Abstract
Background
High endothelial venules (HEV) and tertiary lymphoid structures (TLS) are associated with clinical outcomes of patients with colorectal cancer (CRC). However, because HEV are components of TLS, there have been few studies of the role of the HEV proportion in TLS (HEV/TLS). This study investigated the role of the HEV/TLS and its relationship with the tumor immune microenvironment in CRC.
Methods
A retrospective analysis of 203 cases of tissue pathologically diagnosed as CRC after general surgery was performed at the First Affiliated Hospital of Jinan University from January 2014 to July 2017. Paraffin sections were obtained from the paracancerous intestinal mucosal tissues. The area of HEV and TLS and immune cells were detected by immunohistochemistry. We further divided the positive HEV expression group into the high HEV/TLS group and the low HEV/TLS group by the average area of HEV/TLS. After grouping, the data were also analyzed using the chi-square test, Kaplan-Meier method, and univariate and multivariate Cox proportional risk regression analyses. A correlation analysis of the HEV/TLS and immune cells as well as angiogenesis was performed.
Results
Patients with a high HEV/TLS in CRC tissue were associated with longer OS, DFS and lower TNM stage. Meanwhile, CRC tissue with a high HEV/TLS showed a greater ability to recruit the CD3+ T cells, CD8+ T cells and M1 macrophages and correlated with less angiogenesis. Conclusively, high HEV/TLS links to the favorable prognosis of CRC patients and correlated with anti-tumor immune microenvironment, which can be a potential biomarker for prognosis of CRC patients.
Conclusion
A high HEV/TLS is associated with a favorable prognosis for CRC and is correlated with the anti-tumor immune microenvironment. Therefore, it is a potential biomarker of the CRC prognosis.
High HEV/TLS is associated with a favorable prognosis for CRC.
High HEV/TLS correlated with the anti-tumor immune microenvironment of CRC and can serve as a novel prognostic biomarker.
KEY MESSAGES
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and has the second highest cancer-related mortality worldwide [Citation1]. Several studies have reported that the tumor microenvironment has an important role in tumor immunotherapy; However, the biomarker for evaluating the therapeutic response of immunotherapy in CRC patients remains limited [Citation2]. Microsatellite status, tumor mutation burden [Citation3], POLE/POLD1 mutation [Citation4], tumor-infiltrating lymphocytes [Citation5,Citation6], and programmed death-ligand 1 expression [Citation7] have been reported as biomarkers of CRC for patients who can benefit from immune checkpoint inhibitors [Citation8]. However, the immunotherapy response is heterogeneous and varies among patients. Moreover, it is difficult to accurately predict the effect of immunotherapy on current biomarkers [Citation9]. Therefore, it is necessary to combine multiple indicators or identify novel biomarkers that can more accurately predict the prognosis for and immunotherapy response of CRC patients.
Recently, it has been reported that tertiary lymphoid structures (TLS) can predict the immunotherapy response and improve the effect of immunotherapy and the prognosis for cancer patients [Citation10,Citation11]. TLS are ectopic lymphoid organs that develop with various pathophysiological conditions, including autoimmune and infectious diseases, transplanted organs, inflammatory disorders, and tumors, and create a range of context-dependent effects [Citation12]. TLS comprise a crucial element of the tumor microenvironment, which is mainly formed by T cells, B cells, fibroblastic reticular cell networks, high endothelial venules (HEV), and follicular dendritic cells [Citation12–15]. As a special component of TLS, HEV can express peripheral node addressin and L-selectin ligands [Citation16–18], thereby causing infiltration of immune cells and an anti-tumor effect [Citation19,Citation20]. Accumulating evidence has shown that the presence of HEV is correlated with favorable clinical outcomes of various cancers, including breast cancer [Citation21], pancreatic cancer [Citation22], brain cancer [Citation23], and oral squamous cell carcinoma [Citation24]. However, the opposite effect has been reported for HEV with different cancers. Okayama et al. reported that positive HEV expression was significantly associated with shorter survival times of gastric cancer patients [Citation25]. Previous studies [Citation26,Citation27] have found that HEV are rare and accumulate in the extratumoral area. However, it has been reported that HEV in extratumoral areas results in an unfavorable prognosis for CRC [Citation28]. Furthermore, the HEV is a prognostic biomarker for CRC that is dependent on the presence of tumor-infiltrating lymphocytes [Citation29]. Therefore, the role of HEV in CRC and the relationship between the proportion of HEV in TLS (HEV/TLS) in CRC remain controversial.
During this study, we found that the HEV/TLS is different in different CRC tissues. Therefore, we proposed a novel method involving the use of the HEV/TLS to evaluate the relationship between HEV expression and clinicopathological characteristics of CRC patients. We found that high HEV/TLS can serve as a novel prognostic biomarker and correlated with anti-tumor immune microenvironment in CRC patients.
2. Materials and methods
2.1. Patient cohort and samples
CRC and matched adjacent normal tissues were collected from patients who underwent surgical resection for CRC at the First Affiliated Hospital of Jinan University between January 2014 and July 2017. The inclusion criteria were as follows: admitted to the First Affiliated Hospital of Jinan University for surgical treatment; postoperative pathology confirmed CRC; and complete clinical data and follow-up data were available. The exclusion criteria were as follows: neoadjuvant chemoradiotherapy before surgery; the colorectal tumor was derived from other tumors or the patient had a history of complications with other tumors; and the patient died because of a non-disease-related condition. The adjacent normal tissue samples were 5 cm from the edge of the tumors, and all samples were identified by a pathologist. This study was performed in accordance with the guidelines of the 1975 Declaration of Helsinki and approved by the Medical Ethics Committee of the First Affiliated Hospital of Jinan University.
The baseline characteristics are summarized in . Patients were followed-up at outpatient clinics every 3 months for the first 2 years after surgery. They were also followed-up every 6 months until death or January 2020. When patients refused to come to the hospital for follow-up, a telephone follow-up survey was performed. At each follow-up appointment, patients underwent a physical examination, laboratory investigation, and contrast-enhanced computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis. Overall survival (OS) was calculated from the date of colorectal resection to death or, for those alive, to the date of the last follow-up examination. Disease-free survival (DFS) was defined as the time between the onset of disease and relapse or death caused by disease progression. The median follow-up period was 50.0 months (0–72 months). Tumors were restaged and regraded based on the 8th edition of the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system and the 2017 World Health Organization classification.
Table 1. Baseline characteristics of 203 CRC patients.
2.2. Immunohistochemistry
The following methods and experimental steps to determine the mismatch repair (MMR) status were provided by the Department of Pathology of our hospital. Immunohistochemistry (IHC) staining for MutL protein homolog 1 (MLH1), postmeiotic segregation increased 2 (PMS2), MutS protein homolog 2 (MSH2), and MutS protein homolog 6 (MSH6) was performed for all CRC cases. Antigen retrieval was performed using Tris/EDTA buffer and microwave treatment for 30 min. Sections were incubated overnight with primary antibodies against MLH1 (1:25; M3640; DAKO), MSH2 (IR085, ready-to-use; DAKO), and MSH6 (IR086, ready-to-use; DAKO) at room temperature and PMS2 (1:50; M3647; DAKO) at 4 °C. Sections were incubated at room temperature with Autostainer Link (DAKO) for 30 min, followed by incubation with the corresponding secondary antibody. Detection was performed using the EnVision Detection System with peroxidase (rabbit)/DAB (mouse) (K5007; DAKO). Furthermore, 3,3′-diaminobenzidine was used as the chromogen. The slides were counterstained with hematoxylin, dehydrated, and mounted. Slides were evaluated by two observers blinded to the patient and tumor characteristics. Discrepancies were discussed and reviewed using a multihead microscope until a consensus was reached. Intact IHC staining of MMR proteins was defined as positive staining for all MLH1, MSH2, MSH6, and PMS2 proteins, whereas deficient IHC staining was defined as negative staining of any of these four proteins [Citation30].
Formalin-fixed, paraffin-embedded specimens were obtained from 203 patients. To further assess the tumor immune microenvironment of colorectal tumors, IHC staining was performed using serial sections from the patients. First, 4-μm-thick sections were cut with a microtome; then, the slides were deparaffinized in xylene and rehydrated in ethanol. Antigen retrieval was performed in Tris/EDTA buffer using microwave treatment for 23 min. Endogenous peroxidase was blocked using 3% hydrogen peroxidase for 25 min and washed with phosphate-buffered saline (1:2000; G0002-2L; Servicebio, Wuhan, China). Protein blocking was performed using QuickBlock™ blocking buffer for Immunol staining (P0260; Beyotime Biotechnology, Nanjing, China) for 30 min. The primary antibody was diluted in QuickBlock™ primary antibody dilution buffer for Immunol staining (P0262; Beyotime Biotechnology) and incubated overnight at 4 °C, followed by washing in phosphate-buffered saline and incubation with the secondary antibody (1:400; P0267; Beyotime Biotechnology) for 50 min. Immpress anti-rabbit, anti-rat, or anti-mouse was used depending on the species of the primary antibody. DAB was used to visualize antibody complexes. At least three fields (20×) per area were evaluated for each marker. Quantification of tumor-infiltrating immune cells and vessels was performed by the same pathologist. Slides were stained for MECA-79+ HEV, CD3+ T cells, CD8+ T cells, HLA-DR (M1 macrophages), and CD31+ vessels. The details of the primary antibodies used for IHC and staining conditions are provided in Supplementary Table 1.
2.3. Evaluation of HEV and TLS in CRC
The MECA-79 monoclonal antibody specifically reports HEV-expressed ligands for L-selectin by recognizing a critical sulfation-dependent determinant of these ligands [Citation31]. No uniform standard was available for the histopathological TLS scoring system, and TLS was defined as the cumulative area of ectopic lymphocytes found by IHC according to the criteria of previous studies [Citation32–34]. During our study, we defined a new evaluation criterion: the HEV/TLS. We used ImageJ software to calculate the area of TLS in the red dotted line and calculate the HEV in the TLS in the same way. The HEV/TLS is the area of the HEV in the TLS divided by the area of the TLS.
2.4. Evaluation of tumor-associated lymphocytes and angiogenesis in CRC
Similarly, we evaluated the proportion of tumor-associated lymphocytes by counting the number of CD3+ cells, CD8+ T cells, and HLA-DR+ (M1 macrophages) in TLS. The CD31+ vessel can represent angiogenesis, whereas the CD31 biomarker can nonspecifically label HEV [Citation35,Citation36]. Therefore, the area of angiogenesis is the area of CD31+-labeled blood vessels minus the area of MECA-79+-labeled HEV in the TLS.
2.5. Statistical analysis
All statistical analyses were performed using SPSS software (version 23.0; SPSS Inc., Chicago, IL), GraphPad Prism software (version 8.0; GraphPad, La Jolla, CA), and ImageJ Launcher software (version 1.4.3.67). The optimal threshold of the proportion of HEV expression in TLS was identified by applying the mean from the average expression area of all HEV expressions in the TLS. The Mann-Whitney U test was used to investigate continuous variables with unequal variance. The Spearman correlation or Pearson χ2 test was used to examine correlations. Survival curves were plotted using the Kaplan-Meier method and tested using a log-rank test. Univariate and multivariate Cox proportional risk regressions were performed to evaluate the prognostic factors for OS and DFS. Those found to be significant by the univariate analysis were subjected to multivariate COX proportional risk regressions analyze. A two-tailed p < 0.05 was considered statistically significant.
3. Result
3.1. HEV expression was not associated with clinical outcomes of CRC patients
IHC revealed that HEV was mainly expressed in CRC tissue, but that it was less expressed in the adjacent normal tissue (). Based on the different HEV expressions, we divided the 203 patients into the HEV-negative and HEV-positive expression groups. The clinicopathological characteristics of the two groups are summarized in . The presence of HEV was not associated with the clinicopathological characteristics of CRC patients, such as age (p = 0.143), sex (p = 0.089), location (p = 0.578), T stage (p = 0.626), N stage (p = 0.735), M stage (p = 0.633), TNM stage (p = 0.969), and MMR status (p = 0.955) (). We also examined the association between HEV and the prognosis for patients with CRC. However, our results showed that patients with positive HEV expression did not exhibit longer OS and DFS than those with negative HEV expression ().
Figure 1. Characterization of CRC-associated HEV and the patient survival outcomes. (A) Representative images of immunohistochemistry staining CRC tissues and adjacent tissue showing the expression of HEV (black arrowheads). The solid black line indicates the scale bar, 100 mm. (B–C) Kaplan-Meier curves were used for comparison of OS and RFS among patients with negative or positive HEV expression. Significance was tested by log-rank test. (D–G) Kaplan-Meier curves for OS and RFS in different stage CRC patients with negative or positive HEV expression. Significance was tested by log-rank test. OS: overall survival; DFS: disease-free survival; HEV: high endothelial venules.
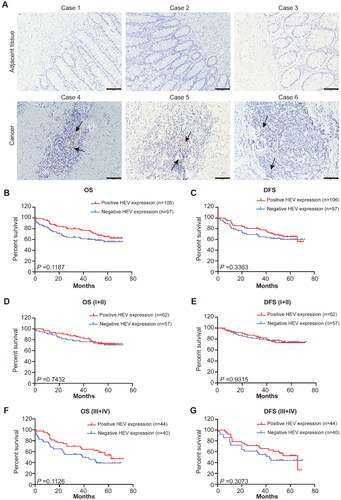
Table 2. Correlation between HEV expression and clinicopathological characteristics of CRC (n = 203).
We systematically analyzed the influence of HEV expression at different clinical stages. By calculating the OS and DFS associated with stage I–II and stage III–IV, we also found that HEV expression had no relationship with OS and DFS (p > 0.05) (, Supplementary Figure S1A and S1B). To investigate the prognostic significance of HEV expression, we conducted univariate and multivariate COX proportional risk regressions analysis ( and ). According to the univariate survival analysis, age, gender, location, MMR status, and HEV expression were not independent factors for OS (p = 0.357, p = 0.087, p = 0.527, p = 0.229, and p = 0.261, respectively) and DFS (p = 0.362, p = 0.102, p = 0.571, p = 0.212, and p = 0.244, respectively). However, the TNM stage was an independent factor for OS (hazard ratio [HR], 2.280; 95% confidence interval [CI]: 1.447–3.593; p = 0.000) and DFS (HR, 2.299; 95% CI, 1.459–3.624; p = 0.000). According to the multivariate survival analysis involving the same parameters, positive HEV expression was not associated with OS (HR, 0.749; 95% CI, 0.477–1.175; p = 0.321) or DFS (HR, 0.745; 95% CI, 0.475–1.169; p = 0.296).
Table 3. Analysis for OS using the univariate and multivariate Cox proportional hazards regression model in the patient with CRC (n = 203).
Table 4. Analysis for DFS using the univariate and multivariate Cox proportional hazards regression model in the patient with CRC (n = 203).
3.2. Correlation between the HEV/TLS and prognosis for CRC
Interestingly, we observed different area proportions of HEV in the TLS in CRC tissues (). Because TLS is a novel predictive biomarker for various cancers, and because HEV is a critical element of TLS, we were interested in whether the HEV/TLS could be a predictive biomarker for CRC. We calculated the area of HEV expression in the TLS and created two groups (low HEV/TLS and high HEV/TLS) based on the results and the mean proportion of the area (4.168%) of CRC patients with positive HEV expression (). Because the status of having no HEV expression has an important role in the analysis of OS, we systematically analyzed the OS and DFS of three groups (negative HEV expression, low HEV/TLS, and high HEV/TLS groups) (). The results showed obvious differences among these groups (OS: p = 0.047; DFS: p = 0.006). According to the subgroup analysis, the OS of the group with a high HEV/TLS was longer than that of the group without HEV expression (p = 0.001) and that of the group with a low HEV/TLS (p = 0.004) (); this outcome was similar to that for DFS () (high HEV/TLS vs. low HEV/TLS: p = 0.001; high HEV/TLS vs. negative HEV expression: p = 0.019). Moreover, the OS and DFS of the groups (low HEV/TLS vs. negative HEV expression) were not statistically significant ().
Figure 2. Characterization of different HEV/TLS and the patient survival outcomes. (A) Representative images of immunohistochemistry staining CRC tissues showing the different HEV/TLS (black arrowheads represent the HEV, black dotted lines are TLS, the top row is low HEV/TLS and the bottom row is high HEV/TLS). The solid black line indicates the scale bar, 100 mm. (B–C) Kaplan-Meier curves were used for comparison of OS and RFS in the CRC patient among three groups (High HEV/TLS vs. Low HEV/TLS vs. negative HEV expression). Significance was tested by log-rank test. (D–G) Kaplan-Meier curves for OS and RFS in different stage CRC patients with high HEV/TLS or low HEV/TLS. Significance was tested by log-rank test. (H–I) Kaplan-Meier curves for OS and RFS in CRC patients in the different group (High HEV/TLS vs. None or Low HEV/TLS). OS: overall survival; DFS: disease-free survival; HEV: high endothelial venules.
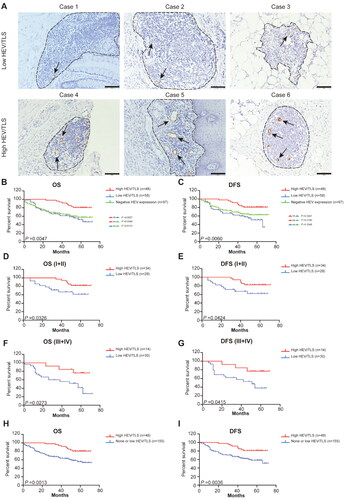
We evaluated the association of the HEV/TLS with OS and DFS at different stages of CRC. Patients with a high HEV/TLS had longer OS and DFS with stage II (Supplementary Figure 1(C,D)), stage I + II (), and stage III + IV () than patients with a low HEV/TLS. However, with other stages, the OS and DFS had no statistical significance (Supplementary Figure 1(C,D)).
To comprehensively understand the association of the HEV/TLS with OS and DFS, we combined patients with a low HEV/TLS and patients with negative HEV expression into one group (none or low HEV/TLS) for analysis. Patients with a high HEV/TLS exhibited significantly longer OS and DFS than patients with negative HEV expression or a low HEV/TLS (OS: p = 0.001; DFS: p = 0.004) ().
3.3. Correlation between the HEV/TLS and clinicopathological characteristics of CRC
We analyzed the correlation between the HEV/TLS and clinicopathological characteristics under two different conditions (none or low HEV/TLS vs. high HEV/TLS). As shown in , a high HEV/TLS was associated with a lower TNM stage (p = 0.049), but not with age, sex, location, and MMR status (p > 0.05).
Table 5. Correlation of HEV/TLS with clinicopathological characteristics (n = 203).
Univariate and multivariate survival COX proportional risk regressions analysis indicated that a high HEV/TLS was an independent prognostic factor for favorable OS (univariate: HR, 0.376, 95% CI, 0.193–0.732, p = 0.004; multivariate: HR, 0.413, 95% CI, 0.211–0.808, p = 0.010) and DFS (univariate: HR, 0.374, 95% CI, 0.192–0.729, p = 0.004; multivariate: HR, 0.412, 95% CI, 0.211–0.805, p = 0.009) ( and ).
Table 6. Analysis for OS using the univariate and multivariate Cox proportional hazards regression model in the patient with the HEV expression (n = 203).
Table 7. Analysis for DFS using the univariate and multivariate Cox proportional hazards regression model in the patient with colorectal cancer (n = 203).
3.4. High HEV/TLS correlated with anti-tumor immune microenvironment
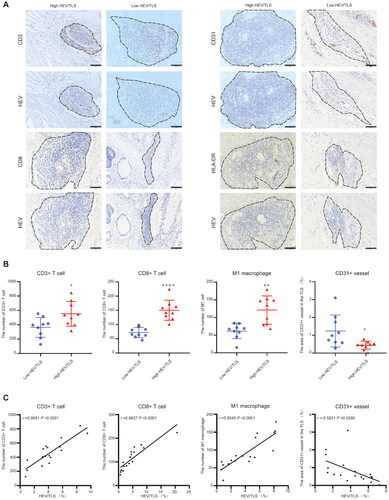
We examined the correlation between the HEV/TLS, tumor-infiltrating immune cells, and angiogenesis in CRC. CRC tissue with a high HEV/TLS could recruit more immune cells, such as CD3+ T cells, CD8+ T cells, and HLA-DR + M1 macrophages, than CRC tissue with low HEV/TLS, which had fewer areas of CD31+ vessels (). According to the correlational analysis, the HEV/TLS was positively correlated with the numbers of CD3+ T cells (r = 0.869; p = 0.000), CD8+ T cells (r = 0.884; p = 0.000), and HLA-DR + M1 macrophages (r = 0.855; p = 0.000), and negatively correlated with the area of CD31+ vessels in the TLS (r = −0.520; p = 0.027) ().
Figure 3. Relationship between different HEV/TLS and the immune cell infiltration in CRC tissue. (A) Representative images of immunohistochemistry staining CRC tissues showing the different HEV/TLS (black dotted lines represent TLS, the top row is low HEV/TLS and the bottom row is high HEV/TLS). The solid black line indicates the scale bar, 100 mm. (B) The number of CD3+, CD8+ T cell and HLA-DR + M1 macrophage and area of CD 31+ vessel was compared between CRC patients with high HEV/TLS or low HEV/TLS (each group, n = 9; *, p < 0.05; **, p < 0.001, ****, p < 0.0001). (C) Correlation analysis between HEV/TLS and the number of CD3+, CD8+ T cell and HLA-DR + M1 macrophage and area of CD 31+ vessel (each group, n = 18).
4. Discussion
Although HEV have been expressed and investigated in various tumors, little is known about their role in CRC. However, the presence of HEV has served as a predictor of a positive prognosis for CRC [Citation21,Citation37,Citation38]. Other studies have indicated that the presence of HEV was not associated with the prognosis of patients with Dukes’ C tumors and did not indicate a favorable prognosis [Citation28]. Therefore, the role of HEV in CRC remains controversial. Our study is the first to construct a novel method involving the use of the HEV/TLS to clarify the role of HEV in TLS and CRC. We also discovered that the HEV/TLS could serve as a prognostic biomarker for CRC. A high HEV/TLS was associated with a lower TNM stage and longer OS and DFS for patients with CRC. Additionally, we demonstrated that a high HEV/TLS in the tumor was correlated with the anti-tumor immune microenvironment, as indicated by increased CD3+ T cells, CD8+ T cells, and M1 macrophage infiltration and attenuated angiogenesis near HEV.
Our study proposed a novel method of evaluating the role of HEV in CRC and the relationship between HEV and TLS. Our results showed that the HEV/TLS can more accurately predict the prognosis for CRC than HEV expression. As a special venule in tumors, HEV can transport immune cells to the tumor tissue and promote the formation of TLS. However, TLS contain many types of lymphocytes, such as CD8+ T cells, CD3+ T cells, and M1 macrophages [Citation13]. The TLS components have different effects on tumors. For example, CD8+ T cells and B cells have an anti-tumor role, whereas Treg cells act as immunosuppressors that decrease the effectiveness of therapy [Citation39,Citation40]. Some studies have reported that TLS are a favorable prognostic biomarker for different cancers, including gastric cancer [Citation41], pancreatic cancer [Citation16], breast cancer [Citation42], liver cancer [Citation43], melanoma [Citation10], and lung cancer [Citation26]. However, peritumoral TLS expression indicates unfavorable clinicopathological characteristics and a worse prognosis for hepatocellular carcinoma [Citation44]. HEV, as a key component of TLS, also have a dual role in various tumors. Some studies reported that HEV serve as independent biomarkers of favorable prognoses for some tumors, including breast cancer [Citation21], melanoma [Citation45] and oral squamous cell carcinoma [Citation24]. However, it has been reported that HEV have the opposite role in gastric cancer [Citation25]. Hence, the prognosis cannot be accurately judged using HEV or TLS alone. Our study showed that a high HEV/TLS can serve as an independent factor for predicting favorable OS and DFS for CRC patients. Our results indicate that the HEV/TLS is more reliable because it involves a ratio of both HEV and TLS, which may eliminate errors in judgment caused by using HEV or TLS alone to evaluate CRC. Using the same standards, the same results can be observed for patients with stage II CRC, which further confirmed our hypothesis that a high HEV/TLS is associated with longer OS and DFS. These results also indicate that a high HEV/TLS is a biomarker of a favorable prognosis for CRC patients.
In the subgroup analysis, our results showed that the HEV/TLS was related to the TNM stage. Previous studies found that the function and morphology of the sentinel lymph node HEV change in the metastatic axillary lymph nodes of patients with breast cancer. The lumen of HEV increases and the wall becomes thinner, thus leading to defective immune cell trafficking, which causes lymph node metastasis [Citation46]. Therefore, the association between a high HEV/TLS and metastasis could explain the predictive value of the HEV/TLS during clinical staging of CRC. This is why the number of patients with stage III-IV disease was lower than the number of patients with stage I-II disease. Although HEV is closely associated with tumor metastasis and clinical stage, further evidence is needed and the potential mechanisms of the TNM stage and HEV/TLS must be investigated. Based on the relationship between HEV and metastasis,previous studies found that a fusion compound of the cytokine LIGHT and a vascular targeting peptide (LIGHT-VTP) can induce HEV to prevent tumor metastasis [Citation47]. This may provide a new treatment pathway for terminal cancer patients in the future.
Our study also revealed that the high HEV/TLS group can recruit more CD3+ T cells, CD8+ T cells, and M1 macrophages than the low HEV/TLS group. These results indicate that a high HEV/TLS is correlated with the ability to recruit immune cells. As reported by previous studies, HEV can recruit T cells with chemokine ligand 21 [Citation48] and B cells with chemokine ligand 19 [Citation20]. Additionally, HEV recruit B cells in an IL-36γ-dependent autocrine manner into the tumor [Citation49]. Tumor-infiltrating lymphocytes have been found to have a favorable impact on the prognosis for CRC [Citation50]. Furthermore, the quantity of CD8+ T cells was found to be associated with favorable clinical outcomes of early CRC [Citation38]. In fact, the more HEV present in the tumor, the more specific pathways there are to help immune cells, especially for the anti-tumor lymphocytes entering the tumor. Our hypothesis was preliminarily confirmed by the results of the correlation analysis of the HEV/TLS and immune cell infiltration performed during our study. Chemokines from the HEV and the lumen of the transport passage formation by HEV in TLS are potential mechanisms for high HEV/TLS recruitment of more anti-tumor lymphocytes. Because of the increase in anti-tumor immune cells, the anti-tumor effect can be enhanced, which can benefit the prognosis for CRC.
We observed less neovascularization near HEV in the high HEV/TLS group. These results indicate a correlation between angiogenesis and HEV. Recent studies have shown that HEV can promote the infiltration of CD8+ T cells, thus restricting endothelial cell proliferation and angiogenesis [Citation51–53]. This may be the reason for the attenuated angiogenesis near HEV. Moreover, combined anti-angiogenic and anti-programmed death-ligand 1 therapy stimulates HEV formation, which phenomenon made the researchers hypothesize that normalization of tumor vessels may be the first step in promoting HEV formation [Citation54]. Finally, HEV recruit immune cells, especially M1 macrophages, which can also inhibit tumor angiogenesis [Citation55]. Therefore, further studies are required to investigate their relationships with cancers.
For limitations, our work only used single-center samples. Studies of pathological tissues and further mechanisms and functions should be performed in vivo and in vitro. Additional studies involving different clinical cohorts are necessary to confirm the clinical significance of the HEV/TLS.
In conclusion, we used the HEV/TLS to predict the prognosis for CRC. We found that a high HEV/TLS can serve as a novel prognostic biomarker for favorable outcomes and is correlated with the anti-tumor immune microenvironment of CRC.
Ethical approval
The studies involving human participants were reviewed and approved by the Institutional Review Board of the First Affiliated Hospital of Jinan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZZ and SL conceived and carried out the experiments; ZZ, SL and ZL wrote the manuscript; ZZ, SL and YZ made the revision; XZ and HD made the data collection; ZZ and ZL made the data analysis; ZZ, ZL and YZ perform the figure making; YP and JP designed study and guide the experiments.
Supplemental Material
Download MS Word (245.4 KB)Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Zhong W, Yu Z, Zhan J, et al. Association of serum levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and disease characteristics in colorectal cancer. Pathol Oncol Res. 2015;21(1):83–95.
- Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30(7):1096–1103.
- Wang F, Zhao Q, Wang YN, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019;5(10):1504–1506.
- Kroemer M, Turco C, Spehner L, et al. Investigation of the prognostic value of CD4 T cell subsets expanded from tumor-infiltrating lymphocytes of colorectal cancer liver metastases. J Immunother Cancer. 2020;8(2):e001478.
- Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn’s-Like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108(8):djw027.
- Li Y, Liang L, Dai W, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55.
- Guren MG. The global challenge of colorectal cancer. Lancet Gastroenterol Hepatol. 2019;4(12):894–895.
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337.
- Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565.
- Bruno TC. New predictors for immunotherapy responses sharpen our view of the tumour microenvironment. Nature. 2020;577(7791):474–476.
- Sautes-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325.
- Colbeck EJ, Ager A, Gallimore A, et al. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol. 2017;8(1830):1830.
- Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560.
- Goc J, Fridman WH, Sautes-Fridman C, et al. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology. 2013;2(12):e26836.
- Hiraoka N, Ino Y, Yamazaki-Itoh R, et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112(11):1782–1790.
- Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773.
- Ivetic A, Hoskins Green HL, Hart SJ. L-selectin: a major regulator of leukocyte adhesion, migration and signaling. Front Immunol. 2019;10:1068.
- Hong SA, Hwang HW, Kim MK, et al. High endothelial venule with concomitant high CD8+ tumor-infiltrating lymphocytes is associated with a favorable prognosis in resected gastric cancer. JCM. 2020;9(8):2628.
- Zeng J, Eljalby M, Aryal RP, et al. Cosmc controls B cell homing. Nat Commun. 2020;11(1):3990.
- Martinet L, Garrido I, Filleron T, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71(17):5678–5687.
- Bahmani B, Uehara M, Ordikhani F, et al. Ectopic high endothelial venules in pancreatic ductal adenocarcinoma: a unique site for targeted delivery. EBioMedicine. 2018;38:79–88.
- He B, Jabouille A, Steri V, et al. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J Pathol. 2018;245(2):209–221.
- Wirsing AM, Ervik IK, Seppola M, et al. Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma. Mod Pathol. 2018;31(6):910–922.
- Okayama H, Kumamoto K, Saitou K, et al. Ectopic expression of MECA-79 as a novel prognostic indicator in gastric cancer. Cancer Sci. 2011;102(5):1088–1094.
- Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417.
- Dieu-Nosjean MC, Goc J, Giraldo NA, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35(11):571–580.
- Bento DC, Jones E, Junaid S, et al. High endothelial venules are rare in colorectal cancers but accumulate in extra-tumoral areas with disease progression. Oncoimmunology. 2015;4(3):e974374.
- Di Caro G, Bergomas F, Grizzi F, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20(8):2147–2158.
- Chao X, Li L, Wu M, et al. Comparison of screening strategies for lynch syndrome in patients with newly diagnosed endometrial cancer: a prospective cohort study in China. Cancer Commun (Lond). 2019;39(1):42.
- Bistrup A, Tsay D, Shenoy P, et al. Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope. Am J Pathol. 2004;164(5):1635–1644.
- He W, Zhang D, Liu H, et al. The high level of tertiary lymphoid structure is correlated with superior survival in patients with advanced gastric cancer. Front Oncol. 2020;10:980.
- Klintrup K, Mäkinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41(17):2645–2654.
- Väyrynen JP, Sajanti SA, Klintrup K, et al. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. 2014;134(9):2126–2135.
- Choe K, Hwang Y, Seo H, et al. In vivo high spatiotemporal resolution visualization of circulating T lymphocytes in high endothelial venules of lymph nodes. J Biomed Opt. 2013;18(3):036005.
- Kennel-De March A, Béné MC, Hurault de Ligny B, et al. Enhanced expression of CD31 and CD54 on tonsillar high endothelial venules in IgA nephropathy. Clin Immunol Immunopathol. 1997;84(2):158–165.
- Milutinovic S, Abe J, Godkin A, et al. The dual role of high endothelial venules in cancer progression versus immunity. Trends Cancer. 2021;7(3):214–225.
- Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951.
- Joshi Nikhil S, Akama-Garren Elliot H, Lu Y, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43(3):579–590.
- Sofopoulos M, Fortis SP, Vaxevanis CK, et al. The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. Cancer Immunol Immunother. 2019;68(11):1733–1745.
- Sakimura C, Tanaka H, Okuno T, et al. B cells in tertiary lymphoid structures are associated with favorable prognosis in gastric cancer. J Surg Res. 2017;215:74–82.
- Liu X, Tsang JYS, Hlaing T, et al. Distinct tertiary lymphoid structure associations and their prognostic relevance in HER2 positive and negative breast cancers. Oncologist. 2017;22(11):1316–1324. Epub 07/12.
- Calderaro J, Petitprez F, Becht E, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70(1):58–65.
- Finkin S, Yuan D, Stein I, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16(12):1235–1244. https://www.nature.com/articles/ni.3290#supplementary-information.
- Martinet L, Le Guellec S, Filleron T, et al. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012;1(6):829–839.
- Qian CN, Berghuis B, Tsarfaty G, et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66(21):10365–10376.
- He B, Johansson-Percival A, Backhouse J, et al. Remodeling of metastatic vasculature reduces lung colonization and sensitizes overt metastases to immunotherapy. Cell Rep. 2020;30(3):714–724.e5.
- Baekkevold ES, Yamanaka T, Palframan RT, et al. The Ccr7 ligand ELC (Ccl19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193(9):1105–1112.
- Weinstein AM, Giraldo NA, Petitprez F, et al. Association of IL-36γ with tertiary lymphoid structures and inflammatory immune infiltrates in human colorectal cancer. Cancer Immunol Immunother. 2019;68(1):109–120.
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964.
- Freeman MR, Schneck FX, Gagnon ML, et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55(18):4140–4145.
- Menzel L, Höpken UE, Rehm A. Angiogenesis in lymph nodes is a critical regulator of immune response and lymphoma growth. Front Immunol. 2020;11:591741.
- Qin Z, Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12(6):677–686.
- Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9(385):eaak9679.
- Zhu C, Kros JM, Cheng C, et al. The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies. Neuro Oncol. 2017;19(11):1435–1446.
