obtained from several biological sources [Citation1]. It is also called quebrachine, aphrodine, corynine, and hydroaerogotocin. Indole-based pharmaceutical agents constitute a significant class of therapeutic molecules, and much progress has been made in their utilization in current therapies [Citation2–4]. Yohimbine has been used as a stimulant and aphrodisiac to improve erectile function [Citation5]. Yohimbine is a prototypical α2-receptor antagonist licenced in Germany (since 1978) and Canada (since 1951) as therapy for erectile dysfunction [Citation6]. Over several decades, it has been used as a therapeutic agent for sexual difficulties and is a comparatively safer agent with few known adverse effects [Citation7]. The hyperadrenergic effects of yohimbine are beneficial in various disease conditions such as erectile dysfunction, myocardial dysfunction, inflammatory disorders, and cancer [Citation8–11]. It also has antidiuretic, anti-adrenaline, mydriatic, serotonin antagonist properties [Citation5]. Yohimbine combines effects of central and peripheral nervous system, including blocking of pre- and postsynaptic α2-adrenergic receptors [Citation12]. The usual recommended dose of pure yohimbine is 5–10 mg thrice a day. The side effects of α-2 adrenergic inhibition are often minor and short-lived. They consist of hypertension, perspiration, chest pain, sleeplessness, anxiety, and palpitations [Citation10]. Despite extensive pharmacological data, the exact mechanism of action of yohimbine is unclear and needs further research.
Chemistry of yohimbine
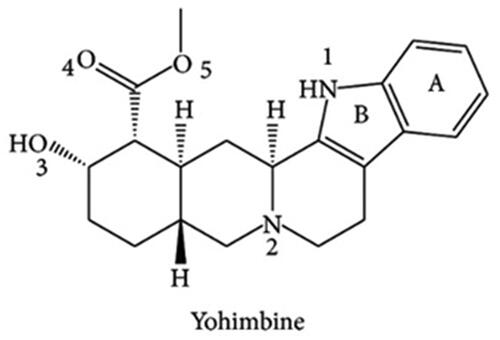
Yohimbine is a nitrogenous organic compound with the chemical formula C21H26N2O3 and a molecular weight of 354.44 g/mol (). It is a 17, α-hydroxyyohimban-16, α-carboxylic acid methyl ester. Tryptophan and the secoiridoid monoterpene secologanin are the sources of this pentacyclic monoterpenoid indole alkaloid [Citation13,Citation14]. Indole is a weak base comprising a pyrrole ring fused to a benzene nucleus and ten π-electrons moving throughout the structure [Citation15]. Natural indole alkaloids have shown high therapeutic potential in preclinical and clinical studies [Citation4,Citation15,Citation16]. Several yohimbine stereoisomers have been identified based on their stoichiometric arrangement. The alkaloids are divided into four subfamilies based on the stereochemical arrangement around the D-ring: normal, allo, pseudo, and epiallo. The representative examples of these subfamilies are yohimbine, rauwolscine, pseudoyohimbine, and reserpine [Citation14]. Yohimbine synthesis is complicated because of the complex generation of the pentacyclic rings containing five chiral centres [Citation17]. It is colourless, weakly basic with a pKa value of 6–7.5, highly soluble in alcohol, and sparingly soluble in water [Citation18]. It is readily soluble in chloroform and ethanol and is sparingly soluble in diethyl ether. It has a melting point of 234 °C and decomposes at 302 °C [Citation19]. Yohimbine hydrochloride undergoes various reactions like acidic and alkaline hydrolysis and photo-degradation [Citation13]. The yohimbine reaction is resistant to acid catalysis and is remarkably stable at very low pH, probably due to the intramolecular H-bonding between the β-hydroxyl group and the ester’s acyl group. Awad et al. [Citation20] studied the kinetics of yohimbine hydrolysis and reported its high stability. Hydrolysis is the main route of degradation of yohimbine hydrochloride at pH 6 and 7 to form yohimbine acid, and it follows first-order kinetics. Around neutral pH, yohimbine hydrochloride is stable enough to develop a transdermal preparation. The yohimbine compounds meet fundamental drug-like criteria with DPQ and quantitative estimates of drug-likeness >0.5 [Citation21].
Isolation of yohimbine using different analytical approaches
Yohimbine hydrochloride (CAS: 146-48-5) was isolated for the first time from the bark of the Pausinystalia yohimbe tree in West Africa [Citation22]. Humans have traditionally used this plant as a stimulant and aphrodisiac, particularly as herbal remedy to help erectile dysfunction. Yohimbine has been also detected in Alchornea floribunda Müll. Arg., Rauvolfia vomitoria, Rauwolfia nitida, Rauwolfia serpentina, Rauvolfia yunnanensis, and other plants [Citation5,Citation23]. A summary of extracted yohimbine from different natural sources is listed in .
Table 1. Extraction of yohimbine from various natural sources.
Synthesis of yohimbine
Several researchers synthesized yohimbine using mainly two general strategies. The first strategy involves creating a DE-ring system and then building a C-ring. On the other hand, building an ABC-ring and annulling a DE-ring are necessary for the second strategy [Citation33]. The synthesis of a racemic mixture of yohimbine was first achieved by a series of steps for constructing a DE trans group of naturally occurring pentacyclic indole derivatives [Citation34]. Aimi et al. [Citation35] synthesized yohimbine by converting some natural oxindoles (pteropodine, isopteropodine, and isorhynchophylline) into corresponding indole alkaloids. Similarly, asymmetric synthesis of yohimbine was achieved by a series of reactions that included the asymmetric intramolecular Michael reaction [Citation36]. Mergott et al. [Citation37] and Herlé et al. [Citation38] reported the catalytic asymmetric total synthesis of (+)-yohimbine using highly enantioselective thiourea-catalyzed acyl-Pictet–Spengler reaction and substrate-controlled intramolecular Diels–Alder reaction [Citation37,Citation38]. The asymmetric synthesis of (+)-yohimbine by Mergott et al. [Citation37] was achieved in 11 steps with a 14% overall yield. On the other hand, the total synthesis of enantiopure (+)-yohimbine by Herlé et al. [Citation38] involved 9 steps with an overall yield of 16% ().
Figure 2. Synthesis mechanism of yohimbine proposed by Herlé et al. [Citation38].
![Figure 2. Synthesis mechanism of yohimbine proposed by Herlé et al. [Citation38].](/cms/asset/468b8f5a-3819-4cdc-ac84-d57edc08e99c/iann_a_2131330_f0002_b.jpg)
Similarly, Feng et al. [Citation17] reported a bicyclic guanidine-catalyzed asymmetric tandem isomerization intramolecular-Diels–Alder reaction for the enantioselective catalytic synthesis of (+)-alpha-yohimbine with yield % in the range of 72–91%. Recently, Miller et al. [Citation14] proposed a concise enantioselective approach for synthesizing yohimbine alkaloids with upto 65% yield. This method provides a powerful strategy for producing various complex alkaloids and related structures for biomedical applications [Citation14].
Spectroscopic characterization of yohimbine
Yohimbine contains a pentacyclic ring structure with five chiral carbon atoms (C3, C15, C16, C17, and C20) and two nitrogen atoms [Citation18]. Multiple chiral centres form several diastereomers, which can be separated under chromatographic conditions [Citation39]. The two stereoisomers of yohimbine, rauwolscine, and corynanthine, differ little in terms of their physical characteristics and how they affect dopamine/serotonin receptors. In addition, yohimbine and reserpine share a similar structural makeup, even with different stereochemistry [Citation40]. Benoin et al. [Citation41] reported UV absorption spectrum of yohimbine in ethanol at a different excitation wavelengths of 286, 278, and 226 nm. Similarly, the UV spectrum of yohimbine in methanol and H2SO4 exhibited three band maxima at 289, 282, and 225 nm [Citation42]. In one study, Farouk et al. [Citation13] reported a characteristic λmax of yohimbine hydrochloride at 279–282 nm in the UV region. On the other hand, Joshi et al. [Citation43] observed the UV spectrum of yohimbine hydrochloride in ethanol solution with band peaks at 289, 280, and 228 nm. The main transitions observed in the UV region are π→ π* and n→ π*. A single band of low intensity in the range of 250–300 nm, with no significant absorption at shorter wavelengths, also suggests the n→ π* transition, which occurs at approximately 228 nm and is caused by the carbonyl group. The experimental analysis of yohimbine hydrochloride by Joshi et al. [Citation43] revealed details about yohimbine molecular structure and biological activities. They reported 54 atoms in the yohimbine hydrochloride molecule, which gives 156 normal modes of vibrations, belonging to an irreducible representation of both Raman and IR active. The equilibrium geometries and harmonic vibrational wavenumbers of all 156 normal molecule modes reveal detailed information about the size, shape, charge density distribution, and structure–activity relationship by mapping electron density iso-surface with electrostatic potential. The CH stretching of the yohimbine ring showed a peak at 3057 and 3031 cm−1 in the Raman and 3087 and 3066 cm−1 in the IR spectrum. On the other hand, the CC stretching vibrations of the ring are observed at 1636, 1571, and 1499 cm−1 in the Raman and 1624, 1572, and 1496 cm−1 in the IR spectrum. The N5H stretching vibration of the second ring showed an intense high peak at 3514 cm−1 in the IR spectrum. On the other hand, the CC stretching of this ring is reported at 1593 cm−1 in the Raman spectrum, having a very high intensity. Similarly, the NC stretching motions are reported high-intensity Raman spectrum at 1295 cm−1 and low-intensity IR spectrum at 1296 cm−1. Six possible stretching vibrations, namely symmetric, asymmetric stretch, deformation modes, rocking modes, wagging modes, and twisting modes, are observed in C18H2 of the third ring. At 2895 cm−1 in the infra-red spectrum and 2945 cm−1 in the Raman spectrum, the symmetric stretching vibration of C18H2 is observed. While C18H2 exhibits wagging motions at 1381 cm−1 in the Raman spectrum and at 1385 cm−1 in the IR spectrum, deformation motions of C18H2 are observed at 1453 cm−1 in the Raman spectrum and 1454 cm−1 in the IR spectrum. In the Raman and IR spectra, the twisting mode of C18H2 is recorded at 1223 and 1221 cm−1 peaks, respectively. In the Raman spectra, C18H2 is found to be rocking at 894 cm−1. A weak asymmetric mode of C11H2 is observed by CH2 and CH vibrations of the fourth ring, with peaks at 2965 and 2960 cm−1 in the Raman and 2960 cm−1 in IR spectrum, whereas the symmetric vibration mode reported peaks at 2911 cm−1 in the Raman spectrum. Deformation modes of C11H2 are reported at 1478 cm−1 with high intensity in the Raman spectra and Twisting modes peaks at 1253/1208 cm−1 in the Raman and 1246/1207 cm−1 in the IR spectrum. In the Raman and IR spectra, the combined vibrations of the C7H and C6H stretching modes are observed at 2876/2894 cm−1 and 2883/2887 cm−1, respectively. Due to the presence of two functional groups, a CH3 and a hydroxyl group, that are joined by a carboxylic group, the fifth ring is assigned with a variety of vibrational modes. It has been observed that the weak band in the IR spectrum at 3670 cm−1 corresponds to the characteristic peak related to the stretching mode of the O1H group. The peaks at 1007 cm−1 in the Raman spectra and 1018/873 cm−1 in the IR spectrum are where the O1C13 stretching wavenumbers are found. There are many associated modes with the CH3 group in this ring, including symmetric and asymmetric stretches, bends, rocks, and torsional modes. The asymmetric CH3 group stretches are reported to have significant Raman intensity at 2989 cm−1 and weak IR intensity at 3024 cm−1. In the IR spectrum, the CH3 symmetric stretches are observed at 2935 cm−1 with a medium intensity. In the 1700–1800 cm−1 range, most the carboxylic group’s distinctive characteristics are typically observed. The peak at 1754 cm−1 with weak intensity in the Raman and strong intensity at 1718 cm−1 in the IR spectra are both well matched to the high contributions of the C O stretching band, which are calculated to be at 1749 cm−1.
Yohimbine’s mass spectroscopic properties revealed fragment ions at m/z 323.1560 caused by CH3OH losses, m/z 337.1912 caused by H2O losses, and m/z 397.2108 caused by C10H12O5 losses. Yohimbine has fragment ions at m/z 224.1266, 212.1262, 158.0941, and 144.0802 because of the retro Diels Alder (RDA) cleavage of the C-ring [Citation44]. Duan et al. [Citation32] reported NMR spectroscopy characteristics of yohimbine. These results were comparable with the NMR data of yohimbine previously observed by Herlé et al. [Citation38]. Yohimbine hydrochloride’s XRD pattern showed that it has a ring system made up of two transfused six-membered rings D and E connected to two planar indole groups A and B by another six-membered ring C [Citation45].
Pharmacology of yohimbine
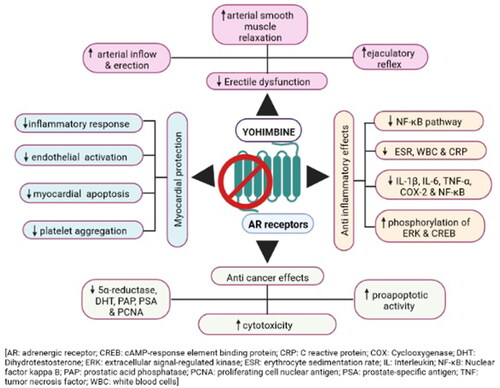
The exact physiological mechanisms underlying yohimbine’s actions remain unknown. However, the available data suggest its role in sympathetic nerve stimulation [Citation6,Citation46]. Yohimbine stimulates the sympathetic nerve by antagonizing the presynaptic α2-adrenergic receptors (ARs). This action often increases the blood norepinephrine (NE) [Citation46]. The most notable activity of yohimbine is the selective inhibition of ARs; it displays a higher affinity for α2 subtypes and a moderate affinity for the α1 subtypes. Binding affinities among the α2 AR subtypes are reported in the following order: α2C (0.88 nM) >α2A (1.4 nM) >α2B (7.1 nM) [Citation5,Citation47]. The mechanism of action of the adrenergic receptor family has been depicted in . Yohimbine also binds other behaviour-related monoaminergic receptors in the following order: α-2 NE > 5HT-1A>, 5HT-1B > 1-D > D3 > D2 receptors [Citation47]. Yohimbine also possesses endothelin-like activities. It influences NO generation and exerts anxiogenic effects via the noradrenergic pathway, activating the hypothalamic–pituitary–adrenal (HPA) stress axis [Citation48,Citation49].
Yohimbine administration also elevates blood epinephrine (EP) and NE in healthy males [Citation46]. It increases the heart rate, systolic blood pressure, alertness, and localized blood flow [Citation50]. An earlier study reported that yohimbine constricts the splanchnic vessels, probably causing blood shunting and hyperaemia of the skeletal muscle [Citation51]. Localized skeletal muscle blood flow modulates the metabolism of the primary energy system and lactic acid production/removal, thus improving anaerobic exercise performance [Citation52]. However, the effect of yohimbine on exercise performance is largely unexplored and requires further investigation.
Yohimbine is rapidly absorbed when consumed orally (absorption half-life: 10 min) and excreted easily (elimination half-life: 30 min) [Citation46]. The therapeutic dose of yohimbine ranges from 50 to 300 ng/mL in the blood [Citation53]. No accurate toxicity or lethal dose data are available for humans except for a few reports on yohimbine fatalities [Citation53]. However, very high concentrations in the blood sample are reported to be toxic. Drevin et al. [Citation53] measured 8000 ng/mL of yohimbine in the blood sample of a 27-year-old male bodybuilder with a history of yohimbine and steroid abuse. The potential of yohimbine against various human disorders is depicted in .
Yohimbine for treating erectile disorder
Over the past decades, yohimbine therapy has been used for sexual difficulties. The global prevalence of erectile disorder (ED) represents a significant sexual difficulty, estimating 300 million men with ED by 2025. Several studies suggest the positive influence of yohimbine on organic impotence [Citation8,Citation54,Citation55]. It is also modestly beneficial over the placebo, particularly in psychogenic erectile disorder, and is generally well tolerated by patients [Citation56]. Guay et al. [Citation54] performed a dose-escalation trial on patients with organic erectile dysfunction and recorded better results when the yohimbine dose was doubled. These patients had minimal side effects, normal cardiovascular responses, and had no significant changes in blood pressure. It was found to be safe for men with medically controlled hypertension. The responders experienced 100% erectile response with satisfactory sexual intercourse compared to the non-responders when 10.8 mg of yohimbine (thrice a day) was administered; the response was independent of age. However, non-smokers and patients with less severe erectile dysfunction responded better. Yohimbine facilitates the ejaculatory function by using the α1- and α2-adrenoceptors of the noradrenergic system at the spinal level [Citation57]. Carro-Juáreza [Citation57] reported yohimibine involvement in a spinal noradrenergic system controlling the ejaculatory reflex in a rat model by antagonizing the AR-α2. Treatment with yohimbine significantly increased male rats’ sexual arousal and mating behaviour without altering the testosterone, FSH, and LH levels [Citation58].
Further, cumulative dose–response curves of yohimbine showed a dose-dependent relaxant effect on isolated rat corpus cavernosum [Citation58]. A recent systematic review and meta-analysis study by Wibowo et al. [Citation8] reported a significant improvement in erectile disorder using yohimbine alone or in combination with other supplements. An earlier study also reported improved erectile function due to the combined treatment of yohimbine and L-arginine, which improved the blood flow, especially in patients with mild to moderate ED [Citation59]. In another study, Senbel and Mostafa [Citation12] reported the yohimbine role in enhancing the impact of sildenafil on the erectile process in a rat model. The combination of the centrally acting yohimbine and the peripheral conditioner sildenafil prolongs the effect of sildenafil on the erectile process without inducing additional hypotension [Citation12].
The smooth muscle constrictor of the corpus cavernosum, relaxant mediators regulated by the central and peripheral nerve systems, and the penis all work together to create the complicated hemodynamic event known as penile erection. The cavernosal tissue and the non-adrenergic non-cholinergic (NANC) system play a major role in erectile response. The degree of erection is determined by the balance between the nitric oxide stimulus generated by non-adrenergic NANC nerves and the counterbalancing of the sympathetic noradrenergic nerves [Citation60]. The action of NE on the α1 and α2 adrenergic receptors in the corpus cavernosum maintains the flaccid state of the human penis [Citation61].
The vasoconstrictor α2-ARs have also been proposed to explain the pro-erectile effects of yohimbine [Citation62]. The mechanical effects of yohimbine (the α2-AR antagonist) induce the pre- and postsynaptic α2-ARs for activating the noradrenergic neurons and releasing NE. This NE, in turn, activates the α-ARs in the endothelium to release NO and prostanoids. These molecules elevate the intracellular cyclic GMP (cGMP) and cyclic CMP, respectively, relaxing the penile smooth muscles. According to Simonsen et al. [Citation63], stimulation of prejunctional α-ARs prevents NO, a NANC neurotransmitter, from being released into penile resistance arteries. Yohimbine inhibits these prejunctional α-ARs, which increases NO production and activates the soluble guanylate cyclase. This raises cGMP levels, which causes the corpus cavernosal smooth muscles to relax [Citation63]. Yohimbine has previously been shown to have a synergistic effect on the in vitro relaxing of rabbit corpus cavernosum and the in vivo enhancement of rabbit penile erection when the NO pathway is simultaneously stimulated and α-ARs are blocked [Citation64]. Kuchakulla et al. [Citation65] reported yohimbine as an effective ingredient in prevalent over-the-counter testosterone and ED supplements. Yohimbine combined with arginine produces a synergistic effect of increasing the penile artery inflow, acting as a reasonable therapeutic option for ED [Citation66]. Thus, the addition of arginine overcomes the disadvantage of the less-dominant α2-adrenergic receptors in the corpus cavernosum. Despite the reported beneficial effect and mechanistic support, clinical guidelines do not recommend yohimbine as the standard treatment [Citation67]. Yohimbine should be reconsidered as a therapeutic, considering its observed benefits and possible clinical value.
Yohimbine for myocardial dysfunction
Despite some progress in current research, septic cardiomyopathy remains a leading cause of death in the intensive care unit [Citation68]. Identifying therapeutic targets and developing an effective therapy for sepsis-induced myocardial dysfunction is challenging. Lipopolysaccharide (LPS) is an essential activator of pathologic events during septic cardiomyopathy [Citation69]. An earlier study suggested the promoting role of NE in LPS-induced TNF-α production in the Kupffer cells via α2A-AR stimulation [Citation70]. Other studies also reported increased inflammatory cytokine production through α2A-AR-mediated mechanism of NE in macrophages [Citation71–73]. Blocking the α2A-AR inhibits inflammatory responses and improves the survival of septic rats [Citation74]. The presence of α2A-AR is confirmed in macrophages and LPS-activated macrophages that adhere to cardiomyocytes by reducing the myocardial contractile function via TNF-α and NO [Citation75]. Hence, α2A-AR inhibition in macrophages protects against LPS-triggered myocardial dysfunction.
In an earlier study, Wang et al. [Citation68] reported a role of yohimbine in reversing LPS-induced reduction in left ventricular ejection fraction, stroke volume, cardiac output, and an increment in the left ventricular end-systolic volume. Furthermore, they observed yohimbine-associated prevention of hepatic injury in LPS-challenged mice. In another study, Yu et al. [Citation69] reported reduced septic cardiomyopathy when α2A-ARs were blocked by increasing the cardiac NE concentration and inhibiting cardiac endothelial activation. Wang et al. [Citation76] also reported the beneficial effect of berberine and yohimbine on LPS-induced myocardial dysfunction in mice by inhibiting myocardial apoptosis and I-κBα phosphorylation in the heart. They observed a significantly reversed LPS-induced reduction in the left ventricular ejection fraction and fractional shortening-like control levels by yohimbine, indicating its protective role in systolic myocardial dysfunction. Yohimbine treatment significantly lowered the TNF-α, caspase 3/7, and IL-1β levels in the heart tissue and suppressed LPS-induced myocardial apoptosis. Yohimbine treatment has been reported to reduce cardiac damage. However, no effect was observed on pulmonary injury in LPS-challenged mice, suggesting its organ-specific protective effect [Citation76,Citation77]. A cardiac presynaptic α2A-AR inhibition indicates the cardioprotective effect of yohimbine via inhibition of cardiac iNOS expression and TNF-α production. Yohimbine inhibits α2A-AR and provides a novel therapeutic strategy for myocardial dysfunction in septic patients.
EP and NE profoundly affect platelet aggregation and atherothrombosis. Platelet activation plays a crucial role in the pathogenesis of acute coronary syndrome [Citation78,Citation79]. In one study, Lahiri et al. [Citation80] reported the potency of yohimbine hydrochloride in inhibiting collagen-EP interactive platelet aggregation. They suggested a significant role of α2A-adrenoreceptors of platelets in precipitating the interactive effect of collagen and EP. In another study, Yokota et al. [Citation81] reported the beneficial effect of α2-AR blocking in effectively inhibiting adrenaline-potentiated platelet aggregation. Moreover, Marketou et al. [Citation82] suggested the role of the aggregation regulator α2B-AR subtype in platelets and its inhibition, offering a novel therapeutic opportunity to prevent atherothrombotic events. Kawano et al. [Citation9] recently reported the antiplatelet effect by co-blocking 5-HT2A and α2-AR receptors on the platelets and suppressing platelet aggregation mediated by ADP.
Anti-inflammatory effects of yohimbine
Abnormal immune responses cause inflammatory diseases, and an imbalance between inflammatory mediators and cells leads to chronic or systemic inflammation [Citation83,Citation84]. The scientific community has lately been interested in identifying anti-inflammatory agents from natural resources. Ou et al. [Citation11] reported anti-inflammatory properties of yohimbine that suppress the NF-κB pathway. Yohimbine reduced the IL-1β or noradrenaline-induced IL-6 upregulation and ameliorated cartilage destruction in condylar processes by suppressing the NF-κB pathway in vitro and in vivo. Moreover, yohimbine-induced α2-AR inhibition upregulated tyrosine hydroxylase (TH). Despite intense investigations on TH regulation, effective drugs to upregulate TH are limited. Previous studies showed that α2-AR activation reduced the cAMP levels and increased the cAMP upregulation of TH. These observations indicate a cAMP-mediated mechanism of anti-inflammatory effects [Citation85–87]. Yohimbine also exerts an anti-inflammatory effect by inhibiting pro-inflammatory cytokines and modulating the antioxidant states in arthritic rats [Citation88]. The expression of COX-2, TNF-α, and NF-ĸB are significantly reduced, and ESR, WBC, and C-reactive protein (CRP) levels are decreased in the rats treated with yohimbine. Moreover, yohimbine provided significant protection from renal ischemia/reperfusion-induced acute kidney injury by blocking of α2 adrenoceptor in a rat model. Recently, Shimokawa et al. [Citation89] also reported the reno-protective effect of yohimbine on LPS-induced acute kidney injury in rats. Yohimbine treatment ameliorates damaged kidney function and LPS-induced low blood pressure; it suppresses cytokine mRNA and iNOS and blocks NF-κB nuclear localization. Yohimbine also enhances the phosphorylation of extracellular signal-regulated kinase (ERK) and cAMP response element-binding protein (CREB) that promotes IL-10 expression. It improved the therapeutic effect of berberine on LPS-induced bacteraemia. This effect is achieved by inhibiting JNK, ERK, NFκB, and other pathways by upregulating IL-10 in a mice model [Citation90]. Thus, the yohimbine–berberine combination is an effective immunomodulating agent. Lin et al. [Citation91] reported that yohimbine inhibits the overproduction of TNF-α, IL-1β, and IL-6 and relieves the severity of pulmonary inflammation induced by endotoxin blockage of α2A AR. Additionally, significant improvements are observed in the oxygenation index, white blood cell count, and lung histopathological scores at each time interval in LPS-treated male Sprague–Dawley rats.
Anticancer effects of yohimbine
The involvement of yohimbine in inflammatory and metastatic pathways has encouraged researchers to evaluate its anticancer potential. Activation of the sympathetic nerves and increased plasma catecholamines indicates higher risk and lower survival of cancer patients due to activated stress response mechanisms [Citation92,Citation93]. Noradrenaline and adrenaline typically orchestrate acute stress reactions and promote other biological processes favourable for stimulating cancer cell proliferation and inhibiting immune surveillance [Citation94]. Several studies reported the probable association of adrenergic stimulation and carcinogenesis in different cancer forms [Citation95–97].
G-protein-coupled receptors (GPCRs) are promising targets for small molecular therapeutics. They constitute the largest protein superfamily in the human genome and mediate cancer events like proliferation and metastasis [Citation98]. GPCRs are involved in different cancer types such as breast, lung, gastric, pancreas, colon, ovarian, prostate, and melanoma [Citation92,Citation99,Citation100]. Blocking the GPCRs increases the overall survival of cancer patients, thus, indicating their clinical relevance [Citation92]. Yohimbine reportedly inhibits native GPCR targets like the α-2B adrenergic receptor, dopamine D2B receptor, and hydroxytryptamine receptor [Citation47]. A recent study reported antagonistic activities of yohimbine and its ring-distorted derivatives against GPCR-mediated cancer [Citation10]. Additionally, the ring-distorted yohimbine analog Y6p has modest (µM) potencies for the neuropeptide S receptor 1, prolactin-releasing hormone receptor, and follicle-stimulating hormone receptor (IC50=8.03 µM, 10.3 µM, and 4.89 µM, respectively). Another yohimbine analog, Y7g, greatly inhibits the oxytocin receptor (OXTR) and the arginine vasopressin receptors (AVPR1A, AVPR1B, and AVPR2), which are thought to be implicated in the evolution of tumours in prostate and small-cell lung cancer [Citation10,Citation101]. In contrast to the well-known AVPR2 antagonist, tolvaptan (IC50 = 7.34 pM), Y7g has an IC50 of 459 nM and inhibits roughly 83% of the AVPR2 action at 20 µM (in a dose-dependent manner). Same research group also suggested anti-inflammatory and hypoxia-inducible factor-dependent anticancer properties of Y7g [Citation102]. Another analog of yohimbine, Y1f, exhibits antagonistic activity against five GPCRs (≥80% inhibition), including the closely related chemokine receptors CCR3 (IC50=8.30 µM), CCR8 (IC50=7.29 µM), CX3C (CX3CR1, IC50=8.47 µM), C-X-C type 4 (CXCR4, IC50=7.94 µM) and 5-hydroxytryptamine (serotonin) receptor (HTR2C, IC50=180 nM). These GPCRs are expressed in different cancers, and novel antagonists could serve as potential therapeutic targets [Citation10]. Recently, one study reported the therapeutic potential of yohimbine hydrochloride against benign prostatic hyperplasia (BPH) by downregulating steroid 5α-reductase type 2 [Citation103]. Administration of 2, 4, and 8 mg/kg of yohimbine inhibits the increase in the prostatic index by 46.7, 55.1, and 69.3%, respectively, in BHP rats. Furthermore, yohimbine significantly reduces the levels of dihydrotestosterone, prostatic acid phosphatase, prostate-specific antigen, proliferating cell nuclear antigen, and steroid 5α-reductase, suggesting its beneficial effects against BPH. Several studies reported the cytotoxicity of yohimbine in different human cancer cell lines [Citation1,Citation104,Citation105]. Zhan et al. [Citation105] reported moderate cytotoxicity of yohimbine-type alkaloid containing a β-carbolinium group on human MCF-7 breast, SWS80 colon, and A549 lung cancer cell lines with IC50 values of 25.5, 22.6, and 26.0 µM, respectively. In addition, Shen et al. [Citation104] reported the proapoptotic activity of yohimbine against the PC-2 and PC-3 pancreatic cancer cell lines. Recently, Yang et al. [Citation1] synthesized novel yohimbine analogs, which showed cytotoxicity against human pancreatic cancer cells (PATU-8988), human gastric cancer cells (SGC-7901), and normal human gastric mucosal cells (GES-1). Yohimbine exerts anticancer effects by inhibiting the GPCRs expressed in the cancer cells [Citation92]. The pharmacological activities and possible mechanisms of action of yohimbine against different human diseases/disorders are highlighted in .
Other therapeutic effects of yohimbine
Yohimbine treatment reportedly improves various clinical manifestations of polycystic ovarian syndrome (PCOS), which is treated with insulin-lowering agents. One study reported yohimbine’s benefits in reducing PCOS parameters like body weight, LH, and insulin in reproductively mature female rabbits [Citation106]. Increased activities of the sympathetic and HPA axes are reported in PCOS; blocking these pathways is a recommended therapeutic option [Citation107]. In another study, yohimbine therapy reduced the LH concentration and normalized the ovulatory cycles by increasing the oestrogen concentration in PCOS rats [Citation108]. Recently, Barnes et al. [Citation46] reported the beneficial effects of acute yohimbine hydrochloride supplementation on repeated supramaximal sprint performance. Yohimbine also generates and maintains power output during repeated sprints, and its use is recommended for athletes and exercisers to attenuate fatigue and improve anaerobic performance. Nasa and Juneja [Citation109] reported using yohimbine for managing acute pesticide ingestion as rescue therapy. They reported a case of a 3-year-old child who accidentally ingested an amitraz solution and showed signs of severe poisoning. The child regained consciousness after yohimbine treatment in 18 h and was successfully weaned off mechanical ventilation without any side effects. Thus, yohimbine (the α2 adrenergic antagonist) worked as an antidote. It enabled faster neurological recovery, prevented bradycardia, and provided hemodynamic stability in severe amitraz poisoning [Citation109]. Another study reported the weight reduction and anorectic action of yohimbine in a high-fat diet-induced obesity model of rats. Yohimbine reduced body weight and improved lipid and carbohydrate profiles without causing serious side effects [Citation110]. Kotańska et al. [Citation111] reported the favourable influence of yohimbine on the imbalanced lipid carbohydrate homeostasis in leptin deficiency and impairment of the α2- and β3-adrenoceptor function. The overall therapeutic potential of yohimbine and its possible mechanism of action are summarized in .
Table 2. Clinical conditions and possible mechanism of yohimbine action.
Metabolism of yohimbine
Yohimbine shows a comparatively rapid absorption with a half-life of 7–11 min when consumed orally. The plasma levels reach their peak after 45–60 min of ingestion. Around 2.24–1.25 L kg−1 of volume is distributed after oral delivery, whereas 0.26 L kg−1 is distributed after intravenous exposure [Citation19]. The bioavailability of yohimbine ranges widely from 10% to 90% in humans [Citation112]. Yohimbine is primarily metabolized in the liver, where it undergoes oxidation to its pharmacologically active metabolite 11-hydroxy-yohimbine; a smaller percentage is oxidized to 10-OH-yohimbine [Citation113]. This metabolic pathway depends on the cytochrome P450 polymorphic variants CYP2D6 and CYP3A4 [Citation112]. CYP2D6 is a critical drug-metabolizing enzyme that metabolizes approximately 20% of the approved drugs across a broad spectrum of clinical disciplines [Citation114]. CYP2D6 is crucial in eliminating yohimbine via 11-hydroxylation; extreme differences exist in yohimbine clearance between the CYP2D6 genotypes. The vast variability observed among individuals in the clinical effects of yohimbine may be attributable to differences in hepatic metabolism. The kidneys excrete yohimbine, and less than 1% of the medication is found in the urine after 24 h [Citation19]. Yohimbine has a half-life of less than 1 h and is rapidly excreted from the plasma.
Toxicological aspects of yohimbine
Despite well-tolerated effects in both young and elderly populations, scientific reports indicate some dose-related toxicity issues of yohimbine. Generally, yohimbine displays specific side effects like nervousness, insomnia, anxiety, increased urinary frequency, dizziness, tremors, headache, hypo/hypertension, bronchospasm, and lupus-like syndrome. In rare cases, patients have gastrointestinal symptoms such as nausea, vomiting, lack of appetite, and diarrhoea. The oral administration of yohimbine increases spontaneous salivary secretion in healthy humans [Citation43]. Yohimbine increases NE and its metabolite (3-methoxy-4-hydroxyphenylglycol) in the plasma resulting in hypnotic dysregulation, the most common endocrine effect of yohimbine HCl. These effects may be accompanied by changes in blood pressure, mood, or pulse [Citation115,Citation116]. Yohimbine HCl improves heart performance and increases cardiac output and blood pressure [Citation117]. This effect is dose-dependent and varies widely among individuals. The concentrations of 4–16.2 mg (as a single dose or thrice daily) generally do not affect the blood pressure and pulse in individuals with normal blood pressure. Moreover, 20–30 mg of yohimbine either has no significant effect or moderately increases the blood pressure without affecting the heart rate [Citation6]. On the other hand, concentrations of 45.5 mg and above occasionally increase the medium arterial blood pressure and, less commonly, the heart rate. If such hemodynamic effects occur, they reach their maximum level after 60–90 min and recede over a few hours [Citation6].
Conclusion and future directions
Yohimbine is a prototypical α2-receptor antagonist used widely as a therapeutic agent against erectile dysfunction for many years. It interacts with other behaviourally relevant monoaminergic receptors, viz. α1, 5HT-1A, 5HT-1B, 1-D, D3, and D2. However, the exact mechanism of yohimbine action is unclear. Interestingly, yohimbine compounds meet fundamental drug-like criteria with DPQ and quantitative estimates of drug-likeness. Yohimbine’s inhibitory activity on the α2-adrenergic receptor is beneficial in various disease conditions such as erectile dysfunction, myocardial dysfunction, inflammatory disorders, and cancer. However, few studies reported toxicological concerns after yohimbine treatment for erectile dysfunctions, albeit at comparatively higher doses. The potential clinical applicability of yohimbine can be assessed by investigating the relative variables and genetic factors using preclinical models followed by clinical studies. Well-designed randomized clinical trials are recommended to evaluate the efficacy and safety of yohimbine as a human pharmaceutical agent. The extraction or neo-synthesis of yohimbine or its analogs with better bioavailability and limited toxicity is also a possible prospect. Some studies also highlighted a synergetic therapeutic effect of yohimbine with other relevant compounds. Therefore, the possibility of using yohimbine as an adjunct therapy for associated clinical conditions should be tested. Perhaps, nanotechnology can enhance the effectiveness and bioavailability of yohimbine in diseased cells by preventing the disruption of normal physiological activities of non-diseased cells and organs. Despite some observed adverse effects, yohimbine use should be reconsidered for its associated benefits and clinical value.
Author contributions
ST, TAZ, and BA: Conception and design, and interpretation of the reported data; NRJ, CKF, MAA, and AA: Drafting of the paper; ST, AMA, AIA, BA, and AA: Revised critically for intellectual content, ARK; and the final approval of the revised version. All authors agree to be accountable for all aspects of the work. All the authors listed in this article meet the criteria for authorship as per the ICMJE.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFPRP: 42-141-1442 and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing not applicable—no new data generated.
Additional information
Funding
References
- Yang H, Poznik M, Tang S, et al. Synthesis of conformationally liberated yohimbine analogues and evaluation of cytotoxic activity. ACS Omega. 2021;6(29):19291–19303.
- Biswal S, Sahoo U, Sethy S, et al. Indole: the molecule of diverse biological activities. 2012;5(1):1–6.
- Kumar S. A brief review of the biological potential of indole derivatives. Future J Pharm Sci. 2020;6(1):121.
- Qin R, Zhao Q, Han B, et al. Indole-based small molecules as potential therapeutic agents for the treatment of fibrosis. Front Pharmacol. 2022;13:845892.
- Hai-Bo L, Yong P, Lu-qi H, et al. Mechanism of selective inhibition of yohimbine and its derivatives in adrenoceptor α2 subtypes. J Chem. 2013;2013:e783058–9.
- Tam SW, Worcel M, Wyllie M. Yohimbine: a clinical review. Pharmacol Ther. 2001;91(3):215–243.
- Ojatula AO, Idu M, Timothy O. Aphrodisiac potentials of Pausinystalia yohimbe (K. Schum.) Pierre ex Beille methanol root extract in male Wistar rats. J Integr Nephrol Androl. 2020;7(2):47.
- Wibowo DNSA, Soebad DM, Soebadi MA, Department of Urology, Universitas Airlangga Faculty of Medicine, Dr. Soetomo General-Academic Hospital, Surabaya, Indonesia. Yohimbine as a treatment for erectile dysfunction: a systematic review and meta-analysis. Turk J Urol. 2021;47(6):482–488.
- Kawano Y, Katsuyama M, Nagata M, et al. Antiplatelet effect of mirtazapine via co-blocking of the 5-HT2A and α2-adrenergic receptors on platelets. Biol Pharm Bull. 2021;44(2):238–244.
- Paciaroni NG, Norwood VM, Ratnayake R, et al. Yohimbine as a starting point to access diverse natural product-like agents with re-programmed activities against cancer-relevant GPCR targets. Bioorg Med Chem. 2020;28(14):115546.
- Ou F, Huang Y, Sun J, et al. Yohimbine ameliorates temporomandibular joint chondrocyte inflammation with suppression of NF-κB pathway. Inflammation. 2021;44(1):80–90.
- Senbel AM, Mostafa T. Yohimbine enhances the effect of sildenafil on erectile process in rats. Int J Impot Res. 2008;20(4):409–417.
- Farouk M, Abd El-Aziz L, El-Gindy AE, et al. Validated methods for determination of yohimbine hydrochloride in the presence of its degradation products. Bull Fac Pharm Cairo Univ. 2011;49(2):67–79.
- Miller ER, Hovey MT, Scheidt KA. A concise, enantioselective approach for the synthesis of yohimbine alkaloids. J Am Chem Soc. 2020;142(5):2187–2192.
- Omar F, Tareq AM, Alqahtani AM, et al. Plant-based indole alkaloids: a comprehensive overview from a pharmacological perspective. Molecules. 2021;26(8):2297.
- Mitra S, Prova SR, Sultana SA, et al. Therapeutic potential of indole alkaloids in respiratory diseases: a comprehensive review. Phytomedicine. 2021;90:153649.
- Feng W, Jiang D, Kee C-W, et al. Bicyclic guanidine catalyzed asymmetric tandem isomerization intramolecular-Diels-Alder reaction: the first catalytic enantioselective total synthesis of (+)-alpha-yohimbine. Chem Asian J. 2016;11(3):390–394.
- EFSA-ANS_Panel. Scientific opinion on the evaluation of the safety in use of Yohimbe (Pausinystalia yohimbe (K. Schum.) Pierre ex Beille). EFSA J. 2013;11(7):3302.
- Garrard A. Yohimbine. In: Wexler P, editor. Encyclopedia of toxicology. 3rd ed. Oxford: Academic Press; 2014. p. 995–996.
- Awad R, Mallah E, Al-Akayleh F, et al. Determination of hydrolysis parameters of yohimbine HCl at neutral and slightly acidic medium. Int J Pharm Pharm Sci. 2015;7(5):134–137.
- Bickerton GR, Paolini GV, Besnard J, et al. Quantifying the chemical beauty of drugs. Nat Chem. 2012;4(2):90–98.
- Obreshkova D, Tsvetkova D. Validation of HPLC method with UV-detection for determination of yohimbine containing products. Pharmacia. 2016;63:3–9.
- Abuzenadah A, Al-Sayes F, Alam S, et al. Identification of potential poly(ADP-ribose)polymerase-1 inhibitors derived from Rauwolfia serpentina: possible implication in cancer therapy. Evid Based Complement Alternat Med. 2022;2022:3787162.
- Singh A, Alvi N. High-performance thin-layer chromatographic quantification of yohimbine in the stem bark of Pausinystalia yohimbe. J Planar Chromat. 2011;24(3):253–256.
- Švorc Ľ, Stanković DM, Mehmeti E, et al. Sensitive electrochemical determination of yohimbine in primary bark of natural aphrodisiacs using boron-doped diamond electrode. Anal Methods. 2014;6(13):4853–4859.
- Chen Q, Li P, Zhang Z, et al. Analysis of yohimbine alkaloid from Pausinystalia yohimbe by non-aqueous capillary electrophoresis and gas chromatography-mass spectrometry. J Sep Sci. 2008;31(12):2211–2218.
- Deshmukh SR, Ashrit DS, Patil BA. Extraction and evaluation of indole alkaloids from Rauwolfia serpentina for their antimicrobial and antiproliferative activities. Int J Pharm Pharm Sci. 2012;4:329–334.
- Zhanjing G, Xiongmin L, Hongmiao H, et al. Optimization of ultrasonic-assisted extraction of yohimbine from Rauvolfia verticillata roots by response surface analysis. Food Sci. 2015;16:66–71.
- Joshi BD, Tandon P, Jain S. Molecular characterization of yohimbine hydrochloride using vibrational spectroscopy and quantum chemical calculations. BIBECHANA. 2012;8:73–80.
- Kumar A, Bhardwaj MK, UA K, et al. Quantitative determination of yohimbine alkaloid in the different part of the Rauvolfia tetraphylla. J Chem Pharm Res. 2011;3(2):907–910.
- Tarkowská D. A fast and reliable UHPLC-MS/MS-based method for screening selected pharmacologically significant natural plant indole alkaloids. Molecules. 2020;25(14):3274.
- Duan WJ, Liu Q, Zhao RX, et al. Preparative separation of two alkaloids from devil pepper radix (Rauvolfia verticillata [Lour.] Baill.) by pH-zone-refining counter-current chromatography. Acta Chromatogr. 2018;30(2):81–84.
- Boğa M, Bingül M, Özkan EE, et al. Chapter 7 – chemical and biological perspectives of monoterpene indole alkaloids from Rauwolfia species. In: Atta-ur-Rahman, editor. Studies in natural products chemistry. Vol. 61. Netherland: Elsevier; 2019. p. 251–299.
- van Tamelen E, Shamma M, Burgstahler A, et al. The total synthesis of yohimbine. J Am Chem Soc. 1958;80(18):5006–5007.
- Aimi N, Yamanaka E, Endo J, et al. Transformation of indole alkaloids—I: conversion of oxindole alkaloids into indole alkaloids. Tetrahedron. 1973;29(14):2015–2021.
- Hirai Y, Terada T, Okaji Y, et al. A novel approach to (+)-yohimbine. Tetrahedron Lett. 1990;31(33):4755–4756.
- Mergott DJ, Zuend SJ, Jacobsen EN. Catalytic asymmetric total synthesis of (+)-yohimbine. Org Lett. 2008;10(5):745–748.
- Herlé B, Wanner MJ, van Maarseveen JH, et al. Total synthesis of (+)-yohimbine via an enantioselective organocatalytic Pictet–Spengler reaction. J Org Chem. 2011;76(21):8907–8912.
- Lucas D, Neal-Kababick J, Zweigenbaum J. Characterization and quantitation of yohimbine and its analogs in botanicals and dietary supplements using LC/QTOF-MS and LC/QQQ-MS for determination of the presence of bark extract and yohimbine adulteration. J AOAC Int. 2015;98(2):330–335.
- Bylund DB, Lavicky M. Yohimbine. In: Enna SJ, Bylund DB, editors. xPharm: the comprehensive pharmacology reference. New York: Elsevier; 2007. p. 1–5.
- Benoin PR, Burnell RH, Medina JD. Alkaloids of Aspidosperma excelsum Benth. Can J Chem. 1967;45(7):725–730.
- Mekkawi AG, Al-Badr AA. Yohimbine. In: Florey K, editor. Analytical profiles of drug substances. Vol. 16. USA: Academic Press; 1987. p. 731–768.
- Joshi BD, Srivastava A, Tandon P, et al. Molecular structure, vibrational spectra and HOMO, LUMO analysis of yohimbine hydrochloride by density functional theory and ab initio Hartree–Fock calculations. Spectrochim Acta A Mol Biomol Spectrosc. 2011;82(1):270–278.
- Kumar S, Singh A, Bajpai V, et al. Structural characterization of monoterpene indole alkaloids in ethanolic extracts of Rauwolfia species by liquid chromatography with quadrupole time-of-flight mass spectrometry. J Pharm Anal. 2016;6(6):363–373.
- Ambady G, Kartha G. Crystal structure and absolute configuration of yohimbine hydrochloride, C21H27ClN2O3. J Cryst Mol Struct. 1973;3(1):37–45.
- Barnes ME, Cowan CR, Boag LE, et al. Effects of acute yohimbine hydrochloride supplementation on repeated supramaximal sprint performance. Int J Environ Res Public Health. 2022;19(3):1316.
- Millan MJ, Newman-Tancredi A, Audinot V, et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35(2):79–95.
- Zheng H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze requires hindbrain noradrenergic neurons that target the anterior ventrolateral bed nucleus of the stria terminalis. Eur J Neurosci. 2013;37(8):1340–1349.
- Ajayi AA, Newaz M, Hercule H, et al. Endothelin-like action of Pausinystalia yohimbe aqueous extract on vascular and renal regional hemodynamics in Sprague Dawley rats. Methods Find Exp Clin Pharmacol. 2003;25(10):817–822.
- Herman AM, Critchley HD, Duka T. The impact of yohimbine-induced arousal on facets of behavioural impulsivity. Psychopharmacology. 2019;236(6):1783–1795.
- Owen JA, Nakatsu SL, Fenemore J, et al. The pharmacokinetics of yohimbine in man. Eur J Clin Pharmacol. 1987;32(6):577–582.
- McMahon S, Jenkins D. Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med. 2002;32(12):761–784.
- Drevin G, Palayer M, Compagnon P, et al. A fatal case report of acute yohimbine intoxication. Forensic Toxicol. 2020;38(1):287–291.
- Guay AT, Spark RF, Jacobson J, et al. Yohimbine treatment of organic erectile dysfunction in a dose-escalation trial. Int J Impot Res. 2002;14(1):25–31.
- Retzler K. Erectile dysfunction: a review of comprehensive treatment options for optimal outcome. J Restor Med. 2019;8(1):e20190104.
- Sommer F, Obenaus K, Engelmann U. Creative-dynamic image synthesis: a useful addition to the treatment options for impotence. Int J Impot Res. 2001;13(5):268–274; discussion 275.
- Carro-Juáreza M, Rodríguez-Manzo G. Yohimbine reverses the exhaustion of the coital reflex in spinal male rats. Behav Brain Res. 2003;141(1):43–50.
- Saad MA, Eid NI, Abd El-Latif HA, et al. Potential effects of yohimbine and sildenafil on erectile dysfunction in rats. Eur J Pharmacol. 2013;700(1–3):127–133.
- Lebret T, Hervé J-M, Gorny P, et al. Efficacy and safety of a novel combination of L-arginine glutamate and yohimbine hydrochloride: a new oral therapy for erectile dysfunction. Eur Urol. 2002;41(6):608–613; discussion 613.
- Cripps SM, Mattiske DM, Pask AJ. Erectile dysfunction in men on the rise: is there a link with endocrine disrupting chemicals? Sex Dev. 2021;15(1–3):187–212.
- Sangiorgi G, Cereda A, Benedetto D, et al. Anatomy, pathophysiology, molecular mechanisms, and clinical management of erectile dysfunction in patients affected by coronary artery disease: a review. Biomedicines. 2021;9(4):432.
- Morton JS, Daly CJ, Jackson VM, et al. α1A-Adrenoceptors mediate contractions to phenylephrine in rabbit penile arteries. Br J Pharmacol. 2007;150(1):112–120.
- Simonsen U, Prieto D, Hernandez M, et al. Prejunctional alpha sub 2-adrenoceptors inhibit nitrergic neurotransmission in horse penile resistance arteries. J Urol. 1997;157(6):2356–2360.
- de Tejada IS, Garvey DS, Schroeder JD, et al. Design and evaluation of nitrosylated α-adrenergic receptor antagonists as potential agents for the treatment of impotence. J Pharmacol Exp Ther. 1999;290(1):121–128.
- Kuchakulla M, Narasimman M, Soni Y, et al. A systematic review and evidence-based analysis of ingredients in popular male testosterone and erectile dysfunction supplements. Int J Impot Res. 2021;33(3):311–317.
- Rhim HC, Kim MS, Park Y-J, et al. The potential role of arginine supplements on erectile dysfunction: a systemic review and meta-analysis. J Sex Med. 2019;16(2):223–234.
- Peak TC, Yafi FA, Sangkum P, et al. Emerging drugs for the treatment of erectile dysfunction. Expert Opin Emerg Drugs. 2015;20(2):263–275.
- Wang Y, Yu X, Wang F, et al. Yohimbine promotes cardiac NE release and prevents LPS-induced cardiac dysfunction via blockade of presynaptic α2A-adrenergic receptor. PLoS One. 2013;8(5):e63622.
- Yu X, Wang Y, Yang D, et al. α2A-adrenergic blockade attenuates septic cardiomyopathy by increasing cardiac norepinephrine concentration and inhibiting cardiac endothelial activation. Sci Rep. 2018;8(1):5478.
- Miksa M, Das P, Zhou M, et al. Pivotal role of the α2A-adrenoceptor in producing inflammation and organ injury in a rat model of sepsis. PLoS One. 2009;4(5):e5504.
- Cong Z, Li D, Lv X, et al. α2A-adrenoceptor deficiency attenuates lipopolysaccharide-induced lung injury by increasing norepinephrine levels and inhibiting alveolar macrophage activation in acute respiratory distress syndrome. Clin Sci. 2020;134(14):1957–1971.
- Duan L, Chen J, Razavi M, et al. Alpha2B-adrenergic receptor regulates neutrophil recruitment in MSU-induced peritoneal inflammation. Front Immunol. 2019;10:501.
- Zhou M, Das P, Simms HH, et al. Gut-derived norepinephrine plays an important role in up-regulating IL-1β and IL-10. Biochim Biophys Acta. 2005;1740(3):446–452.
- Zhang F, Wu R, Qiang X, et al. Antagonism of alpha2A-adrenoceptor: a novel approach to inhibit inflammatory responses in sepsis. J Mol Med. 2010;88(3):289–296.
- Simms MG, Walley KR. Activated macrophages decrease rat cardiac myocyte contractility: importance of ICAM-1-dependent adhesion. Am J Physiol. 1999;277(1):H253–H260.
- Wang Y-y, Li H-m, Wang H-D, et al. Pretreatment with berberine and yohimbine protects against LPS-induced myocardial dysfunction via inhibition of cardiac I-[kappa]B[alpha] phosphorylation and apoptosis in mice. Shock. 2011;35(3):322–328.
- Zhang H-q, Wang H-d, Lu D-X, et al. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock. 2008;29(5):617–622.
- Theofilis P, Sagris M, Oikonomou E, et al. Factors associated with platelet activation-recent pharmaceutical approaches. Int J Mol Sci. 2022;23(6):3301.
- Jabir NR, Siddiqui AN, Firoz CK, et al. Current updates on therapeutic advances in the management of cardiovascular diseases. Curr Pharm Des. 2016;22(5):566–571.
- Lahiri P, Roy S, Sardar P, et al. Platelet responsiveness to yohimbine hydrochloride and MRS2179 in the context of the interaction between collagen and epinephrine in acute coronary syndrome. Blood Cells Mol Dis. 2009;43(1):105–110.
- Yokota S-I, Hikasa Y, Mizushima H. Effects of imidazoline and non-imidazoline α-adrenergic agents on rabbit platelet aggregation. Pharmacology. 2013;91(3–4):135–144.
- Marketou ME, Kintsurashvili E, Androulakis NE, et al. Blockade of platelet alpha2B-adrenergic receptors: a novel antiaggregant mechanism. Int J Cardiol. 2013;168(3):2561–2566.
- Liu Y-Z, Wang Y-X, Jiang C-L. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316.
- Jabir NR, Tabrez S. Cardiovascular disease management through restrained inflammatory responses. Curr Pharm Des. 2016;22(7):940–946.
- Kindler S, Samietz S, Houshmand M, et al. Depressive and anxiety symptoms as risk factors for temporomandibular joint pain: a prospective cohort study in the general population. J Pain. 2012;13(12):1188–1197.
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18(10):1394–1404.
- Lorenz J, Schäfer N, Bauer R, et al. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthr Cartil. 2016;24(2):325–334.
- Neha N, Ansari MM, Khan HA. Yohimbine hydrochloride ameliorates collagen type-II-induced arthritis targeting oxidative stress and inflammatory cytokines in Wistar rats. Environ Toxicol. 2017;32(2):619–629.
- Shimokawa T, Yoneda K, Yamagata M, et al. Yohimbine ameliorates lipopolysaccharide-induced acute kidney injury in rats. Eur J Pharmacol. 2020;871:172917.
- Li H, Wang Y, Zhang H, et al. Yohimbine enhances protection of berberine against LPS-Induced mouse lethality through multiple mechanisms. PLoS One. 2012;7(12):e52863.
- Lin Y, Zhu X, Yao W-Z, et al. Yohimbine protects against endotoxin-induced acute lung injury by blockade of alpha 2A adrenergic receptor in rats. Chin Med J. 2011;124(7):1069–1074.
- Amaro F, Silva D, Reguengo H, et al. β-Adrenoceptor activation in breast MCF-10A cells induces a pattern of catecholamine production similar to that of tumorigenic MCF-7 cells. Int J Mol Sci. 2020;21(21):7968.
- Dai S, Mo Y, Wang Y, et al. Chronic stress promotes cancer development. Front Oncol. 2020;10:1492.
- Qiao G, Chen M, Bucsek MJ, et al. Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front Immunol. 2018;9:164.
- Coelho M, Moz M, Correia G, et al. Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol Rep. 2015;33(5):2513–2520.
- Barbieri A, Bimonte S, Palma G, et al. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int J Oncol. 2015;47(2):527–534.
- Lin Q, Wang F, Yang R, et al. Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS One. 2013;8(4):e61435.
- Alhosaini K, Azhar A, Alonazi A, et al. GPCRs: the most promiscuous druggable receptor of the mankind. Saudi Pharm J. 2021;29(6):539–551.
- Dal Monte M, Calvani M, Cammalleri M, et al. β-Adrenoceptors as drug targets in melanoma: novel preclinical evidence for a role of β3-adrenoceptors. Br J Pharmacol. 2019;176(14):2496–2508.
- Yazawa T, Kaira K, Shimizu K, et al. Prognostic significance of β2-adrenergic receptor expression in non-small cell lung cancer. Am J Transl Res. 2016;8(11):5059–5070.
- Zhong M, Boseman ML, Millena AC, et al. Oxytocin induces the migration of prostate cancer cells: involvement of the Gi-coupled signaling pathway. Mol Cancer Res. 2010;8(8):1164–1172.
- Paciaroni NG, Ratnayake R, Matthews JH, et al. A tryptoline ring distortion strategy leads to complex and diverse biologically active molecules from the indole alkaloid yohimbine. Chemistry. 2017;23(18):4327–4335.
- Zhao Y, Zhang Y, Li Y, et al. Yohimbine hydrochloride inhibits benign prostatic hyperplasia by downregulating steroid 5α-reductase type 2. Eur J Pharmacol. 2021;908:174334.
- Shen S-G, Zhang D, Hu H-T, et al. Effects of alpha-adrenoreceptor antagonists on apoptosis and proliferation of pancreatic cancer cells in vitro. World J Gastroenterol. 2008;14(15):2358–2363.
- Zhan G, Miao R, Zhang F, et al. Cytotoxic yohimbine-type alkaloids from the leaves of Rauvolfia vomitoria. Chem Biodivers. 2020;17(12):e2000647.
- Sajjad S, Tobassum S, Farooq U, et al. Effects of naloxone and yohimbine in polycystic ovary syndrome: a rabbit model study. Turk J Med Sci. 2016;46(4):1265–1270.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520.
- Zangeneh FZ, Mohammadi A, Ejtemaeimehr S, et al. The role of opioid system and its interaction with sympathetic nervous system in the processing of polycystic ovary syndrome modeling in rat. Arch Gynecol Obstet. 2011;283(4):885–892.
- Nasa P, Juneja D. Acute pesticide ingestion managed with yohimbine as a rescue therapy. Indian J Crit Care Med. 2016;20(12):739–741.
- Dudek M, Knutelska J, Bednarski M, et al. A comparison of the anorectic effect and safety of the alpha2-adrenoceptor ligands guanfacine and yohimbine in rats with diet-induced obesity. PLoS One. 2015;10(10):e0141327.
- Kotańska M, Marcinkowska M, Knutelska J, et al. Yohimbine improves lipid and carbohydrate profiles without reduction in body weight in obese leptin-deficient ob/ob mice. J Pre-Clin Clin Res. 2018;12(2):67–71.
- Vay M, Meyer MJ, Blank A, et al. Oral yohimbine as a new probe drug to predict CYP2D6 activity: results of a fixed-sequence phase I trial. Clin Pharmacokinet. 2020;59(7):927–939.
- Vay M, Sauter M, Mikus G, et al. Quantification of microdosed oral yohimbine and its major metabolite in human plasma in the picogram range. Bioanalysis. 2019;11(16):1459–1467.
- Taylor C, Crosby I, Yip V, et al. A review of the important role of CYP2D6 in pharmacogenomics. Genes. 2020;11(11):1295.
- Grasing K, Sturgill MG, Rosen RC, et al. Effects of yohimbine on autonomic measures are determined by individual values for area under the concentration—time curve. J Clin Pharmacol. 1996;36(9):814–822.
- Sturgill MG, Grasing KW, Rosen RC, et al. Yohimbine elimination in normal volunteers is characterized by both one- and two-compartment behavior. J Cardiovasc Pharmacol. 1997;29(6):697–703.
- Le Corre P, Parmer RJ, Kailasam MT, et al. Human sympathetic activation by alpha2-adrenergic blockade with yohimbine: bimodal, epistatic influence of cytochrome P450-mediated drug metabolism. Clin Pharmacol Ther. 2004;76(2):139–153.