 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The epidemiologic studies investigating the association of birthweight and genetic factors with gastrointestinal cancer remain scarce. The study aimed to prospectively assess the interactions and joint effects of birthweight and genetic risk levels on gastrointestinal cancer incidence in adulthood.
Methods
A total of 254,997 participants were included in the UK Biobank study. We used multivariate restricted cubic splines and Cox regression models to estimate the hazard ratios (HRs) and 95% confidential intervals (CI) for the association between birthweight and gastrointestinal cancer risk, then constructed a polygenic risk score (PRS) to assess its interaction and joint effect with birthweight on the development of gastrointestinal cancer.
Results
We documented 2512 incident cases during a median follow-up of 8.88 years. Compare with participants reporting a normal birthweight (2.5–4.5 kg), multivariable-adjusted HR of gastrointestinal cancer incidence for participants with high birthweight (≥4.5 kg) was 1.17 (95%CI: 1.01–1.36). Such association was remarkably observed in pancreatic cancer, with an HR of 1.82 (95%CI: 1.26–2.64). No statistically significant association was observed between low birth weight and gastrointestinal cancers. Participants with high birthweight and high PRS had the highest risk of gastrointestinal cancer (HR: 2.95, 95%CI: 2.19–3.96).
Conclusion
Our findings highlight that high birthweight is associated with a higher incidence of gastrointestinal cancer, especially for pancreatic cancer. Benefits would be obtained from birthweight control, particularly for individuals with a high genetic risk.
The epidemiologic studies investigating the association of birthweight and genetic factors with gastrointestinal cancer remain scarce.
This cohort study of 254,997 adults in the United Kingdom found an association of high birthweight with the incidence of gastrointestinal cancer, especially for pancreatic cancer, and also found that participants with high birthweight and high polygenic risk score had the highest risk of gastrointestinal cancer.
Our data suggests a possible effect of in utero or early life exposures on adulthood gastrointestinal cancer, especially for those with a high genetic risk.
KEY MESSAGES
1. Introduction
Gastrointestinal cancer has represented over one-quarter of the global cancer incidence, with an estimated 5.5 million new cases worldwide in 2020 [Citation1]. It includes mouth cancer, esophagus cancer, stomach cancer, liver cancer, biliary duct cancer, pancreatic cancer, and colorectal cancer. In recent years, colorectal, liver, and stomach cancers have been the second, third, and fourth leading causes of cancer deaths, just behind lung cancer [Citation1,Citation2]. Moreover, cancers of mouth, esophagus, biliary tract, and pancreas have also become a growing global concern due to increasing incidence and poor prognosis [Citation3–7]. Generally, several factors including age, sex, family history, smoking, and alcohol intake have been reported to contribute to the development of gastrointestinal cancer [Citation8]. However, more than 20% of liver cancers, more than 40% of colon and stomach cancers, more than 60% of gallbladder cancers, and more than 70% of pancreatic cancers could not be explained by these known risk factors [Citation8]. In view of the high incidence and aggressive nature of gastrointestinal cancer, to identify the ‘high risk population’ as early as possible is crucial for strategies of cancer prevention.
As a widely used measure of fetal growth and intrauterine malnutrition, birthweight has been reported to predict the risk of several diseases in infancy and adulthood [Citation9]. Substantial evidence supports the associations of birthweight with increased risk of metabolic disease, such as type 2 diabetes [Citation10–14] and cardiovascular disease [Citation15–18], which may share etiological pathways with gastrointestinal cancer [Citation19–21]. However, results on birthweight and gastrointestinal cancer risk remain inconsistent, with linear [Citation22,Citation23], non-linear [Citation22,Citation24,Citation25], and null associations [Citation26,Citation27] reported. For example, in the studies from the Copenhagen School Health Records Register (CSHRR), birthweight was reported to be positively associated with the risk of colon but inversely associated with rectal cancer [Citation22], positively associated with the risk of liver cancer in women but not in men [Citation25]. Studies on other sites (mouth, esophagus, biliary duct, etc.) are too limited to draw a conclusion.
Accumulating evidences have shown that genetic factors are crucial in the development of gastrointestinal cancer [Citation28–30]. A Nordic study on twins estimated that inherited genes contribute to the risk of 28% of stomach, 35% of colorectal, and 36% of pancreas cancer, respectively [Citation29]. To date, many risk genetic variants have been identified by genome-wide association studies (GWAS), but each variant accounts for only a small fraction of the genetic risk of cancer [Citation31]. Therefore, polygenic risk scores (PRS) are developed to consider the accumulative effects of these genetic variants with a small effect in a risk model and have been shown to be effective in predicting cancer risk and other clinical outcomes [Citation32,Citation33]. It is well-known that common complex traits or diseases result from a combination of both environmental and genetic factors and the interactions between them. Birthweight is a physical measure that combines effects from both non-genetic and genetic factors. Several studies have reported that genetic factors could modify associations between birthweight and diseases [Citation34,Citation35]. However, the association of birthweight and genetic factors with gastrointestinal cancer is still not clear.
2. Methods
2.1. Study population
This prospective study used the publicly available data from UK Biobank (https://www.ukbiobank.ac.uk/). From 2006 to 2010, the UK Biobank recruited over 500,000 people aged 40–69. Participants went to one of 22 assessment centers in England, Wales, and Scotland and filled out self-reported touchscreen questionnaires as well as provided biological samples for various types of testing [Citation36]. Data were collected including comprehensive personal and exposure information, and physical measurements. The UK Biobank cohort was granted ethics approval from the North West Multicenter Research Ethics Committee. All the participants provided informed written consent.
In this study, we excluded the participants who reported a history of cancer at baseline (n = 46,333) and those who had missing birthweight data (n = 200,928), leaving 254,997 participants in the final analysis (Supplementary Figure S1).
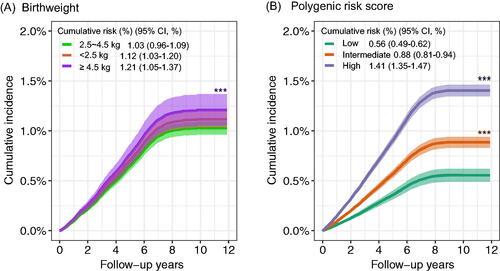
Figure 1. The cumulative risk of gastrointestinal cancer incidence according to birthweight and genetic risk. PY: person-years. (A) The cumulative risk of gastrointestinal cancer incidence in birthweight during follow-up. (B) The cumulative risk of gastrointestinal cancer incidence in low, intermediate, and high genetic risk groups during follow-up.
2.2. Birthweight
At baseline, participants were required to report their own birthweight via a verbal interview at the UK Biobank Assessment Centre (UK Biobank field ID: 20022). The Interviews were conducted by the trained nurses. Birthweight were first recorded in kilograms (kg) or in ounces and pounds, and then all converted into kg.
2.3. Genotyping
Participants were genotyped using the Applied Biosystems UK BiLEVE Axiom Array (807,411 markers tested for 49,950 participants) or Applied Biosystems UK Biobank Axiom Array (825,927 markers tested for 438,427 participants) by Affymetrix. These arrays share more than 95% of single-nucleotide polymorphisms (SNP) tested. Imputation was performed with SHAPEIT3 and IMPUTE3 based on merged UK10K and 1000 Genomes phase 3 panels.
2.4. PRS calculation
The PRS of gastrointestinal cancer was calculated based on previous literature [Citation31]. Briefly, we extracted each gastrointestinal cancer-related single nucleotide polymorphisms (SNPs) and effect sizes (odds ratios [ORs]) reported in the literature (summarized in Table S1) [Citation31]. In our study, we constructed the PRSs by using the risk-associated SNPs for oral, colorectal, esophageal, pancreatic, and stomach cancers. The corresponding age-standardized incidence rates (hk) of UK population were extracted [Citation37]. The PRSi,k of a certain gastrointestinal cancer (k) of ith individual is calculated by multiplying the dosage of the risk allele of each SNP and the corresponding weight [log (ORs)] and then adding them up. Subsequently, the PRS of gastrointestinal cancer is the sum of the products of PRSi,k and hk, which is defined as follows:
Table 1. Characteristics of study participants according to birthweight at baseline.a
2.5. Follow-up and outcomes
Incident cancer cases from the UK Biobank cohort were recorded in linkage to national cancer and death registries. Participants were followed up from their initial visit to the end of October 2015 in Scotland and the end of March 2016 in England and Wales.
The diagnosis of gastrointestinal cancer was confirmed by electronic health records and was coded according to the 10th Revision of the International Classification of Diseases (ICD-10). For oral (C03–C06), esophageal (C15), stomach (C16), colorectal (C18–20), liver (C22), biliary tract (C23–24), and pancreatic (C25) malignancies, separate analyses were carried out. According to the anatomical sub-site, colorectal cancer cases were divided into two groups: malignancies of the colon (C18) and rectum (C19–20).
2.6. Covariates
Covariates were obtained from questionnaires at baseline and included as follows: age at recruitment, gender, race, income, family history of cancer, smoking status, alcohol consumption, aspirin intake, menopause status, and the intake of oily fish and processed meat. Body mass index (BMI) was derived by weight (kg) and height (m). Physical activity was measured by total metabolic equivalent task (MET) hours per week for all activity including walking, and moderate and vigorous activity.
2.7. Statistical analysis
Followed-up time was calculated from the baseline to the first record of gastrointestinal cancer diagnosis or censoring. Censoring was defined as the date of death, withdrawal from the study, or the end of follow-up, whichever occurred first.
Multivariate restricted cubic splines were used to examine the dose-response association between birthweight and gastrointestinal cancer risk, with p < 0.05 as significant nonlinearity. We also calculated the hazard ratios (HRs) and 95% confidence intervals (CIs) to assess the associations of birthweight, and genetic factors with gastrointestinal cancer risk, using multivariate Cox proportional hazard models. The proportional hazards assumption was tested using a cross-product term of log(time) and birthweight, and no violations were observed (p > 0.05). Multivariate models were adjusted by the following covariates: sex, age at recruitment, race, income, family history of cancer, body mass index, alcohol status, smoking status, physical activity, take aspirin, menopause status, and the top genetic principal components of each gastrointestinal cancer.
In view of the nonlinear association between birthweight and gastrointestinal cancer risk (Supplementary Figure S2(A)), we categorized birthweight into three groups: low (<2.5 kg), normal (2.5–4.5 kg), and high (≥4.5 kg), according to the international standards. The PRS was categorized into low (tertile 1), intermediate (tertile 2), and high (tertile 3). We also conducted the stratified analyses by sex (male, female), age (<60, ≥60 years), tobacco smoking (never, smoker), alcohol drinking (non-excessive, excessive), BMI (<25, ≥25 kg/m2), aspirin use (no, yes), physical activity (<29.5, ≥29.5 METS-hours/week), and family history of cancer (no, yes), respectively. p-Values for interaction were tested by introducing a product term of the two variables studied in the regression models. To test the joint effects of birthweight and genetic factors on gastrointestinal cancer risk, we categorized the participants into 9 groups according to birthweight and the tertiles of PRS. The relative excess risk due to interaction (RERI) and the attributable proportion because of the interaction (AP) were estimated to test for statistical significance of additive interactions by using Cox proportional hazards regression models [Citation38]. The 95% CIs of the RERI and AP were calculated by randomly selecting 5000 bootstrap samples from the estimation datasets [Citation39]. Statistical analyses in our study were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and R 4.0.2. A two-sided p < 0.05 was considered statistically for all the tests, if not specified.
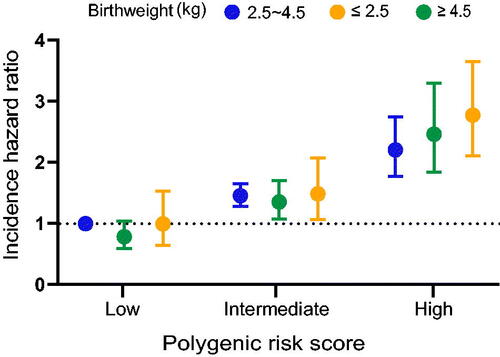
Figure 2. The joint effects of birthweight and genetic factors on gastrointestinal cancer incidence. The hazard ratios (95% CIs) of gastrointestinal cancer incidence within joint categories of birthweight and PRS. Cox proportional hazard models were used with adjustment for sex (male, female), age at recruitment (years), race (White, non-white race), income (<18,000, 18,000–30,999, 31,000–51,999, 52,000–100,000, >100,000, and not known), family history of cancer (yes, no), body mass index (kg/m2), alcohol intake frequency (never, special occasions only, one to three times a month, once or twice a week, three or four times a week, and daily or almost daily), physical activity (in quartiles), take aspirin (yes, no), menopause status (yes, no, and not sure), oily fish (never, <1 occasion per week, =1 occasion per week, 2–4 occasions per week, 5–6 occasions per week, ≥7 occasions per week) and processed meat (never, <1 occasion per week, =1 occasion per week, 2–4 occasions per week, 5–6 occasions per week, ≥7 occasions per week).
3. Results
During a median follow-up of 8.88 years, there were 2512 incident cases of gastrointestinal cancer among 254,997 participants for analysis. We used multivariate restricted cubic splines and found that there was a nonlinear association between birthweight and gastrointestinal cancer risk (Supplementary Figure S2(A)). Of 2512 incident cases, 257 (10.2%) were born with low birthweight (<2.5 kg) and 198 (7.9%) were born with high birthweight (≥4.5 kg). Other baseline characteristics of participants based on the birthweight groups are shown in .
We performed Cox proportional hazard models to investigate the association of birthweight with overall and each classification of gastrointestinal cancer risk (). Participants with high birthweight had an increased risk of gastrointestinal cancer (multivariable HR: 1.17, 95%CI: 1.01–1.36) compared with those with normal birthweight. Such association was remarkably observed in pancreatic cancer (multivariable HR: 1.82, 95%CI: 1.26–2.64). Consistent with overall gastrointestinal cancer, the association was U-shaped between birthweight and pancreatic cancer risk by multivariate restricted cubic splines (Supplementary Figure S2(B)). No statistically significant association was observed between low birth weight and gastrointestinal cancers.
Table 2. Association between birthweight and gastrointestinal cancer risk.
We also conducted cumulative risk analysis according to birthweight and PRS, respectively (). For birthweight, there was a consistent trend of increasing cumulative risk for gastrointestinal cancer incidence over follow-up time. The cumulative incidence was highest for participants with high birthweight. For PRS of gastrointestinal cancer, a similar trend was also observed, and higher PRS were related to higher gastrointestinal cancer risk. In the joint analysis of birthweight and PRS, we identified that the risk of gastrointestinal cancer was significantly increased with increasing PRS (). Compared with participants with normal birthweight and low PRS, those with high birthweight and high PRS had the highest risk of gastrointestinal cancer (HR: 2.95, 95%CI: 2.19–3.96).
Interactions between birthweight and PRS on gastrointestinal cancer incidence were further investigated, and a border additive interaction was found (). For high birthweight participants with high PRS, the RERI was 0.57 (95%CI: −0.10–1.24, p = 0.095); and AP showed that the additive interaction accounted for 21% (95%CI: 1–42%, p = 0.061) of gastrointestinal cancer risk in these high-risk participants.
Table 3. Additive interactions between birthweight and PRS on gastrointestinal cancer risk.
Finally, we conducted subgroup analyses to examine associations of birthweight with gastrointestinal cancer risk and their interactions based on covariates (Supplementary Figure S3). Results demonstrated no significant association or interaction between these factors and birthweight on gastrointestinal cancer risk.
4. Discussion
In this prospective cohort study based on the UK Biobank, we identified that high birthweight (≥4.5 kg) was significantly associated with an increased risk of gastrointestinal cancer, especially in pancreatic cancer. No association has been found between low birthweight (<2.5 kg) and gastrointestinal cancers. Moreover, we also found that individuals with high birthweight and high PRS might have the highest cancer risk. Our findings have revealed a harmful role of high birthweight in the development of gastrointestinal cancer, which provides new evidence for the protection of fetal growth in cancer prevention. Benefits would be obtained from birthweight control, particularly for those with more risk genetic variants.
Several prospective studies have been performed on birthweight and gastrointestinal cancers, but the results are inconsistent. The study conducted in the Danish Civil Registration System [Citation26] and the Women’s Health Initiative (WHI) [Citation27] reported no association of birthweight with the risk of gastrointestinal cancers. However, the Uppsala Birth Cohort Study reported a linear association that one standard deviation increase in birthweight was associated with a 13% increase in rates of digestive cancers [Citation23]. In addition, some studies showed nonlinear results. A Danish cohort study reported a sex-specific association between birthweight of 3.75–5.50 kg and liver cancer risk in women but not in man, as compared with birthweights between 3.25 and 3.75 kg [Citation25]. A study from the CSHRR reported that birthweight is positively associated with the risk of adult colon cancer, whereas the results for rectal cancer were inverse only in birthweights over 3.5 kg [Citation22]. Non-linear result between birthweight and incident colorectal cancer was also observed in the European Prospective Investigation of Cancer in Norfolk study, with an adjusted HR of 2.57 for having neonatal macrosomia at birth [Citation24]. In this study, we supported the nonlinear association that high birthweight increased the risk of gastrointestinal cancer, especially for pancreatic cancer.
Although the mechanisms of the association between birthweight and gastrointestinal cancer risk remain unclear, it is confirmed that restricted early life development has a long-term structural and functional influence on individuals’ predisposition to an increased risk of complex diseases, such as metabolic diseases [Citation40,Citation41]. Previous prospective studies have reported that increased birthweight might increase the risk of adult obesity [Citation42,Citation43], type 2 diabetes [Citation10–14], and cardiovascular disease [Citation15–18]. These diseases may have overlapping mechanisms with gastrointestinal cancer, such as inflammation and insulin resistance [Citation19–21]. Another potential mechanism underlying the association between high birthweight and gastrointestinal cancer might be through hormone levels [Citation44]. High birthweight may be associated with a high hormonal exposure in utero [Citation25], whereas estrogen have numerous effects on various organs and diseases specific to the gastrointestinal tract [Citation45]. In addition, Adipose tissue is known to be related to pancreatic inflammation, insulin resistance, β cell dysfunction, and diabetes [Citation46]. These processes have been suggested to play a role in adulthood pancreatic cancer and may contribute to the association between birth weight and pancreatic cancer. Overweight in early childhood may also be associated with later pancreatic cancer through a correlation between childhood and adult BMI or through an independent process that stimulates the early steps of carcinogenesis [Citation47]. In addition, genes may be involved in the embryonic development of the pancreas and have been reported to be associated with BMI as well as pancreatic cancer [Citation48,Citation49]. This supports the role of early life exposures affecting the pancreas in the pathway leading to the development of pancreatic cancer later in life.
Cancer is a class of complex chronic diseases influenced by long-term interactions of genetic factors and environmental actors [Citation50,Citation51]. PRS involving multiple SNPs can better predict the individual genetic risk of certain cancer than a single SNP which can only explain part of tumor susceptibility [Citation52–54]. Several studies used integrated approaches for disease risk [Citation34,Citation55–58] or mortality [Citation59] estimation by adding PRS information to routinely used risk predictors, finding significant improvement [Citation34,Citation55] or improving the prediction power of such predictors [Citation56,Citation57,Citation60].
Birthweight reflects a complex interplay of genetic, nutritional, and other environmental factors that affect growth within the womb [Citation9]. It is likely a summative indicator of these factors, rather than a causative factor, in relation to the risks of chronic diseases [Citation44]. To our knowledge, this is the first study to investigate the interaction between birthweight and genetic factors on the risk of gastrointestinal cancer. Our findings showed that a combination of high genetic risk and high birthweight enhanced the risk of gastrointestinal cancer. Our data provided initial evidence for the observed additive interactions between genetic factors and birthweight, and may help to suggest that birthweight control may potentially influence gastrointestinal cancer development, particularly in high genetic risk populations. Future research is needed to confirm our findings in other populations and clarify the underlying biological mechanisms.
Strengths of this study include its prospective cohort design, long follow-up time with a high follow-up rate, and the ability to control for multiple gastrointestinal cancer risk factors. However, several potential limitations merit consideration. First, GWASs data for liver cancer in the European population were not available, so we are unable to include any genetic information about liver cancer in the analysis of PRSs. Our results related to liver cancer need to be updated in the future. Second, the birthweight information was self-reported in this cohort, and could not be validated. However, validity has been demonstrated for the correlation of categories of self-reported birthweight to medical record information [Citation60,Citation61]. As observed by earlier investigations, this problem does not appear to change the general relationship between birthweight and gastrointestinal cancers [Citation22,Citation27]. Third, the mean age of the participants was around 60 years old at baseline, survivor bias was unavoidable, although no statistically significant interactions between age and birthweight were found in our stratified analyses. Fourth, information, such as gestational age was scarce. Birthweight has traditionally been thought of as a combination of postnatal nutrition and gestational age at birth. As a result, information bias is unavoidable, particularly when it comes to data on maternal exposure and ‘catch-up growth’ in low birthweight newborns. Nonetheless, we found no evidence of an association between low birthweight and gastrointestinal cancer. Although we could not distinguish whether the results were influenced by undernutrition or prematurity, the possibility that their association was overestimated has a limited impact on our study. Finally, because the majority of covariates were only available at baseline, we cannot capture changes in possible confounders over time.
5. Conclusion
In conclusion, this large prospective cohort study highlights that high birthweight is associated with a higher incidence of gastrointestinal cancer. This research suggests a possible effect of in utero or early life exposures on adulthood gastrointestinal cancer, especially for those with a high genetic risk.
Author contributions
RZ: conception and design. RZ, HH, and LL: analysis of the data. LL: the drafting of the paper. All the authors: acquisition or interpretation of data, revising it critically for intellectual content, and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (1.2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Source data is accessible via application to the UK Biobank (https://www.ukbiobank.ac.uk/).
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26(2):107–118.
- Luo G, Zhang Y, Guo P, et al. Global patterns and trends in pancreatic cancer incidence: age, period, and birth cohort analysis. Pancreas. 2019;48(2):199–208.
- Castro FA, Koshiol J, Hsing AW, et al. Biliary tract cancer incidence in the United States-demographic and temporal variations by anatomic site. Int J Cancer. 2013;133(7):1664–1671.
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357.
- Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551.
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54.
- Research WCRFAIfC. Height and birthweight and the risk of cancer; 2018. Available from: https://www.wcrf.org/dietandcancer/height-and-birthweight/
- Harder T, Rodekamp E, Schellong K, et al. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165(8):849–857.
- Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–2897.
- Li Y, Ley SH, Tobias DK, et al. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: prospective cohort study. BMJ. 2015;351:h3672.
- Norris SA, Osmond C, Gigante D, et al. Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care. 2012;35(1):72–79.
- Li Y, Qi Q, Workalemahu T, et al. Birth weight, genetic susceptibility, and adulthood risk of type 2 diabetes. Diabetes Care. 2012;35(12):2479–2484.
- Liang J, Xu C, Liu Q, et al. Association between birth weight and risk of cardiovascular disease: evidence from UK biobank. Nutr Metab Cardiovasc Dis. 2021;31(9):2637–2643.
- Smith CJ, Ryckman KK, Barnabei VM, et al. The impact of birth weight on cardiovascular disease risk in the women’s health initiative. Nutr Metab Cardiovasc Dis. 2016;26(3):239–245.
- Eriksson JG, Forsen T, Tuomilehto J, et al. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322(7292):949–953.
- Lawlor DA, Ronalds G, Clark H, et al. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen children of the 1950s prospective cohort study. Circulation. 2005;112(10):1414–1418.
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86(3):s836–s842.
- Janssen J. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int J Mol Sci. 2021;22(15):7797.
- Godsland IF. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin Sci. 2009;118(5):315–332.
- Smith NR, Jensen BW, Zimmermann E, et al. Associations between birth weight and colon and rectal cancer risk in adulthood. Cancer Epidemiol. 2016;42:181–185.
- McCormack VA, dos Santos Silva I, Koupil I, et al. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115(4):611–617.
- Sandhu MS, Luben R, Day NE, et al. Self-reported birth weight and subsequent risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(9):935–938.
- Zimmermann E, Berentzen TL, Gamborg M, et al. Sex-specific associations between birth weight and adult primary liver cancer in a large cohort of Danish children. Int J Cancer. 2016;138(6):1410–1415.
- Ahlgren M, Wohlfahrt J, Olsen LW, et al. Birth weight and risk of cancer. Cancer. 2007;110(2):412–419.
- Spracklen CN, Wallace RB, Sealy-Jefferson S, et al. Birth weight and subsequent risk of cancer. Cancer Epidemiol. 2014;38(5):538–543.
- Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315(1):68–76.
- Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85.
- Graff RE, Moller S, Passarelli MN, et al. Familial risk and heritability of colorectal cancer in the Nordic twin study of cancer. Clin Gastroenterol Hepatol. 2017;15(8):1256–1264.
- Zhu M, Wang T, Huang Y, et al. Genetic risk for overall cancer and the benefit of adherence to a healthy lifestyle. Cancer Res. 2021;81(17):4618–4627.
- Martin AR, Kanai M, Kamatani Y, et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51(4):584–591.
- Sugrue LP, Desikan RS. What are polygenic scores and why are they important? JAMA. 2019;321(18):1820–1821.
- Moldovan A, Waldman YY, Brandes N, et al. Body mass index and birth weight improve polygenic risk score for type 2 diabetes. J Pers Med. 2021;11(6):582.
- Hubinette A, Cnattingius S, Ekbom A, et al. Birthweight, early environment, and genetics: a study of twins discordant for acute myocardial infarction. Lancet. 2001;357(9273):1997–2001.
- Collins R. What makes UK biobank special? Lancet. 2012;379(9822):1173–1174.
- Cancer registration statistics: England 2018 final release [cited 2022 Oct 16]. Available from: https://www.gov.uk/government/statistics/cancer-registration-statistics-england-2018-final-release
- Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–236.
- Assmann SF, Hosmer DW, Lemeshow S, et al. Confidence intervals for measures of interaction. Epidemiology. 1996;7(3):286–290.
- Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(10):787–794.
- Wang T, Huang T, Li Y, et al. Low birthweight and risk of type 2 diabetes: a mendelian randomisation study. Diabetologia. 2016;59(9):1920–1927.
- Monasta L, Batty GD, Cattaneo A, et al. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010;11(10):695–708.
- Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev. 2011;12(7):525–542.
- Aarestrup J, Bjerregaard LG, Meyle KD, et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int J Obes. 2020;44(7):1546–1560.
- Chen C, Gong X, Yang X, et al. The roles of estrogen and estrogen receptors in gastrointestinal disease. Oncol Lett. 2019;18(6):5673–5680.
- Alempijevic T, Dragasevic S, Zec S, et al. Non-alcoholic fatty pancreas disease. Postgrad Med J. 2017;93(1098):226–230.
- Nogueira L, Stolzenberg-Solomon R, Gamborg M, et al. Childhood body mass index and risk of adult pancreatic cancer. Curr Dev Nutr. 2017;1(10):e001362.
- Li D, Duell EJ, Yu K, et al. Pathway analysis of genome-wide association study data highlights pancreatic development genes as susceptibility factors for pancreatic cancer. Carcinogenesis. 2012;33(7):1384–1390.
- Pierce BL, Austin MA, Ahsan H. Association study of type 2 diabetes genetic susceptibility variants and risk of pancreatic cancer: an analysis of PanScan-I data. Cancer Causes Control. 2011;22(6):877–883.
- Wang WY, Barratt BJ, Clayton DG, et al. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet. 2005;6(2):109–118.
- Irigaray P, Newby JA, Clapp R, et al. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61(10):640–658.
- Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018;19(9):581–590.
- Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28(R2):R133–R142.
- Mavaddat N, Michailidou K, Dennis J, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104(1):21–34.
- Riveros-Mckay F, Weale ME, Moore R, et al. Integrated polygenic tool substantially enhances coronary artery disease prediction. Circ Genom Precis Med. 2021;14(2):e003304.
- Liu W, Zhuang Z, Wang W, et al. An improved genome-wide polygenic score model for predicting the risk of type 2 diabetes. Front Genet. 2021;12:632385.
- Sun L, Pennells L, Kaptoge S, et al. Polygenic risk scores in cardiovascular risk prediction: a cohort study and modelling analyses. PLOS Med. 2021;18(1):e1003498.
- Mars N, Koskela JT, Ripatti P, et al. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. 2020;26(4):549–557.
- Meisner A, Kundu P, Zhang YD, et al. Combined utility of 25 disease and risk factor polygenic risk scores for stratifying risk of all-cause mortality. Am J Hum Genet. 2020;107(3):418–431.
- Jaworowicz DJ, Nie J, Bonner MR, et al. Agreement between self-reported birth weight and birth certificate weights. J Dev Orig Health Dis. 2010;1(2):106–113.
- Allen DS, Ellison GT, dos Santos Silva I, et al. Determinants of the availability and accuracy of self-reported birth weight in middle-aged and elderly women. Am J Epidemiol. 2002;155(4):379–384.
