Abstract
Background
Cytopenia is one of the most common adverse events following the CAR-T cell infusion, affecting the quality of life and potentially leading to life-threatening bleeding and infection. This study aimed to systematically review the cytopenias following anti-CD19 CAR-T therapy and further analyse the contributing factors.
Methods
Databases including PubMed, MEDLINE, Embase and Cochrane were systematically searched on 8 May 2022. A random-effect meta-analysis was used to estimate the incidence of cytopenia, and subgroup analyses were applied to explore heterogeneity.
Results
A total of 68 studies involving 2950 patients were included in this study. The overall incidence of all grade anaemia, thrombocytopenia, neutropenia, leukopoenia, lymphocytopenia and febrile neutropenia was 65%, 55%, 78%, 62%, 70% and 27%, respectively, and the corresponding cytopenias of grade 3 or worse were 33%, 31%, 61%, 45%, 46%, and 21%, respectively. Subgroup analysis showed increased incidence of cytopenias in subgroups with lower median age, proportion of males (<65%) and proportion of bridging therapy (<80%) and in the subgroup with a median line of prior therapy ≥3. In terms of disease and therapeutic target, cytopenias were more frequent in ALL patients and in dual-target CAR-T therapies (targeting CD19 in combination with other targets). Furthermore, CAR-T products manufactured by lentiviral vectors and those with the costimulatory domain of CD28 were more likely to cause haematological toxicity. No significant differences were observed in cytopenia between patients treated with CAR-T products with murine and humanized scFv.
Conclusion
In conclusion, neutropenia is the most frequent cytopenia after CAR-T therapy, both in all grades or grade ≥3. The incidence of cytopenias following CAR-T therapy is influenced by the age, sex, disease and number of prior therapy lines of the patients, as well as the target and costimulatory domain of CAR-T cells, and viral vectors used for manufacturing.
Neutropenia is the most frequent cytopenia after CAR-T therapy.
The clinical characteristics of the patients, the design of CAR-T cells and the protocol of CAR-T treatment can influence the occurrence of cytopenias following the CAR-T therapy.
KEY MESSAGES
1. Introduction
Genetically engineered T cells expressing chimeric antigen receptor (CAR), known as CAR-T cells, can recognize antigens specifically and activated directly without the need for antigen presentation by major histocompatibility complex (MHC) molecules. Over the past decade, the efficacy of CAR-T therapy in tumours, especially in B cell malignancies, has been demonstrated in a large number of clinical trials. CD19 is the most studied and the first commercialized target in CAR-T therapy, and commercialized anti-CD19 CAR-T products like axicabtagene-ciloleucel (axi-cel), tisagenlecleucel (tisa-cel) have been applied for years [Citation1]. The field of CAR-T therapy is rapidly evolving, whereas treatment-related adverse events (AEs) remain inevitable. Toxicities including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) have been largely reported, whereas cytopenia, which can occur in up to 90% of patients, has not been well characterized [Citation2]. Cytopenias not only affect the quality of life and increases the cost of treatment, but may also result in life-threatening bleeding and infection, and, additionally, limit the application of salvage therapies once the disease progresses. In a study by Sarah et al. [Citation3], prolonged neutropenia and thrombocytopenia after CAR-T infusion were associated with a shorter one-year overall survival.
Cytopenia is one of the most common AEs following anti-CD19 CAR-T therapy. In a single-centre, phase 1 b/2 study of relapsed or refractory B cell malignancies, neutropenia, thrombocytopenia, and anaemia occurred in 94%, 80%, and 51% of patients, respectively. Interestingly, nearly half of patients experienced biphasic cytopenias following CAR-T therapy, with the first phase occurring shortly after the infusion, followed by a second reduction after initial recovery [Citation3,Citation4]. Cytopenias were observed in up to 93% of patients beyond day 21 following infusion, and 62%, 44%, and 17% of patients experienced late neutropenia, thrombocytopenia, and anaemia, respectively, 42 days after cell administration [Citation4]. According to the ZUMA-1 trial, a multi-centre, phase 1–2 study of the safety and activity of axi-cel in adult refractory large B-cell lymphoma (LBCL), grade 3 or worse neutropenia, thrombocytopenia and anaemia occurred in 11%, 7%, and 3% of patients at three months after axi-cel [Citation5]. A long-term follow-up of axi-cel reported a 67.7% incidence of cytopenias at day 360 post cell infusion, with all grades of neutropenia, thrombocytopenia, and anaemia accounting for 25.8%, 38.7%, and 22.6%, and that, in addition, corresponding cytopenias of grade 3 or worse were found in 9.7%, 3.2%, and 3.2% of patients [Citation6]. One real-world analysis in a large series of LBCL patients (n = 356) displayed that grade 4 neutropenia occurred in 81% of patients, with a median duration of 13 days, and 17% with persisting grade 4 neutropenia at day 28 post-infusion [Citation7].
The mechanism of delayed haematopoietic reconstitution and biphasic pattern of cytopenia following CAR-T infusion remains largely unexplored, with the following mechanisms postulated based on the current study. First, previous treatments, including chemotherapy, radiotherapy, immunotherapy, haematopoietic stem cell transplantation (HSCT), and others, can lead to the injury of the bone marrow microenvironment composed of haematopoietic and stromal cells. The compromised haematopoietic microenvironment predisposes patients to delayed haematopoiesis after receiving lymphodepletion and CAR-T cell infusion. Second, CAR-T cells themselves can mediate robust immune responses. The activated CAR-T cells, monocytes and macrophages release a large number of cytokines that further act on bystander immune cells, which in turn trigger a loop of inflammation called a cytokine storm [Citation8], thereby compromising the haematopoietic microenvironment [Citation4,Citation9]. However, little is known about the characteristics and influencing factors of cytopenias following CAR-T therapy. To provide a comprehensive overview of cytopenias following the anti-CD19 CAR-T therapy, we searched the database for a meta-analysis and performed subgroup analyses to clarify the critical factors for therapy-related cytopenias.
2. Methods
This meta-analysis was registered in the International Prospective Register of Systematic Reviews (CRD42022329286). The methods used in this study were conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1. Search strategy
We searched the databases including PubMed, Web of Science, Embase and Cochrane on 8 May 2022. The search strategy used in this work was a combination of Medical Subject Headings (MeSH) terms and free words. The entire search strategies are listed in the Supplementary Material.
2.2. Eligibility criteria
Inclusion criteria: Patients with B-cell malignancies, including B-cell acute lymphoblastic leukaemia (B-ALL), chronic lymphocytic leukaemia (CLL), B-cell non-Hodgkin’s lymphoma (B-NHL), and multiple myeloma (MM), who were treated with anti-CD19 CAR-T therapy were included in this meta-analysis. Both prospective and retrospective studies were eligible for inclusion, either single-centre or multi-centre.
Exclusion criteria: (1) Studies with fewer than three patients, without reporting haematological adverse events, and with insufficient data. (2) Studies that used a cocktail approach (combining CAR T cells therapy with other kinds of treatment). (3) Systematic reviews, abstracts from conferences, basic experimental researches, irrelevant studies and studies published in languages other than English. Studies with the same registration number of clinical trials were screened to exclude reports with smaller sample sizes.
2.3. Article selection and data extraction
All retrieved articles were imported into Endnote X9, and duplicate entries were removed using Endnote X9 and manual identification methods. The articles were then screened independently by two authors (Y. X. and J. Z.), who first screened papers by title and abstract, and articles that could not be distinguished by the abstract were subject to full-text reading. The following data were extracted independently by the two authors mentioned above: first author, year of publication, registration number of the clinical trial, number of patients included, sex, median age, disease, target, previous treatment lines, prior HSCT, bridging therapy, costimulatory domain, the origin of single-chain antibody fragment variable (scFv), type of viral vector, AE criteria adopted, as well as the number of patients who developed anaemia, thrombocytopenia, neutropenia, leukopoenia, lymphopenia and febrile neutropenia. Data from two authors were compared, and discrepancies among them were discussed and negotiated with a third author (J. L.).
2.4. Assessment of study quality and publication bias
Methodological quality was evaluated using the Methodological Index of Non-Randomized Studies (MINORS) scale [Citation10]. Publication bias was assessed using funnel plots and confirmed by Egger’s test.
2.5. Statistical analysis
Stata software (version 16.0) was utilized for this meta-analysis. The I2 test and Q test were used to measure heterogeneity. The random-effects model was applied when statistical heterogeneity was significant (p < .10 or I2>50%), whereas a fixed-effects model was chosen when statistical heterogeneity was not significant (p ≥ .10 or I2 ≤ 50%). Effects were presented as event rates with pooled dominance ratios and 95% confidence intervals (CI). The Clopper–Pearson formula was used to determine confidence intervals. The publication bias was then evaluated using a funnel plot and Egger’s test, and p > .05 means there was no discernible publication bias. Sensitivity analysis was applied to assess the stability and reliability of the pooled meta-analysis results, and the corresponding studies were removed on a case-by-case basis. Subgroup analyses were performed to explore sources of heterogeneity, and a Z test was used to compare the combined incidence between subgroups. All tests were two-sided, and p < .05 was considered statistically significant.
3. Results
3.1. Basic characteristics of the studies
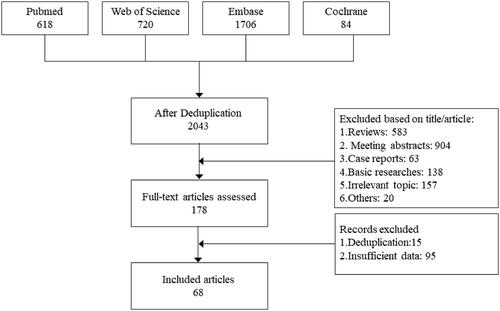
A total of 3128 articles were retrieved from the databases, and 1085 duplicate entries were removed by Endnote X9 and manual identification. After screening titles and abstracts, 178 articles remained, which were subsequently subjected to full-text reading and alignment of clinical trial registration numbers to screen for eligible articles. Finally, a total of 68 studies involving 2950 patients were included in this meta-analysis [Citation4,Citation11–77]. A flow chart of the literature selection procedure is shown in , and the clinical characteristics and quality rating of the included studies are listed in and Supplementary Table. The median MINORS score for the 68 studies was 12 (range 11–13), suggesting a moderate quality of evidence for this work.
Table 1. Clinical characteristics for the included studies.
3.2. Pooled rates of cytopenias
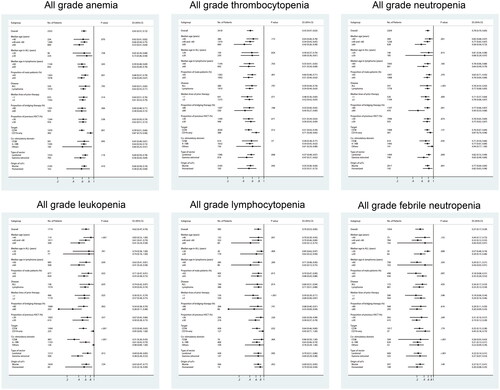
Among the 68 articles, the incidence of anaemia, thrombocytopenia, leukopoenia, lymphopenia, neutropenia and febrile neutropenia was described in 58, 61, 54, 32, 24 and 22 articles, respectively. The incidence of grade ≥3 anaemia, thrombocytopenia, neutropenia, leukopoenia, lymphopenia and febrile neutropenia was described in 65, 66, 61, 38, 23 and 23 articles, respectively.
As shown in , the overall incidence of all grade anaemia, thrombocytopenia, neutropenia, leukopoenia, lymphocytopenia and febrile neutropenia was 65% (95% CI: 57–72%), 55% (95% CI: 47–63%), 78% (95% CI: 70–85%), 62% (95% CI: 47–76%), 70% (95% CI: 53–85%), and 27% (95% CI: 17–39%), respectively. The incidence of grade ≥3 anaemia, thrombocytopenia, neutropenia, leukopoenia, lymphocytopenia and febrile neutropenia was 33% (95% CI: 26–39%), 31% (95% CI: 26–36%), 61% (95% CI: 53–69%), 45% (95% CI: 34–56%), 46% (95% CI: 31–62%), and 21% (95% CI: 14–29%), respectively.
3.3. Subgroup analysis
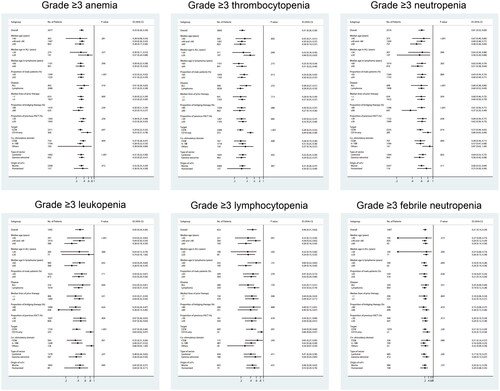
Subgroup analyses were performed for median age, the proportion of male patients, disease, target, median lines of prior therapy, the proportion of bridging therapy, the proportion of previous HSCT, costimulatory domain, type of vector, and species of scFv origin ( and ). Since the I2 is greater than 50% according to the Q test, the random-effect model was adopted for the subgroup analysis.
Patients were divided into three subgroups according to age, with younger patients more likely to experience haematological toxicity, especially neutropenia (p = .001), leukopoenia (p < .001) and lymphocytopenia (p = .001), as well as grade ≥3 neutropenia (p < .001), grade ≥3 leukopoenia (p < .001) and grade ≥3 lymphocytopenia (p = .001). Considering the differences in the age of onset of different diseases, we conducted further subgroup analyses of different diseases. In patients with ALL, we discovered that subgroup with a median age of ≥20 years tend to have a high risk of cytopenia than the subgroup with a median age of <20 years after CAR-T therapy, the grade ≥3 thrombocytopenia in particular (p = .049). On the contrary, in patients with B-NHL, younger patients (with a median age of <60 years) present a trend of more frequent cytopenia and a significantly higher incidence of leukopoenia (p = .039). Compared with the subgroup with a higher proportion of male patients, the subgroup with a lower proportion of males appeared to have an increased risk of developing haematological toxicity, notably anaemia (p = .001), leukopoenia (p = .022), grade ≥3 anaemia (p < .001), grade ≥3 thrombocytopenia(p = .013), and grade ≥3 febrile neutropenia (p = .028).
In terms of disease type, patients with ALL were more likely to develop anaemia, thrombocytopenia, leukopoenia and febrile neutropenia, whereas patients with NHL were more likely to have neutropenia and lymphocytopenia; however, no statistical differences were achieved between groups. Significantly, anaemia (p = .001), grade ≥3 anaemia (p = .047), thrombocytopenia (p = .012), grade ≥3 thrombocytopenia (p < .001), leukopoenia (p < .001), grade ≥3 leukopoenia (p < .001), lymphocytopenia (p = .032), grade ≥3 lymphocytopenia (p = .001) and grade ≥3 neutropenia (p = .019) were more frequent in patients receiving dual-target CAR-T therapied than those treated with single-targeted CAR-T cells.
Subgroup analysis of structures and manufacturing of CAR-T cells showed that CAR-T cells with a costimulatory domain of CD28 were more likely to cause haematological toxicity than those with a 4-1BB domain, and the differences were significant in the analysis of febrile neutropenia (p < .001). Moreover, products manufactured by lentiviral vectors presented a higher incidence of thrombocytopenia (p = .008), neutropenia (p = .009), leukopoenia (p = .012), febrile neutropenia (p < .001) and grade ≥3 anaemia (p < .001) than those by gamma retroviral vectors. No significant differences were observed in cytopenia between patients treated with CAR-T cells with murine or humanized scFv.
In terms of prior treatment, we discovered that patients with median lines of prior chemotherapy ≥3 tend to develop cytopenias more frequently, especially leukopoenia (p = .025), lymphopenia (p < .001) and grade ≥3 leukopoenia (p = .024). Subgroups with lower bridging rates were more likely to experience cytopenia, particularly neutropenia (p = .001) and leukopoenia (p = .002). No significant differences were observed in the incidence of cytopenias between subgroups with different rates of prior HSCT ratios.
3.4. Sensitivity analysis and publication bias assessment
To evaluate the robustness of this pooled meta-analysis, sensitivity analysis on the incidence of haematological toxicity using the ‘leave-one-out’ method was conducted. We found that the pooled effect sizes were not significantly affected after excluding each study individually, suggesting the stability and reliability of this work (Supplementary Figure).
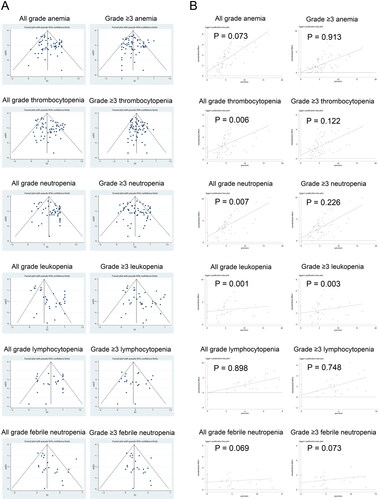
To determine if publication bias affected the overall incidence of anaemia, thrombocytopenia, neutropenia, leukopoenia, febrile neutropenia, and lymphocytopenia, the funnel plots and Egger’s test were performed. Publication bias occurred in the groups of thrombocytopenia, neutropenia, leukaemia and grade ≥3 leukaemia (). Nevertheless, the trim and fill method (without adding new studies) indicated the reliability of the results.
4. Discussion
Despite the extensive application and encouraging efficacy of anti-CD19 CAR-T in B-cell malignancies, cytopenias remain the most common adverse effects following CAR-T therapy, which greatly affects the quality of life of patients. Of the 68 studies involving 2950 patients included in the study, neutropenia is the most frequent cytopenia after CAR-T therapy, both in all grades or grade ≥3. Subgroup analysis showed that patients with ALL tended to have a higher incidence of cytopenia, which may be related to the bone marrow infiltration and intensive prior therapy in ALL patients. Although elderly patients are more likely to develop post-treatment cytopenias due to the insufficient haematopoietic reserve [Citation78], this study showed a higher incidence of cytopenias in younger patients after CAR-T therapy, possibly due to the predominance of ALL in young patients treated with CAR-T therapy. Interestingly, the subgroup with a higher proportion of male patients had a lower incidence of cytopenias (except for febrile neutropenia), that is, female patients may have a higher risk of cytopenia after CAR-T therapy. However, the mechanisms by which male patients are more prone to febrile neutropenia remain unknown, and the increased risk of infection due to a history of smoking in males may be a contributing factor [Citation79].
With the wide application of single-targeted CAR-T therapies, antigen loss is well recognized as one of the mechanisms to evade CAR-T therapy [Citation80]. Multi-targeted CAR-T therapies have been proved to partially overcome the immune escape caused by the down-regulation or loss of tumour antigens, yet, a more robust immune response, such as CRS, may also be elicited [Citation81]. Dual-targeted CAR-T therapies that combine CD19 and other targets, including CD20, CD22, or BCMA, were also included in this meta-analysis. Three types of multi-targeted approaches were observed in this study, involving tandem CAR-T [Citation17,Citation18,Citation22,Citation26,Citation44,Citation62], bicistronic vector transduced CAR-T [Citation19] and the coadministration of two separate products with different targets [Citation28,Citation45,Citation59]. We found that dual-targeted therapies induced more frequent cytopenias than single-target products, suggesting that cytopenias after CAR-T therapy may be mediated by enhanced immune responses.
Since bone marrow injury could be induced by chemotherapy and HSCT, we subsequently analysed the impact of prior therapy on cytopenias after CAR-T cell infusion. It is observed that cytopenias, especially leukopoenia and lymphopenia, were more frequent in heavily pre-treated patients. Although HSCT has been proved to cause bone marrow injury, the incidence of cytopenias did not differ significantly between subgroups with different prior HSCT ratios, which were consistent with the findings of Luo et al. [Citation82]. Due to the limitations of the data, the type of HSCT, source of the donor and pre-treatment regimen were not further analysed, which may overlook the vital influencing factors of HSCT on post-CAR-T cytopenias. Bridging therapy prior to CAR-T infusion refers to treatment delivered between apheresis and lymphodepletion, involving chemotherapy, immunotherapy and radiotherapy [Citation83]. We were surprised to find that, contrary to our common knowledge, subgroups with lower rates of bridging therapy tended to develop cytopenias more frequently, including grade 3 leukopoenia and neutropenia. Despite the inferior survival conferred by bridging therapy in some studies, which may be explained by the selection bias given that patients with heavy tumour burden were more likely to receive bridging therapy [Citation84,Citation85], our results suggest that it would not increase the risk of delayed haematopoietic recovery after CAR-T infusion, and the underlying mechanism remains unknown. We speculate that the decreased tumour burden may provide the possibility of better reconstruction of the haematopoietic niche and alleviation of severe CRS. Due to the differences in clinical study design, as bridging therapy was given to almost all patients in some studies [Citation25,Citation65] while not permitted in some others [Citation15,Citation48]; thus, inevitable heterogeneity of baseline characteristics and treatment selection do exist across clinical studies, and head-to-head studies are warranted to clarify the effect of bridging therapy, as well as the aforementioned HSCT, on haematopoietic recovery following CAR-T therapy.
The structure, manufacturing and species of scFv origin of CAR-T cells can influence the immune response and efficacy of CAR-T therapy [Citation86]. A single-centre study on NHL showed complete haematopoietic recovery rates of 42% and 100% for Axi-cel (with the CD28 costimulatory domain) and Tisa-cel (with the 4-1BB costimulatory domain) at three months after infusion [Citation87]. A previous meta-analysis showed that products with the CD28 costimulatory domain had a significantly higher incidence of thrombocytopenia and anaemia than those with the 4-1BB domain [Citation82]. In the present work, we noticed that products with the CD28 domain also had a trend to develop lymphopenia and febrile neutropenia more frequently, while the incidence of neutropenia was similar for both domains, possibly due to the fact that CAR-T cells with CD28 domains exhibit a higher incidence of CRS that can lead to fever. Therefore, CAR-T products with the 4-1BB domain may be a preferable option for patients at high risk of developing cytopenias. To date, the vast majority of scFv of CAR-T products are derived from mice, and only nine studies involving 277 patients in this meta-analysis adopted humanized scFv. The immunogenicity of non-humanized scFv can induce an anti-CAR immune response that may influence the persistence of CAR-T cells [Citation88], but its effect on haematopoietic reconstitution remains unexplored. The present work suggests that it may have no effect on haematopoietic reconstitution after CAR-T therapy. Currently, CAR-T manufacturing relies primarily on retrovirus vectors, including lentiviral and gamma retroviral vectors, to transduce CAR into T cells [Citation89]. We found that products manufactured by lentiviral vectors presented a higher incidence of leukopoenia, neutropenia and thrombocytopenia, but the mechanisms involved remain unclear.
There are still some limitations to this study. First, we did not analyse the influence factors on haematopoietic recovery time by reason of limited data in the publications. Second, due to the considerable variation in regimens and dosage of lymphodepletion across clinical studies, the effect of lymphodepletion on haematopoietic reconstitution after CAR-T infusion was not analysed in this study. Lymphodepletion, the chemotherapy prior to CAR-T cell administration, enables better engraftment and expansion of CAR-T cells [Citation83]. High-dose lymphodepletion regimens may contribute to the release of beneficial cytokine and are associated with better treatment response [Citation90], yet lead to stronger haematologic toxicity. Last, this work is limited by being a systematic review that includes studies with heterogeneous designs and populations. Moreover, this study is also methodologically limited due to the fact that only a relatively small number of articles screened described leukopoenia and most of the matched articles did not present data for all six categories of cytopenia, thus leading to the occurrence of partial publication bias. Therefore, larger scale clinical studies, especially prospective and head-to-head studies, are warranted to further define the contributing factors of delayed haematopoietic reconstitution in CAR-T therapy so as to optimize the structure, manufacturing and therapeutic schedule of CAR-T therapy.
5. Conclusion
In conclusion, cytopenia is one the most common adverse events following CAR-T therapy, compromising patients’ quality of life and potentially being life-threatening. The occurrence of cytopenia may be related to clinical characteristics of patients such as disease, age, sex, prior treatment, as well as the design of CAR-T cells, including target, costimulatory domain and type of viral vector. Further studies are needed to confirm our findings and enable a better appreciation of haematopoietic reconstruction after CAR-T therapy.
Author contributions
Conception and design: All author
Collection and assembly of data: Y.X., J.Z. and Jing.L.
Data analysis and interpretation: Y.X., J.Z., Jing.L., L.Z., J.L., L.F. and L.C.
Manuscript writing: Y.X. and J.Z.
Manuscript review & editing: L.C. and L.F.
Final approval of manuscript: All authors.
Supplemental Material
Download MS Word (15.7 KB)Supplemental Material
Download MS Word (24.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Haslauer T, Greil R, Zaborsky N, et al. CAR T-cell therapy in hematological malignancies. Int J Mol Sci. 2021;22(16):8996.
- Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34–48.
- Nagle SJ, Murphree C, Raess PW, et al. Prolonged hematologic toxicity following treatment with chimeric antigen receptor T cells in patients with hematologic malignancies. Am J Hematol. 2021;96(4):455–461.
- Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650.
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42.
- Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978–986.
- Bethge WA, Martus P, Schmitt M, et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood. 2022;140(4):349–358.
- Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145–161.
- Schaefer A, Huang Y, Kittai A, et al. Cytopenias after CD19 chimeric antigen receptor T-Cells (CAR-T) therapy for diffuse large B-Cell lymphomas or transformed follicular lymphoma: a single institution experience. Cancer Manag Res. 2021;13:8901–8906.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Gill SI, Frey NV, Hexner E, et al. Anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia. Blood Adv. 2022;online ahead of print:2022007317.
- Geyer MB, Rivière I, Sénéchal B, et al. Autologous CD19-targeted CAR T cells in patients with residual CLL following initial purine analog-based therapy. Mol Ther. 2018;26(8):1896–1905.
- Neelapu SS, Dickinson M, Munoz J, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28(4):735–742.
- Locke FL, Miklos DB, Jacobson CA, All ZUMA-7 Investigators and Contributing Kite Members, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544.
- Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103.
- Shah NN, Johnson BD, Schneider D, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–1575.
- Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–1431.
- Cordoba S, Onuoha S, Thomas S, et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med. 2021;27(10):1797–1805.
- Ortíz-Maldonado V, Rives S, Castellà M, et al. CART19-BE-01: a multicenter trial of ARI-0001 cell therapy in patients with CD19+ relapsed/refractory malignancies. Mol Ther. 2021;29(2):636–644.
- Liu R, Cheng Q, Kang L, et al. CD19 or CD20 CAR T cell therapy demonstrates durable antitumor efficacy in patients with central nervous system lymphoma. Hum Gene Ther. 2022;33(5-6):318–329.
- Wei G, Zhang Y, Zhao H, et al. CD19/CD22 dual-targeted car t-cell therapy for relapsed/refractory aggressive b-cell lymphoma: a safety and efficacy study. Cancer Immunol Res. 2021;9(9):1061–1070.
- Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554.
- Yan ZX, Li L, Wang W, et al. Clinical efficacy and tumor microenvironment influence in a dose-escalation study of anti-CD19 chimeric antigen receptor T cells in refractory B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 2019;25(23):6995–7003.
- Sesques P, Ferrant E, Safar V, et al. Commercial anti-CD19 CAR T cell therapy for patients with relapsed/refractory aggressive B cell lymphoma in a European center. Am J Hematol. 2020;95(11):1324–1333.
- Hu Y, Zhou Y, Zhang M, et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2021;27(10):2764–2772.
- Roddie C, Dias J, O'reilly MA, et al. Durable responses and low toxicity after fast off-rate cd19 chimeric antigen receptor-t therapy in adults with relapsed or refractory b-cell acute lymphoblastic leukemia. J Clin Oncol. 2021;39(30):3352–3363.
- Wang N, Hu X, Cao W, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135(1):17–27.
- Gu R, Liu F, Zou D, et al. Efficacy and safety of CD19 CAR T constructed with a new anti-CD19 chimeric antigen receptor in relapsed or refractory acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):122.
- Ghorashian S, Kramer AM, Onuoha S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25(9):1408–1414.
- Zhang H, Liu M, Li Q, et al. Evaluation of the safety and efficacy of humanized anti-CD19 chimeric antigen receptor T-cell therapy in older patients with relapsed/refractory diffuse large B-cell lymphoma based on the comprehensive geriatric assessment system. Leuk Lymphoma. 2022;63(2):353–361.
- Myers RM, Li Y, Leahy AB, et al. Humanized CD19-Targeted chimeric antigen receptor (CAR) T cells in CAR-Naive and CAR-Exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J Clin Oncol. 2021;39(27):3044–3055.
- Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143–155.
- Gauthier J, Gazeau N, Hirayama AV, et al. Impact of CD19 CAR T-cell product type on outcomes in relapsed or refractory aggressive B-NHL. Blood. 2022;139(26):3722–3731.
- Cheng Z, Wei R, Ma Q, et al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in patients with B cell leukemia. Mol Ther. 2018;26(4):976–985.
- Ramos CA, Rouce R, Robertson CS, et al. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol Ther. 2018;26(12):2727–2737.
- Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10(8):79.
- Lyu C, Cui R, Wang J, et al. Intensive debulking chemotherapy improves the short-term and long-term efficacy of anti-CD19-CAR-T in refractory/relapsed DLBCL with high tumor bulk. Front Oncol. 2021;11:706087.
- Wang Y, Li H, Song X, et al. Kinetics of immune reconstitution after anti-CD19 chimeric antigen receptor T cell therapy in relapsed or refractory acute lymphoblastic leukemia patients. Int J Lab Hematol. 2021;43(2):250–258.
- Shah BD, Bishop MR, Oluwole OO, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood. 2021;138(1):11–22.
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory Mantle-Cell lymphoma. N Engl J Med. 2020;382(14):1331–1342.
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. The Lancet. 2021;398(10299):491–502.
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet (London, England. 2020;396(10254):839–852.
- Zhang Y, Wang Y, Liu Y, et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1–2 tria. Leukemia. 2022;36(1):189–196.
- Wang Y, Cao J, Gu W, et al. Long-term follow-up of combination of B-cell maturation antigen and CD19 chimeric antigen receptor T cells in multiple myeloma. J Clin Oncol. 2022;40(20):2246–2256.
- Frey NV, Gill S, Hexner EO, et al. Long-Term outcomes from a randomized dose optimization study of chimeric antigen receptor modified T cells in relapsed chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2862–2871.
- Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–1813.
- Maschan M, Caimi PF, Reese-Koc J, et al. Multiple site place-of-care manufactured anti-CD19 CAR-T cells induce high remission rates in B-cell malignancy patients. Nat Commun. 2021;12(1):7200.
- Liu X, Zhang Y, Li K, et al. A novel dominant-negative PD-1 armored anti-CD19 CAR T cell is safe and effective against refractory/relapsed B cell lymphoma. Transl Oncol. 2021;14(7):101085.
- Sun M, Xu P, Wang E, et al. Novel two-chain structure utilizing KIRS2/DAP12 domain improves the safety and efficacy of CAR-T cells in adults with r/r B-ALL. Mol Ther Oncolytics. 2021;23:96–106.
- Ying Z, He T, Wang X, et al. Parallel comparison of 4-1BB or CD28 Co-stimulated CD19-targeted CAR-T cells for B cell non-Hodgkin’s lymphoma. Mol Ther Oncol. 2019;15:60–68.
- Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285–295.
- Siddiqi T, Soumerai JD, Dorritie KA, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2022;139(12):1794–1806.
- Kato K, Makita S, Goto H, et al. Phase 2 study of axicabtagene ciloleucel in japanese patients with relapsed or refractory large B-cell lymphoma. Int J Clin Oncol. 2022;27(1):213–223.
- Chen X, Li X, Liu Y, et al. A phase i clinical trial of chimeric antigen receptor-modified T cells in patients with relapsed and refractory lymphoma. Immunotherapy. 2020;12(10):681–696.
- Fan L, Wang L, Cao L, et al. Phase I study of CBM.CD19 chimeric antigen receptor T cell in the treatment of refractory diffuse large B-cell lymphoma in Chinese patients. Front Med. 2022;16(2):285–294.
- Zhou X, Tu S, Wang C, et al. Phase I trial of Fourth-Generation anti-CD19 chimeric antigen receptor T cells against relapsed or refractory B cell Non-Hodgkin lymphomas. Front Immunol. 2020;11:564099.
- Enblad G, Karlsson H, Gammelgård G, et al. A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res. 2018;24(24):6185–6194.
- Sang W, Shi M, Yang J, et al. Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma. Cancer Med. 2020;9(16):5827–5838.
- Hu Y, Wu Z, Luo Y, et al. Potent anti-leukemia activities of chimeric antigen receptor-modified T cells against CD19 in chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res. 2017;23(13):3297–3306.
- Talleur A, Qudiemat A, Métais JY, et al. Preferential expansion of CD8+ CD19-CAR T cells postinfusion and the role of disease burden on outcome in pediatric B-ALL. Blood Adv. 2022; online ahead of print:2021006293.
- Zhang Y, Li J, Lou X, et al. A prospective investigation of bispecific CD19/22 CAR T cell therapy in patients with relapsed or refractory B cell Non-Hodgkin lymphoma. Front Oncol. 2021;11:664421.
- Sim AJ, Jain MD, Figura NB, et al. Radiation therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2019;105(5):1012–1021.
- Ying Z, Yang H, Guo Y, et al. Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China. Cancer Med. 2021;10(3):999–1011.
- Kadauke S, Myers RM, Li Y, et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-Cell acute lymphoblastic leukemia: a prospective clinical trial. J Clin Oncol. 2021;39(8):920–930.
- Ying Z, Huang XF, Xiang X, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25(6):947–953.
- Brudno JN, Lam N, Vanasse D, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med. 2020;26(2):270–280.
- Geyer MB, Rivière I, Sénéchal B, et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight. 2019;5(9):e122627.
- Bishop MR, Dickinson M, Purtill D, et al. Second-Line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–639.
- Pan J, Zuo S, Deng B, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. 2020;135(5):387–391.
- Heng G, Jia J, Li S, et al. Sustained therapeutic efficacy of humanized anti-CD19 chimeric antigen receptor T cells in relapsed/refractory acute lymphoblastic leukemia. Clin Cancer Res. 2020;26(7):1606–1615.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England). 2015;385(9967):517–528.
- Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325–332.
- Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134(26):2361–2368.
- Brudno JN, Somerville RPT, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–1121.
- Schuster SJ, Bishop MR, Tam CS, JULIET Investigators, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448.
- Yamashita M, Iwama A. Aging and clonal behavior of hematopoietic stem cells. Int J Mol Sci. 2022;23(4):1948.
- Huttunen R, Heikkinen T, Syrjanen J. Smoking and the outcome of infection. J Intern Med. 2011;269(3):258–269.
- Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–385.
- Shah NN, Maatman T, Hari P, et al. Multi targeted CAR-T cell therapies for B-Cell malignancies. Front Oncol. 2019;9:146.
- Luo W, Li C, Zhang Y, et al. Adverse effects in hematologic malignancies treated with chimeric antigen receptor (CAR) T cell therapy: a systematic review and meta-analysis. BMC Cancer. 2022;22(1):98.
- Amini L, Silbert SK, Maude SL, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022;19(5):342–355.
- Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium [J]. J Clin Oncol. 2020;38(27):3119–3128.
- Pinnix CC, Gunther JR, Dabaja BS, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4(13):2871–2883.
- Huang R, Li X, He Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13(1):86.
- Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787.
- Wagner DL, Fritsche E, Pulsipher MA, et al. Immunogenicity of CAR T cells in cancer therapy. Nat Rev Clin Oncol. 2021;18(6):379–393.
- Labbe RP, Vessillier S, Rafiq QA. Lentiviral vectors for T cell engineering: clinical applications, bioprocessing and future perspectives. Viruses. 2021;13(8):1528.
- Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116.