?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Epstein-Barr virus (EBV)-associated hemophagocytic lymphohistiocytosis (EBV-HLH) is a common subtype of HLH with heterogeneous clinical presentations from self-limited to death, of which adults are worse than children.
Objective
To establish predictors of mortality risk in adult EBV-HLH patients for timely and appropriate treatment.
Methods
Patients with confirmed EBV-HLH admitted to Beijing Friendship Hospital from January 2015 to December 2019 were enrolled and statistical analysis of their laboratory test results was performed.
Results
Among 246 adult patients with EBV-HLH, the deceased were older (p < 0.05), with fewer blood cells (p < 0.05), poorer renal function (p < 0.01), higher levels of procalcitonin (PCT) (p < 0.01), as well as soluble interleukin-2 receptor (sCD25) (p < 0.01). The overall median survival time of patients was 135 days, 87 days for patients without transplantation and 294 days with transplantation (p < 0.001). A combined index of sCD25, PCT, and estimated glomerular filtration rate (eGFR) was obtained to predict prognosis, named the Improved HLH index (IH index), and patients were divided into three groups meeting IH- (i.e. sCD25 ≤ 18,000 pg/mL, PCT ≤ 1.8 ng/mL, eGFR ≥ 90 mL/min/1.73m2), IH1+ (i.e. only sCD25 > 18,000 pg/mL or only eGFR < 90 mL/min/1.73m2), and IH2+ (i.e. the rest), respectively. In patients with the HScore ≥ 169 or meeting HLH-04, those meeting IH2+ had significantly worse prognoses than those who met IH1+ or IH- (p < 0.001). In the group meeting IH + or IH2+, patients who received allo-HSCT had better prognoses than those who did not (p < 0.05), but there was still a significant difference in prognosis among the three groups in transplanted patients (p < 0.001).
Conclusion
The IH index can early identify adult patients with a poor prognosis of EBV-HLH, initiating timely and appropriate treatment.
A combined index of sCD25, PCT, and eGFR was obtained to predict prognosis, named the Improved Hemophagocytic Lymphohistiocytosis index (IH index).
IH index can early identify adult patients with a poor prognosis of EBV-HLH, initiating timely and appropriate treatment.
KEY MESSAGES
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a group of syndromes with primary or acquired immune abnormalities, mainly manifested by fever, hepatosplenomegaly, hemocytopenia, and elevated ferritin [Citation1]. It progresses rapidly with a poor prognosis, and patients can develop coagulation dysfunction, liver failure, central nervous system involvement, multi-organ failure, and other manifestations, which can be life-threatening [Citation2]. According to the cause, HLH can be divided into primary and secondary. Secondary HLH (sHLH) is mostly seen in adults [Citation3] and Epstein-Barr virus (EBV)-associated HLH is one of its major subtypes, especially in Asian countries [Citation4]. Previous research models suggest that the clinical manifestations of HLH vary according to genetic polymorphisms and mutations [Citation5]. For EBV-HLH, it may be more heterogeneous due to primary infection, chronic active EBV infection, or underlying primary HLH [Citation6]. A small number of patients can achieve long-term clinical remission with HLH-94 treatment regimens, but more often progress to relapsed/refractory [Citation7]. The prognosis of HLH in adults is poor compared to that of children [Citation5]. However, clinical and pathology-based prognostic indicators for EBV-HLH have not yet been established [Citation8], so this study aimed to establish clinical indicators for prognostic prediction in adult EBV-HLH patients.
Methods
Patients
This was a prospective study and adult patients with confirmed EBV-HLH admitted to Beijing Friendship Hospital from January 2015 to December 2019 were enrolled in this study. It was approved by the Ethics Committee of Beijing Friendship Hospital. Written informed consent was obtained from the patients following the Helsinki Declaration.
Inclusion criteria: HLH was diagnosed according to the HLH-2004 diagnostic criteria [Citation9], i.e. meeting at least five of the eight HLH-2004 diagnostic criteria: (1) temperature 38.5 °C and above; (2) splenomegaly; (3) two or three lines of hemocytopenia, i.e. hemoglobin (HB) <90 g/L, platelets (PLT) <100 × 10^9/L or neutrophils (N) <1 × 10^9/L; (4) triacylglycerol (TG) ≥3 mmol/L or fibrinogen (Fbg) <1.5 g/L; (5) serum ferritin (SF) ≥500 µg/L; (6) phagocytosis found in bone marrow, spleen, liver or lymph nodes; (7) soluble CD25 (sCD25) ≥2400 U/mL; (8) low or absent natural killer (NK) cell activity. All patients with HLH were screened for cytotoxic functions, including NK cell activity and degranulation function assays, and protein expression corresponding to HLH-deficient genes, such as perforin, granzyme B, SAP, and XIAP. Genetic sequencing was performed to exclude primary HLH in patients with significant abnormalities of the above, as well as in patients with recurrent HLH. EBV infection was identified by biopsy of tissue ICH staining for EBER and/or real-time PCR of patient blood to diagnose. Exclusion criteria: (1) Incomplete clinical data. (2) Primary HLH or other etiology-related HLH such as lymphoma combined with EBV infection.
Clinical data
General information about the patient diagnosed with EBV-HLH (e.g. gender, age), as well as laboratory results at the time of the patient’s initial admission, including HLH-2004 diagnostic indicators, EBV DNA copies in plasma and peripheral blood mononuclear cells (PBMC), inflammatory indicators (C-reactive protein(CRP), procalcitonin (PCT), Erythrocyte sedimentation rate (ESR), etc.), liver function indicators (γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), bilirubin levels, etc.), renal function indicators (creatinine (Cr), blood urea nitrogen (BUN), and the estimated glomerular filtration rate (eGFR) based on the simplified MDRD formula [Citation10], etc.), as well as blood electrolyte levels (potassium, calcium, sodium, etc.). All tests are done within 3 days. The HScore is shown in [Citation11].
Table 1. The Hscore [Citation10].
Follow up
Telephone follow-up was performed based on hospitalization information. Starting point: The day the patient was admitted to the hospital; end point: 30 September 2021, or the date of death or loss to follow-up.
Statistical analysis
With IBM SPSS Statistics 26.0 software, the measurement data with normal distribution were expressed as ±s. Independent samples t-test was used for comparison between two groups; non-normally distributed data were expressed as median (range), and Mann Whitney U test was used for comparison between two groups; count data were expressed as [cases (%)], and χ2 test was used for comparison between groups. Among these indicators, those with an area under the curve (AUC) >0.6 were selected by receiver operating characteristic (ROC) curves, and their optimal cut-off point, i.e. the point with the highest Youden index [Citation12] (sensitivity + specificity-1), were calculated. Single factor Cox proportional risk regression analysis was used to verify indicators associated with prognosis. A composite ROC curve was produced using multiparametric binary logistic regression and Hazard ratio (HR) was obtained using multiple-factor COX regression analysis. Kaplan-Meier curves and Log-Rank tests were used to compare survival curve differences. P < 0.05 was considered a statistically significant difference.
Results
General characteristics
A total of 246 EBV-HLH adult patients were studied in this study, as shown in . When comparing the clinical characteristics of patients in the survival and death groups, it was found that although there were no significant gender differences, the latter were significantly older (p = 0.035); the routine blood test showed that the latter had significantly fewer blood cells, with median leukocyte counts of 2.53 × 10^9/L in the former and 1.93 × 10^9/L in the latter (p = 0.018), median neutrophil counts 1.36 × 10^9/L in the former and 1.08 × 10^9/L in the latter (p = 0.018), and median platelet counts 89 × 10^9/L in the former and 57 × 10^9/L in the latter (p = 0.001). Among inflammatory parameters, the median CRP level was 6.78 mg/L in the survivors and significantly higher in the dead at 16.5 mg/L (p < 0.001); the median PCT level was 0.37 ng/mL in the former and slightly higher at 0.52 ng/mL in the latter (p = 0.01). Comparing renal function, the median level of Cr was 52.2 μmol/L in the survivors and a slightly higher 57.8 μmol/L in those who died (p = 0.003); the median level of BUN was 4.56 mmol/L in the former and 5.8 mmol/L in the latter (p = 0.001); and the mean eGFR was 119.85 mL/min/1.73m2 in the latter, which was lower than 140.20 mL/min/1.73m2 in the former (p = 0.002). For electrolyte levels, the mean potassium value was 3.77 mmol/L in the survivors and 3.92 mmol/L in the decreased (p = 0.029), and the mean blood sodium value was 136.2 mmol/L in the former and 134.5 mmol/L in the latter (p = 0.015). Regarding HLH-related specific indicators such as sCD25 median level was 14,277 pg/mL in the former and significantly higher at 30,200 pg/mL in the latter (p < 0.001). The dead also showed more abnormalities in other indicators, such as lower albumin with a median of 29.2 g/L (p = 0.008), higher serum β2M with a median of 4.79 mg/L (p = 0.002) and higher LDH with a median of 537 U/L (p = 0.036).
Table 2. Clinical characteristics of adult patients with EBV-HLH.
Survival
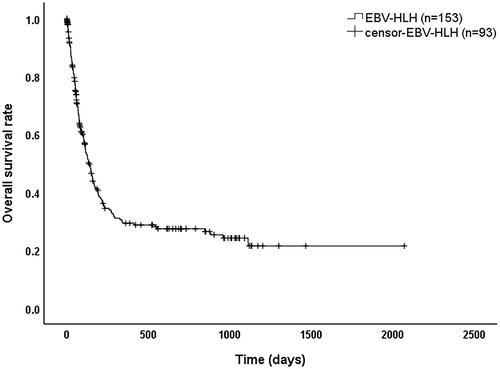
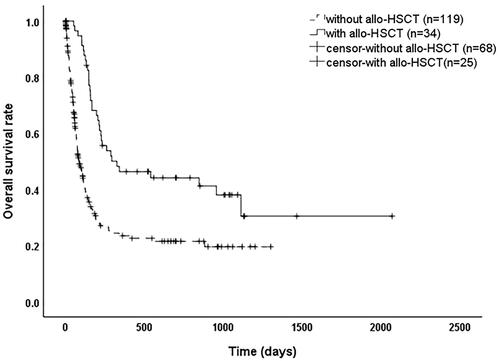
As shown in , 246 EBV-HLH patients with a median survival time of 135 days (95% confidence interval (CI): 107–163 days) and overall survival rates of 41.1%, 29.3%, and 27.4% at 6 months, 1 year, and 2 years, respectively. As shown in , the median survival time was 294 days (95% CI: 0–647 days) for patients who received a transplant and 87 days (95% CI: 63–111 days) for those who did not (p < 0.001).
Improved hemophagocytic lymphohistiocytosis index (IH index)
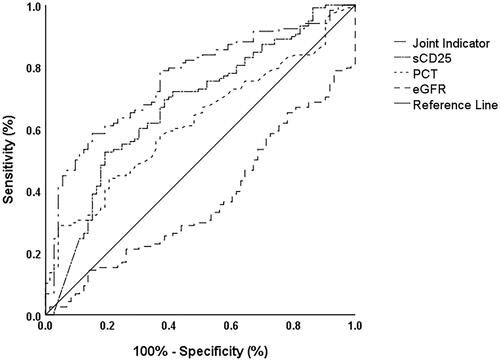
As shown in , for the indicators that were significantly different between the survival and death groups in , we found that PLT, CRP, Cr, BUN, eGFR, CO2, β2M, PCT, and sCD25 had a better ability to predict survival with the AUC greater than 0.6 (0.629, 0.647, 0.612, 0.624, 0.620, 0.626, 0.624, 0.602 and 0.674, respectively) (p < 0.05). The optimal cut-off value of sCD25 was 27,779 pg/mL with poor sensitivity at 0.531, and the latter improved to 0.688 when adjusted to 18,175 pg/mL, while the specificity was acceptable (0.615). Further single factor Cox regression analysis, as shown in , verified that PLT (HR [95%CI] = 0.995 (0.992–0.997), p < 0.001), CRP (HR [95%CI] = 1.007 (1.004–1.010), p < 0.001), Cr (HR [95%CI] = 1.010 (1.006–1.013), p < 0.001), BUN (HR [95%CI] = 1.012 (1.001–1.023), p = 0.033), eGFR (HR [95%CI] = 0.995 (0.992–0.999), p = 0.007), CO2 (HR [95%CI] = 0.901 (0.861–0.943), p < 0.001), β2M (HR [95%CI] = 1.075 (1.032–1.118), p < 0.001), PCT (HR [95%CI] = 1.029 (1.018–1.040), p < 0.001), and sCD25 (HR [95%CI] = 2.484 (1.700–3.629), p < 0.001) were significantly associated with poor prognosis. Then in , sCD25 (OR [95%CI] = 3.335 (1.728–6.435), p < 0.001) and PCT (OR [95%CI] = 6.779 (1.946–23.616), p = 0.003) were used as categorical variables and eGFR (OR [95%CI] = 0.991 (0.985–0.998), p = 0.010) as a continuous variable to establish a joint index by a binary logistic regression for predicting the prognosis with an AUC of 0.762 (CI: 0.694–0.830, p < 0.001), as shown in . Considering that the optimal cut-off value of the ROC curve for eGFR was 133 mL/min/1.73m2, which had less value for clinical application, its cut-off value was artificially adjusted to 90 mL/min/1.73m2 and verified by multi-factor Cox regression analysis that it was still an independent risk factor for poor prognosis (HR [95%CI] = 1.740 (1.172–2.584), p = 0.006) as shown in . At last, we obtained a combined index of sCD25 (HR [95%CI] = 2.362 (1.556–3.587), p < 0.001), PCT (HR [95%CI] = 1.707 (1.118–2.606), p = 0.013), and eGFR to predict prognosis, named the Improved Hemophagocytic Lymphohistiocytosis index (IH index) with a C-index of 0.665 (95%CI: 0.642–0.688).
Table 3. ROC curves of each clinical index.
Table 4. Single factor Cox proportional risk regression analysis of indicators associated with prognosis.
Table 5. binary logistic regression analysis to establish joint indicators to predict prognosis.
Table 6. Multi-factor Cox proportional risk regression analysis of indicators associated with prognosis.
IH index identifies patients with poor prognosis
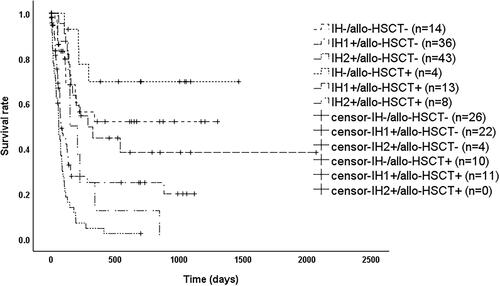
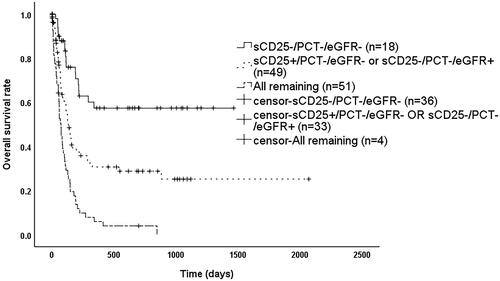
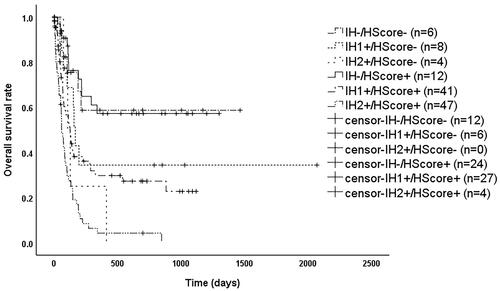
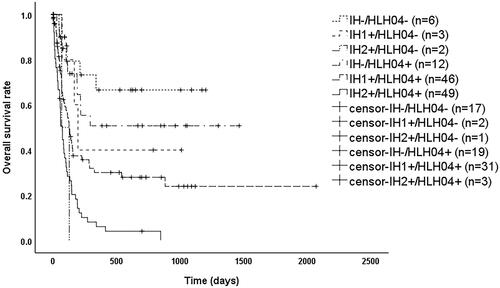
As shown in , patients had significantly better overall survival (OS) when sCD25 ≤ 18,000 pg/mL, PCT ≤1.8 ng/mL, and eGFR ≥90 mL/min/1.73m2 (p < 0.05). OS was significantly worse (p < 0.05) when PCT >1.8 ng/mL, or when any two or more of sCD25 > 18,000 pg/mL, PCT >1.8 ng/mL and eGFR <90 mL/min/1.73m2 were met. In contrast, when only sCD25 > 18,000 pg/mL or only eGFR <90 mL/min/1.73m2, OS was between the above two groups and significantly different (p < 0.05). Therefore, we reclassified patients into three groups based on the above results as shown in , where patients satisfying IH- (i.e. sCD25 ≤ 18,000 pg/mL, PCT ≤1.8 ng/mL, eGFR ≥90 mL/min/1.73m2) had a significantly better prognosis than those meeting IH1+ (i.e. only sCD25 > 18,000 pg/mL or only eGFR <90 mL/min/1.73m2) with a median survival time of 133 days (95% CI: 97–169 days) (p = 0.001) or IH2+ (i.e. the rest) with a median survival time of 74 days (95% CI: 57–91 days) (p < 0.001), while patients meeting IH1+ had a significantly better prognosis than IH2+ (p < 0.001). As shown in , among patients with HScore ≥169, those who met IH- had a significantly better prognosis than those who met IH1+ (p = 0.002) or IH2+ (p < 0.001), while those meeting IH1+ with a median survival time of 127 days (95% CI: 86–168 days), which was better than those who met IH2+ with a median survival time of 61 days (95% CI: 43–79 days) (p < 0.001). As shown in , among patients who met the HLH04 criteria, those meeting IH- had a significantly better prognosis than those meeting IH1+ (p = 0.013) or IH2+ (p < 0.001), while those who met IH1+ had a median survival time of 127 days (95% CI: 99–155 days), which was better than those who met IH2+ with a median survival time of 74 days (95% CI: 55–93 days) (p < 0.001).
Transplantation improves prognosis in high-risk patients met IH index
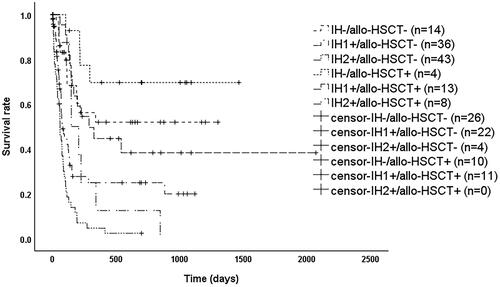
As shown in , among patients meeting IH1+, the median survival time was 78 days (95% CI: 48–108 days) for those without allo-HSCT and 288 days (95% CI: 99–477 days) with allo-HSCT (p = 0.009); among patients meeting IH2+, the median survival time was 60 days (95% CI: 46–74 days) for those without allo-HSCT and 149 days (95% CI: 67–231 days) with allo-HSCT (p = 0.011); while there was no significant difference between patients meeting IH- with allo-HSCT or not (p = 0.191). After stratification according to transplantation, overall survival (OS) remained significantly different between patients eligible for IH- and IH1+ (p < 0.001), patients eligible for IH- and IH2+ (p < 0.001), and patients eligible for IH1+ and IH2+ (p = 0.001).
Discussion
In this study, we combined the index of sCD25, PCT, and estimated glomerular filtration rate (eGFR) to predict prognosis, named the Improved HLH index (IH index), which can early identify adult patients with a poor prognosis of EBV-HLH, initiating timely and appropriate treatment.
Hemophagocytic lymphohistiocytosis is a life-threatening hyperinflammatory syndrome, with one study showing that more than 10% of patients with HLH die within 2 months of diagnosis due to internal organ bleeding, opportunistic infections due to neutropenia, or multi-organ failure [Citation13]. HLH can be classified into various subtypes according to its etiology. Among them, EBV-HLH has a heterogeneous disease course, with clinical manifestations ranging from self-limiting to fatal [Citation6]. In this study, among 246 EBV-HLH patients with a median survival time of 135 days (95% CI: 107–163 days), 153 dead patients had worse clinical presentation compared to survivors, including older age, fewer blood cells, higher levels of inflammatory markers, serum β2M and LDH, lower protein, poorer renal function, and more electrolyte disturbances (p < 0.05), as well as higher levels of HLH-related indicators such as sCD25(p < 0.001). Although there were no significant differences in NK cell activity and degranulation function (p > 0.05), more than half (93 patients, 54.7%) of the patients had reduced CD107a, and the overall median levels of NK cell activity were below the normal range. Mutations leading to abnormal NK cell and CTL cell function are frequently found in primary HLH (pHLH) [Citation14]. Whereas high expression of the NK cell suppressor receptor NKG2A and low expression of the activator receptor NKG2D and less secretion of IFN-γ and CD107a upon CTL stimulation have been observed in patients with sHLH [Citation15], there were no currently confirmed HLH-related genetic alterations in patients with sHLH, and the mechanisms causing such changes at the epigenetic level need further investigation.
EBV, a herpes virus, infects almost all people during their lifetime and persists in the body after the acute phase. It can form latent infections by infecting B cells integrated into genes [Citation16]. EBV DNA quantification and analysis of EBV-specific T-cell recombination levels during EBV reactivation for predicting relapse in asymptomatic individuals with clinical applications [Citation17]. EBV copies in plasma and PBMC of patients with EBV-HLH in this study were significantly higher than the normal range, but they were not significantly different between surviving and deceased patients (p > 0.05), which may indicate that the cause of death in EBV-HLH patients is mainly related to the inflammatory state rather than persistent EBV infection. Eradication of EBV infection may prevent HLH from becoming relapsing and refractory, but stabilizing the highly inflammatory state in patients is the key to saving critically ill HLH patients. Once the inflammatory "storm phase" is over, the symptoms plateau or even disappear in some patients. However, because etoposide-based HLH-94 regimens are not effective in clearing EBV, EBV-HLH often becomes relapsed and refractory, and allo-HSCT is the only option to cure relapsed and refractory patients [Citation18]. In this study, the overall median survival time for patients with EBV-HLH was 135 days, with a 1-year survival rate of only 29.3%. The median survival time was 3 times longer in patients who received allo-HSCT than in those who did not (p < 0.001).
However, how identifying these critically ill patients early to facilitate effective and rapid clinical decision-making is a challenge that remains to be addressed. Previous studies have found that HScore, an important tool used for the early identification of HLH, is associated with the prognosis of critically ill patients with HLH [Citation19]. Ferritin also contributes to the early identification of critically ill patients with HLH [Citation20], and high ferritin levels are often associated with poor prognosis [Citation21]. However, there is little difference in HScore cut-off values between subtypes, which may limit its application in determining the prognosis of different subtypes; ferritin in adult HLH is often greater than 2000 ng/mL in the HLH-04 diagnostic criteria [Citation22], and adult hyperferritinemia is not limited to HLH [Citation23]. As an acute-phase protein, ferritin has predictive validity for short-term prognosis [Citation24], but its prediction of long-term prognosis is unclear. Therefore, this study aimed to establish an independent predictive index of poor prognosis in adult patients with EBV-HLH.
In this study, comparing the AUC and C-index of clinical indicators with significant differences between patients in the survival and death groups, we found that PLT, PCT, CRP, Cr, BUN, eGFR, β2M and sCD25 had a diagnostic sensitivity and specificity. Among them, the critical values of sCD25, PCT and eGFR were taken as 18,000 pg/mL, 1.8 ng/mL and 90 mL/min/1.73m2, respectively, with AUC of 0.762 (p < 0.001). sCD25 is a pro-inflammatory factor, which is currently measured mainly by enzyme-linked immunoassay, and a new protein biochip has been developed for serum IgG antibodies against IL-2, sCD25, and Ag-Ab complexes of sCD25 with similar capabilities to conventional methods [Citation25]. A previous study on malignancy-associated HLH (M-HLH) showed that sCD25 combined with ferritin could better identify patients with poor prognosis including M-HLH and non-HLH patients with high inflammatory status [Citation26]. sCD25 was able to predict prognosis in patients with EBV-HLH in this study, whether it applies to all types of HLH needs to be further investigated. PCT is often used for the early detection of systemic bacterial infections and is usually negative in viral infections [Citation27]. Serum PCT levels are also significantly elevated in severe inflammation and are positively correlated with disease severity and mortality [Citation28]. The production of PCT during inflammation is associated with bacterial endotoxins and inflammatory cytokines (TNF, IL-6) [Citation29]. In this study, on the one hand, due to the intense inflammatory state of HLH patients, a large number of cytokines were released; on the other hand, due to the low number of neutrophils, they were prone to the combination of multiple bacterial infections and even sepsis; therefore, the PCT was significantly elevated, and had some predictive power for patient prognosis. HLH is often combined with multi-organ failure, where acute kidney injury (AKI) is associated with acute tubular necrosis, underperfusion, tumor lysis syndrome, or HLH-related glomerulopathy [Citation30], and abnormally high serum concentrations of nephrotoxic interleukin-6 (IL-6) in late HLH contribute to progression to renal failure [Citation31]. However, cytotoxic chemotherapy can temporally deteriorate organ damage making regimens difficult to implement. Fortunately, ruxolitinib has been shown efficacy in treating HLH without much toxicity in clinical trials [Citation32]. eGFR is commonly used in the diagnosis and staging of chronic kidney disease (CKD) and in determining drug doses [Citation33]. In this study, we found significant differences in age and creatinine levels between the survival and death groups (p < 0.05), and still significant differences in eGFR according to the simple MDRD formula (p = 0.002), which further multi-factor Cox regression analysis confirmed to be an independent risk factor for poor prognosis in patients with EBV-HLH (p = 0.006). Therefore, it is essential to assess the renal function of patients for selecting treatment and predicting prognosis.
A combined index of sCD25, PCT, and estimated glomerular filtration rate (eGFR) was obtained to predict prognosis, named the Improved HLH index (IH index), and patients were divided into three groups meeting IH- (i.e. sCD25 ≤ 18,000 pg/mL, PCT ≤ 1.8 ng/mL, eGFR ≥ 90 mL/min/1.73m2), IH1+ (i.e. only sCD25 > 18,000 pg/mL or only eGFR < 90 mL/min/1.73m2), and IH2+ (i.e. the rest), respectively. In patients with the HScore ≥ 169 or meeting HLH-04, there was a significant difference in prognosis among these three groups (p < 0.05). Therefore, the IH index could further identify patients with poor prognoses who meet the diagnostic criteria of HLH-04 and HScore ≥ 169 in clinical applications.
The only cure for primary HLH is HSCT, which is also required for certain types of sHLH, such as chronic active Epstein-Barr virus (CAEBV) [Citation34] and refractory EBV-HLH [Citation6]. In the group meeting IH + or IH2+, patients who received allo-HSCT had a better OS than those who did not (p < 0.05). However, there was no significant difference between patients meeting IH- with allo-HSCT or not (p = 0.191), while a significant difference still in OS among the three groups of patients who received allo-HSCT (p < 0.001). It is suggested that transplantation improves prognosis in high-risk patients eligible for the IH index and has some value for clinical decision-making.
Conclusion
The IH index has value in clinical practice for early identification of patients with HLH at high risk of poor prognosis, and further validation by more clinical studies is needed in the future.
Ethical approval
Ethical approval for this study was obtained from the Bioethics Committee of Beijing Friendship Hospital, Capital Medical University (2020-P2-096-01). Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW designed the research. SY, LH, RZ, ML, ZH, HZ, ZW and YW analyzed and interpreted the data. SY wrote the manuscript. All authors contributed to the article and approved the submitted version.
Supplemental Material
Download MS Word (21.1 KB)Disclosure statement
The authors have no relevant financial or non-financial interests to disclose.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- Risma KA, Marsh RA. Hemophagocytic lymphohistiocytosis: clinical presentations and diagnosis. J Allergy Clin Immunol Pract. 2019;7(3):824–832.
- Canna SW, Marsh RA. Pediatric hemophagocytic lymphohistiocytosis. Blood. 2020;135(16):1332–1343.
- Yao S, Wang Y, Sun Y, et al. Epidemiological investigation of hemophagocytic lymphohistiocytosis in China. Orphanet J Rare Dis. 2021;16(1):342.
- Ishii E. Hemophagocytic lymphohistiocytosis in children: pathogenesis and treatment. Front Pediatr. 2016;4:47.
- Kim YR, Kim DY. Current status of the diagnosis and treatment of hemophagocytic lymphohistiocytosis in adults. Blood Res. 2021;56(S1): S17–S25.
- Imashuku S, Morimoto A, Ishii E. Virus-triggered secondary hemophagocytic lymphohistiocytosis. Acta Paediatr. 2021;110(10):2729–2736.
- Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269–276.
- El-Mallawany NK, McClain KL. Checkmate for EBV-HLH. Blood. 2020;135(11):782–784.
- Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131.
- Sitter T. Kreatinin und glomeruläre filtrationsrate (GFR): serie laborparameter, folge 6 [serum creatinine and estimated glomerular filtration rate]. MMW Fortschr Med. 2021;163(10):50–51.
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620.
- Fluss R, Faraggi D, Reiser B. Estimation of the youden index and its associated cutoff point. Biom J. 2005;47(4):458–472.
- Morimoto A, Nakazawa Y, Ishii E. Hemophagocytic lymphohistiocytosis: pathogenesis, diagnosis, and management. Pediatr Int. 2016;58(9):817–825.
- Hutchinson M, Tattersall RS, Manson JJ. Haemophagocytic lymphohisticytosis-an underrecognized hyperinflammatory syndrome. Rheumatology. 2019;58(Suppl 6):vi23–vi30.
- Gao Z, Wang Y, Wang J, et al. The inhibitory receptors on NK cells and CTLs are upregulated in adult and adolescent patients with secondary hemophagocytic lymphohistiocytosis. Clin Immunol. 2019;202:18–28.
- Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol. 2019;72(10):651–658.
- Annels NE, Kalpoe JS, Bredius RG, et al. Management of Epstein-Barr virus (EBV) reactivation after allogeneic stem cell transplantation by simultaneous analysis of EBV DNA load and EBV-specific T cell reconstitution. Clin Infect Dis. 2006;42(12):1743–1748.
- La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477.
- Gualdoni GA, Hofmann GA, Wohlfarth P, et al. Prevalence and outcome of secondary hemophagocytic lymphohistiocytosis among SIRS patients: results from a prospective cohort study. JCM. 2019;8(4):541.
- Debaugnies F, Mahadeb B, Nagant C, et al. Biomarkers for early diagnosis of hemophagocytic lymphohistiocytosis in critically ill patients. J Clin Immunol. 2021;41(3):658–665.
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23(3):95–104.
- Sarangi R, Pathak M, Padhi S, et al. Ferritin in hemophagocytic lymphohistiocytosis (HLH): current concepts and controversies. Clin Chim Acta. 2020;510:408–415.
- Otrock ZK, Hock KG, Riley SB, et al. Elevated serum ferritin is not specific for hemophagocytic lymphohistiocytosis. Ann Hematol. 2017;96(10):1667–1672.
- Hua Z, He L, Zhang R, et al. Serum ferritin is a good indicator for predicting the efficacy of adult HLH induction therapy. Ann Med. 2022;54(1):283–292.
- Liu Q, Liu SS, Li SG, et al. Establishment of a protein biochip to detect serum IgG antibodies against IL-2 and soluble CD25 in hemophagocytic lymphohistiocytosis. Clin Chim Acta. 2018;487:256–263.
- Zoref-Lorenz A, Murakami J, Hofstetter L, et al. An improved index for diagnosis and mortality prediction in malignancy-associated hemophagocytic lymphohistiocytosis. Blood. 2022;139(7):1098–1110.
- Cleland DA, Eranki AP. Procalcitonin. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Becker KL, Nylén ES, White JC, Jr., et al. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89(4):1512–1525.
- Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(Suppl 1):S57–S61.
- Aulagnon F, Lapidus N, Canet E, et al. Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am J Kidney Dis. 2015;65(6):851–859.
- Cui Y, Shi J, Lu G, et al. Prognostic death factors in secondary hemophagocytic lymphohistiocytosis children with multiple organ dysfunction syndrome receiving continuous renal replacement therapy: a multicenter prospective nested case-control study. Ther Apher Dial. 2022;26(5):1023–1029.
- Yan WL, Yang SL, Zhao FY, et al. Ruxolitinib is an alternative to etoposide for patient with hemophagocytic lymphohistiocytosis complicated by acute renal injury: a case report. J Oncol Pharm Pract. 2022;28(1):222–227.
- Inker LA, Titan S. Measurement and estimation of GFR for use in clinical practice: core curriculum 2021. Am J Kidney Dis. 2021;78(5):736–749.
- Bergsten E, Horne A, Hed Myrberg I, et al. Stem cell transplantation for children with hemophagocytic lymphohistiocytosis: results from the HLH-2004 study. Blood Adv. 2020;4(15):3754–3766.