Abstract
Background
The circadian clock regulates the function of the immune system, the replication of viruses, and the magnitude of infections. The aim of this study was to analyse whether hospital attendance in Coronavirus disease 2019 (COVID-19) patients presents a diurnal variation.
Methods
Data from the electronic medical records of 1094 COVID-19 patients who presented to a Health Centre in Qatar during the month of July 2020 was retrospectively analysed. The following demographic (i.e. time of day (TOD), sex, age), clinical (i.e. cycle threshold (CT), temperature, oxy-haemoglobin saturation and resting heart-rate), biochemical (i.e. uraemia, glycaemia and albuminia) and haematological (i.e. leukocytes, erythrocytes ad platelets) parameters were collected.
Results
Univariate analysis showed a significant effect of TOD on hospital admission (p < 0.001), with patients attending the health care centre more during the active behavioural phase (08h00-00h00) compared to the resting phase (00h00-08h00). COVID-19 infection blunted the circadian rhythms of core body temperature, neutrophils and leukocytes family and shifted the circadian rhythms of resting heart-rate and uraemia. Correlation analysis showed a near perfect negative correlation between the age of patients and the TOD (r=–0.97), with older patients attending the care centre earlier during the day.
Conclusion
COVID-19 infection affected the circadian rhythms of the host through disrupting the circadian rhythms of core temperature and innate immunity mediators. Old patients attend the health care centre earlier compared to younger ones. However, CT during polymerase chain reaction-test was unaffected by the TOD, which limits the conclusion that COVID-19 viral infection exhibits diurnal variation.
Introduction
The Coronavirus disease 2019 (COVID-19), which has been declared a public health epidemic of global outrage by the World Health Organisation (WHO), is among the most shocking diseases in recent years [Citation1]. There were nearly 620 M laboratory reported cases and over 6.5 M deaths worldwide as of 26 September 2022 (https://www.worldometers.info/coronavirus/). Even though COVID-19 can affect people of all ages, older people are at a higher risk of deleterious consequences, as well as a greater fatality rate [Citation2,Citation3]. For instance, in a cohort of 8516 American veterans, hospital admission during the high load period of intensive care units was associated with two-fold mortality compared to patients treated during the low load period [Citation4]. Understanding the origins of this temporal distribution will increase the efficiency of health care intervention [Citation1]. However, little is known about the circadian distribution of COVID-19 patient admissions.
Almost all physiological and behavioural functions fluctuate over the 24-h cycle. This cycle, namely the circadian rhythm, is generated by the suprachiasmatic nucleus in the hypothalamus and coordinated by the peripheral circadian clock located in distal tissue by way of hormones, clock genes and body temperature [Citation5,Citation6]. Circadian rhythmicity is an evolutionarily conserved pathway that serves to adapt the organism to the 24-h environmental day [Citation7,Citation8]. Because the exposition of the host to pathogens varies across the 24-h cycle, the circadian clock anticipates environmental daily changes in order to optimize the immune response [Citation8–10]. Several types of illness exhibit diurnal variation [Citation11,Citation12]. For instance, respiratory diseases (viral and allergic rhinorrhoea, nocturnal asthma, and chronic pulmonary obstructive disease) display circadian variation with symptoms and/or gravity requiring hospital attendance occurring during the night [Citation11]. Consequently, mediators of the immune system have been shown to exhibit diurnal variations in the blood and tissues, including immune cells, antibodies, cytokines, chemokines and hormones [Citation10]. During the active phase, immune cells migrate to the peripheral tissues in a major anti-microbial response, and the circulating number of immune cells increases during the night [Citation13].
Clinical manifestations of COVID-19 seem to be comparable, with some differences, to influenza [Citation14]. As previous research confirmed that influenza infections and the severity of symptoms are affected by the time of the day (TOD) [Citation11], it seems prudent to study the effect of TOD on COVID-19 patient hospital attendance. Importantly, a recent study reported a diurnal variation in the positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test, with a peak around 14h00 [Citation15]. It has been consistently reported that circadian rhythm regulation differs between males and females [Citation16], with females displaying smaller diurnal amplitude of temperature [Citation17] and higher diurnal amplitude of melatonin [Citation7] compared to males. Further, males and females sleep differently [Citation18] and respond differently to sleep deprivation [Citation17–19]. Indeed, the COVID-19 and its associated lockdown affected the sleep-wake cycle [Citation20–22], with female being more disrupted than males [Citation23–25]. However, little is known concerning the existence of a sex-based time course of COVID-19 patients’ hospital attendance. Both physiological sleep and endogenous melatonin secretion, which present sex-based differences, play an important role in innate immunity regulation [Citation26,Citation27]. The observation that COVID-19 patients reported highly disturbed sleep-wake cycles [Citation24] may be indicative of an infection-based circadian disruption. In addition, older people show reduced circadian flexibility and could be more affected when facing a circadian disrupter [Citation16].
Therefore, the current study aimed to investigate the diurnal distribution of hospital attendance in COVID-19 positive patients. The second aim was to study the sex and age differences in COVID-19 positive patients attending hospital. Based on the existing literature, it was hypothesized that the COVID-19 could also exhibit diurnal variation in hospital attendance, which may be affected by sex and age.
Patients and methods
Study design
In this study, we retrospectively analysed data from the electronic medical records of 1049 COVID-19 positive patients who had presented to Rawdat Al Khail Health Centre, Doha, Qatar (RAK-HC) during the period between 1 July and 31 July 2020. This study was approved by the institutional review board at Primary Health Care Corporation (PHCC-Ref No. PHCC/DCR/2020/08/091) in the spirit of the Helsinki Declaration (64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013). All respondents provided written consent for anonymous data use for research purposes and publications. The data was handled in an anonymous way and in line with the ‘General Data Protection Regulation’ (gdpr-info.eu).
Patient population and data collection
This retrospective observational study included all COVID-19 positive patients who presented to RAK-HC during the month of July 2020. The parameters looked at were time of registration of the patients, when they presented to RAK-HC for COVID-19 testing, and demographic data (i.e. age and sex) of patients. Patients presenting to RAK-HC with symptoms of probable COVID-19 underwent oropharyngeal and nasopharyngeal swabs by trained healthcare professionals to test for SARS-Cov-2 by reverse transcription polymerase chain reaction (RT-PCR) testing at the laboratory in Hamad Hospital, which is the government designated testing laboratory in Qatar. Only COVID-19 positive patients were included in this study.
Demographic (i.e. TOD, age and sex), clinical [i.e. cycle threshold (CT), core body temperature, resting heart-rate and oxy-haemoglobin saturation (SpO2)], biochemical (i.e. albumin, plasma glucose and urea) and haematological (i.e. erythrocytes, leukocytes, neutrophils and platelets) data were collected from the electronic medical records after all patient identifiable data being removed as per the research study requirements.
Statistical analysis
The statistical tests were processed in GraphPad Prism 6 (GraphPad Software, San Diego, CA). The quantitative data was expressed as mean ± standard deviation (SD). Comparison between different TOD [six time frames (00h00-04h00; 04h00-08h00; 08h00-12h00; 12h00-16h00; 16h00-20h00 and 20h00-00h00)] was assessed by a One way analysis of variance (ANOVA). Sex-based comparison at different TOD was assessed by a Two way ANOVA [six time frames (00h00-04h00; 04h00-08h00; 08h00-12h00; 12h00-16h00; 16h00-20h00 and 20h00-00h00) * sex (male/female)]. Once the ANOVA indicates a significant effect or interaction, a Bonferroni post-hoc test was performed to compare data by pair. Pearson product-moment correlation ‘Pearson r’ was performed between the selected demographic, clinical, biochemical and haematological parameters. ‘Pearson r’ was considered ‘high’ when it was > 0.70, ‘good’ when it was between 0.50 and 0.70, ‘fair’ if it was between 0.30 and 0.50 and ‘weak or no association’ if it was < 0.30 [Citation28]. The accepted level of significance was p < 0.05.
Results
Participants
The overall sample included 1094 patients. However, since data were acquired under crisis conditions, some were missing, and not all the patients have complete files. shows the number of participants and missing data for each parameter.
Univariate analysis
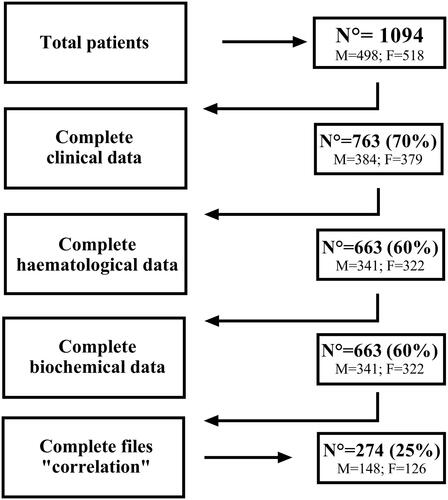
The power analysis for univariate analysis was a posteriori calculated and showed an observed power of 1. The number of COVID-19 patients attending the health care centre was higher between 08h00 and 00h00 compared to 00h00 and 08h00 (all p < 0.001) with no difference between sexes (). CT, core body temperature, leukocytes and neutrophils were not affected by the TOD (). However, resting heart-rate and SpO2 were lower between 00h00-04h00 and heart-rate was higher at 20h00-00h00 compared to other TODs. Erythrocytes (at; 08h00-12h00) and platelets (at; 12h00-16h00) count were higher compared to other TODs. In addition, uraemia and glycaemia levels were higher between 00h00 and 04h00 compared to other TODs. summed the univariate effects of TOD on the selected clinical, haematological, and biochemical data.
Figure 2. Patients’ distribution frequency per TOD (n = 1094). Data was processed using a One-way ANOVA with *** Means a significant difference compared to 00-04 h for male, female and overall. ### Means a significant difference compared to 04-08 h for male, female and overall.
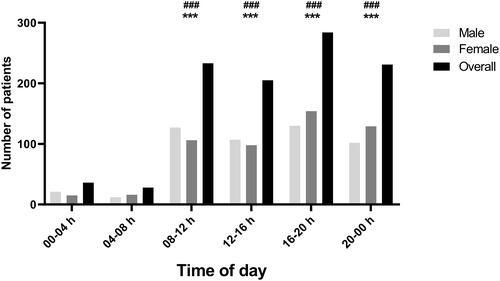
Table 1. Clinical, haematological and biochemical data at different TOD (in hours) in COVID-19 patients (n = 1094).
Multivariate analysis
The power analysis for multivariate analysis was a posteriori calculated and showed a strong observed power of 0.96. Male patients showed higher erythrocytes and uraemia compared to females (all; p < 0.05), and females showed higher SpO2 compared to males (all; p < 0.05) at different TODs. Mean and SD of different clinical, haematological, and biochemical data according to TOD and sex are presented in .
Figure 3. Male-Female comparison of different clinical, haematological and biochemical data at different TOD. Data was assessed using a two-way ANOVA followed by Bonferroni post-hoc test. *, ** and *** Significant sex-based difference at p < 0.05, p < 0.01 and p < 0.001. •, •• and ••• Means significant TOD difference in females at p < 0.05, p < 0.01 and p < 0.001. #, ## and ### means significant TOD difference in males at p < 0.05, p < 0.01 and p < 0.001.
Correlation analysis
The power analysis for the correlation analysis was a posteriori calculated and showed an observed power of 0.92. The correlation was performed on 274 complete files. There was a near perfect reverse correlation between the age of patients and TOD of hospital admission (r=–0.97; p < 0.001). Pearson ‘r’ matrix is presented in .
Table 2 Correlation-coefficient (r) between different clinical, haematological and biochemical data (n = 274).
Discussion
The main outcome of this study was that hospital attendance in COVID-19 patients displays a diurnal variation, with patients attending hospital most in the active behavioural phase (between 08h00 and 00h00) compared to resting behavioural phase of the day (00h00-08h00). A strong negative correlation was found between the age of patients and the TOD of hospital attendance, with older patients attending the hospital earlier during the day (00h00-04h00). However, the TOD of hospital attendance was not affected by the sex of patients. In addition, COVID-19 infection upregulated the circadian rhythms of several haematological, clinical and biochemical parameters.
The current results showed that COVID-19 patients attend the hospital most during the active phase (08h00-00h00) compared to the resting phase (00h00-08h00). A recent study reported that SARS-CoV-2 positive tests were TOD dependent with a peak around 14h00 [Citation15]. Although the former study reported on ∼86,000 patients from 130 different clinical locations and over 20 weeks, the reported TOD pattern showed similar trends as the current study. Therefore, the current findings validate the assumptions reported by McNaughton et al. [Citation15]. In the same way, diurnal variation in the severity of local and systemic symptoms of common cold and influenza has been reported to be greater in the early morning [Citation29]. Similarly, cough frequency in rhinorrhoea patients is more prominent during the initial hours after awakening from nocturnal sleep [Citation12]. This could be consequent to symptoms aggravation during the previous night. It was reported that asthma is more severe during the night time [Citation29], probably because of the infection-induced disruption in innate immunity [Citation30]. It is well known that innate and adaptive immunities are under tight circadian control, with circulation peak occurring during the early night [Citation30–34]. However, the current results showed that the circadian rhythms of neutrophils and leukocytes families were blunted in COVID-19 positive patients attending the hospital. In cases of viral infection, clock gene expression is disturbed due to systemic inflammation [Citation35,Citation36]. The proinflammatory cytokine interferon is released chronically during viral infection, which has the ability to interfere with circadian regulation [Citation37]. This could indicate that SARS-Cov-2 infection affected clock gene expression, namely the brain and muscle ARNT-like 1, which dampened the diurnal variation of immune cells activities [Citation36]. A near perfect reverse correlation (r = −97) was found between age and TOD in hospital attendance, with older patients attending the health care centre earlier during the day compared to younger patients. For instance, morningness preference (shift of rest/activity rhythm to the morning) is associated with advanced age [Citation16,Citation38], which could explain why older patients attend the health care centre at an earlier TOD compared to younger patients. Also, ageing is the most significant risk factor for developing severe outcomes and fatality in COVID-19 patients [Citation2]. Indeed, developing severe COVID-19 outcomes could be related to the age-related immunosenescence [Citation2], which could be defined as a chronic, low grade activation of proinflammatory process within the aged-organism [Citation16]. The current data showed that oldest patients attended the care centre between 00h00 and 04h00. This could be explained by the increased asthma symptoms during the night [Citation29] and the age-related decrease in physiological reserve in the respiratory system [Citation2] that has aggravated COVID-19 symptoms, requiring immediate hospital attendance in older patients.
The current results showed that the CT (i.e. viral load) was unaffected by the TOD. Therefore, the assumption that COVID-19 hospital attendance is affected by the TOD could be somehow misleading. Contrarily, a recent study showed that the viral load was lower during the day in SARS-CoV-2 patients [Citation15]. In fact, only female patients showed a trend towards lower CT values between 16h00 and 20h00, but this difference was not significant. These conflicting results might be explained by the sample size, which is larger from different locations and with a longer collection period in the above mentioned study [Citation15]. Indeed, further research on larger sample size and different cohorts is required to confirm, or deny, the current results.
It has consistently been reported that core body temperature displays a strong circadian rhythmicity [Citation39,Citation40], which was blunted in the current study by COVID-19 infection. This could be explained by the infection-induced fever, which is associated with improved survival and resolution of infection [Citation41]. In addition, COVID-19 infection shifted the circadian rhythms of resting heart-rate. Physiological resting heart-rate is higher during the morning [Citation40,Citation42] but in the current study, heart-rate was higher during the night (20h00-00h00) compared to the other TODs. Indeed, weak but highly significant correlations were found between CT on the one hand, and core body temperature and heart-rate on the other hand. In addition, SARS-Cov-2 infection shifted the circadian rhythms of uraemia, which was higher 00h00-04h00 compared to all other TODs. Contrarily, physiological uraemia was reported to be lower at this TOD (00h00-04h00) and higher between 08h00-14h00 [Citation40]. The current finding showed that the circadian rhythm of glycaemia was upregulated by the COVID-19 infection only in female patients. Physiological glycaemia is higher during the beginning of the active phase of the day [Citation43], which was true only for male patients in the current study (i.e. higher glycaemia between 08h00 and 12h00). However, glycaemia was higher between 00h00 and 04h00 in female patients. Although the origin of this upregulation is not clear, it could be associated with the inflammation-induced fever. Actually, it has been reported that a 1 °C increase in core body temperature requires a 10%–12% increase in metabolic rate [Citation41]. Taken together, these findings strengthen the fact that the SARS-Cov-2 infection disrupted circadian rhythms regulation, probably via circadian gene expression channels [Citation36,Citation44]. Contrary to the strong relationship between the time of hospital attendance and the age of the patient, the TOD of hospital attendance was not related to the sex of the patients. Actually, the sex-based difference in SpO2 persisted in the current study, with females showing higher SpO2 compared to males, similarly to earlier reports [Citation45]. Moreover, males showed higher erythrocytes level compared to females at different TODs, similarly to physiological haematocrit concentration reported elsewhere [Citation46]. This could be indicative of a negligible effect of sex in regard to SARS-Cov-2 infection.
The current findings could be of relevance in the understanding of the temporal pattern of the COVID-19 pandemic. Patients attend health care centres at different TOD and for various reasons. Whether the presentation at a particular TOD has an effect on their overall outcome during the disease process is a key question. Identifying such times will help clinicians to prioritize, escalate, and manage such patients appropriately so that they receive the best possible outcome for their condition. Also, adjusting staffing numbers in health centres when more patients are present could be helpful in dealing more effectively as well as help improve efficiency whilst dealing with the challenges of this pandemic.
To the authors’ finest knowledge, this is the first study to investigate the diurnal variations in symptoms of COVID-19 patients. Diurnal variation in symptoms of cold and influenza has been reported in the literature [Citation11]. Although the current results showed that COVID-19 patients’ hospital admission is influenced by the TOD, the findings do not conclusively support that COVID-19 symptoms follow the same circadian pattern. First, the CT was not influenced by the TOD. Second, the time of viral infection may have affected the time of clinical presentation in these patients [Citation11], which is beyond the scope of this study. Besides, the relatively long incubation period of SARS-CoV-2 makes it difficult to identify retrospectively the onset time of infection. Indeed, the causal relationship between TOD and COVID-19 remains unclear and still to be explored. Furthermore, a longitudinal follow up of patients’ vitals and the development of their symptoms would be very helpful to a better understanding of this pandemic. No exclusion criteria were applied in the current study, which could limit the current assumptions. In addition, there was no control group (e.g. patient attending the healthcare centre for other reasons) in the current study. Indeed, the lack of comparison could lead to biased conclusions. Thus, further studies on larger scales could generate a better understanding of the relationship.
Conclusion
COVID-19 patients’ attendance at the health care centre was found to be associated with the rest/activity pattern. However, the CT was unaffected by the TOD, which limits the conclusion that COVID-19 symptoms’ gravity is modulated by the TOD. Older patients attend the health care centre earlier during the day compared to younger patients. Furthermore, COVID-19 dampened the circadian variation in core body temperature, circulating leukocytes and neutrophils and shifted the circadian variation of heart-rate and uraemia.
Consent to participate
All participants provided informed consent before participating in the study.
Consent for publication
All patients provided consent for anonymous data use for research purposes and publications. All authors approved of the final version to be published and agree to be accountable for any part of the work.
Acknowledgments
Open Access funding provided by the Qatar National Library. No external funding related to the project has been received.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data analysed and reported in this study are available from the first author on reasonable request.
References
- Zhao X, Zhang T, Li B, et al. Job-related factors associated with changes in sleep quality among healthcare workers screening for 2019 novel coronavirus infection: a longitudinal study. Sleep Med. 2020;75:21–26.
- Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205.
- Dergaa I, Abubaker M, MOHAMMED AR, et al. Age and clinical signs as predictors of COVID-19 symptoms and cycle threshold value: red flags in triage algorithms in primary care. Libyan J Med. 2022. In press.
- Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US department of veterans affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266.
- Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111–139.
- Buhr ED, Takahashi JS. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol. 2013;217:3–27.
- Cain SW, Dennison CF, Zeitzer JM, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296.
- Sengupta S, Brooks TG, Grant GR, et al. Accounting for time: Circadian rhythms in the time of COVID-19. J Biol Rhythms. 2021;36(1):4–8.
- Bryson J. Circadian rhythm sleep-wake disorders and the COVID-19 pandemic. J Clin Sleep Med. 2020;16(8):1423.
- Mazzoccoli G, Vinciguerra M, Carbone A, et al. The circadian clock, the immune system, and viral infections: the intricate relationship between biological time and host-virus interaction. Pathogens. 2020;9.
- Smolensky MH, Portaluppi F, Manfredini R, et al. Diurnal and twenty-four hour patterning of human diseases: cardiac, vascular, and respiratory diseases, conditions, and syndromes. Sleep Med Rev. 2015;21:3–11.
- Smolensky MH, Portaluppi F, Manfredini R, et al. Diurnal and twenty-four hour patterning of human diseases: acute and chronic common and uncommon medical conditions. Sleep Med Rev. 2015;21:12–22.
- Diallo AB, Coiffard B, Leone M, et al. For whom the clock ticks: clinical chronobiology for infectious diseases. Front Immunol. 2020;11:1413–1457.
- Osman M, Klopfenstein T, Belfeki N, et al. A comparative systematic review of COVID-19 and influenza. Viruses. 2021;13:1–14.
- McNaughton CD, Adams NM, Hirschie Johnson C, et al. Diurnal variation in SARS-CoV-2 PCR test results: test accuracy may vary by time of day. J Biol Rhythms. 2021;36(6):595–601.
- Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. 2017;127(2):437–446.
- Romdhani M, Hammouda O, Smari K, et al. Total sleep deprivation and recovery sleep affect the diurnal variation of agility performance: the gender differences. J Strength Cond Res. 2021;35(1):132–140.
- Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110.
- Armitage R, Smith C, Thompson S, et al. Sex differences in Slow-Wave activity in response to sleep deprivation. Sleep (Rochester). 2001;4:33–41.
- Romdhani M, Fullagar HHK, Vitale JA, et al. Lockdown duration and training intensity affect sleep behavior in an international sample of 1,454 elite athletes. Front Physiol. 2022;13:904711–904778.
- Romdhani M, Ammar A, Trabelsi K, et al. Ramadan observance exacerbated the negative effects of COVID-19 lockdown on sleep and training behaviors: a international survey on 1,681 Muslim athletes. Front Nutr. 2022;9:925092.
- Dergaa I, Ammar A, Souissi A, et al. COVID-19 lockdown: impairments of objective measurements of selected physical activity, cardiorespiratory and sleep parameters in trained fitness coaches. Excli J. 2022;21:1084–1098.
- Romdhani M, Rae DE, Nedelec M, et al. COVID-19 lockdowns: a worldwide survey of circadian rhythms and sleep quality in 3911 athletes from 49 countries, with data-driven recommendations. Sports Med. 2022;52(6):1433–1448.
- Jahrami HA, Alhaj OA, Humood AM, et al. Sleep disturbances during the COVID-19 pandemic: a systematic review, meta-analysis, and meta-regression, sleep. Sleep Med Rev. 2022;62:101591.
- Trabelsi K, Ammar A, Masmoudi L, et al. Sleep quality and physical activity as predictors of mental wellbeing variance in older adults during COVID-19 lockdown: ECLB COVID-19 international online survey. J Environ Res Public Health. 2021;18:4329.
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199–210.
- Irwin MR, Opp MR. Sleep-Health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2016;42(1):96.
- Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. Boston: Houghton Mifflin; 2003
- Smith A, Tyrrell D, Coyle K, et al. Diurnal variation in the symptoms of colds and influenza. Chronobiol Int. 1988;5(4):411–416.
- Scheiermann C, Gibbs J, Ince L, et al. Clocking in to immunity. Nat Rev Immunol. 2018;18(7):423–437.
- Man K, Loudon A, Chawla A. Immunity around the clock. Science 2016;354(6315):999–1003.
- Reglero-Real N, Rolas L, Nourshargh S. Leukocyte trafficking: time to take time seriously. Immunity. 2019;50(2):273–275.
- He W, Holtkamp S, Hergenhan SM, et al. Circadian expression of migratory factors establishes Lineage-Specific signatures that guide the homing of leukocyte subsets to tissues. Immunity 2018;49(6):1175–1190.
- Adrover JM, del Fresno C, Crainiciuc G, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 2019;50(2):390–402.
- Haspel JA, Chettimada S, Shaik RS, et al. Circadian rhythm reprogramming during lung inflammation. Nat Commun. 2014;5:4753.
- Fujimura A, Ushijima K, Smolensky MH. COVID-19 infection at nighttime. Chronobiol. Int. 2020;0:726–727.
- Kwak Y, Lundkvist GB, Brask J, et al. Interferon-γ alters electrical activity and clock gene expression in suprachiasmatic nucleus neurons. J Biol Rhythms. 2008;23(2):150–159.
- Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: Differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405–415.
- Waterhouse J, Drust B, Weinert D, et al. The circadian rhythm of core temperature: Origin and some implications for exercise performance. Chronobiol Int. 2005;22(2):207–225.
- Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267(3 Pt 2):R819–829.
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–349.
- Shiotani H, Umegaki Y, Tanaka M, et al. Effects of aerobic exercise on the circadian rhythm of heart rate and blood pressure. Chronobiol Int. 2009;26(8):1636–1646.
- Kalsbeek A, La Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab. 2014;3(4):372–383.
- Sehirli AÖ, Chukwunyere U, Aksoy U, et al. The circadian clock gene Bmal1: role in COVID-19 and periodontitis. Chronobiol Int. 2021;38(6):779–784.
- Levental S, Picard E, Mimouni F, et al. Sex-linked difference in blood oxygen saturation. Clin Respir J. 2018;12(5):1900–1904.
- Murphy WG. The sex difference in haemoglobin levels in adults-Mechanisms, causes, and consequences. Blood Rev. 2014;28(2):41–47.