Abstract
Children’s Vitamin D (VitD) fortification and supplementation are diminishing due to less outdoor exercise and insufficient VitD intake (low exogenous intake and endogenous malabsorption induced by gastrointestinal disease). Consequently, children in many developed countries suffer from VitD deficiency, which may contribute to many paediatric disorders. Our review briefly introduced the metabolic process of VitD, summarized the role of VitD in paediatric diseases such as autism, obesity, rickets and asthma. We sought to identify the link between VitD deficiency and these diseases.
The metabolic process of VitD
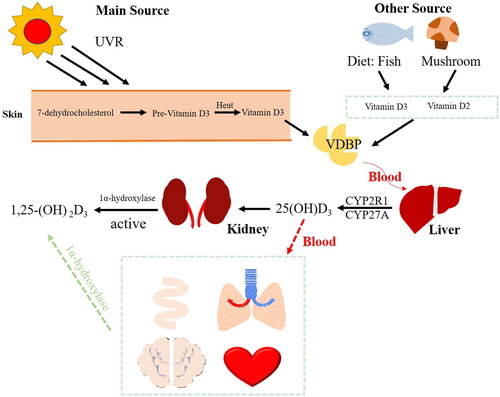
Vitamin D (VitD) is a sterol derivative. In humans, it is mainly obtained from exposure to ultraviolet rays (UVR) or food sources (fishes or mushrooms) [Citation1]. 7-dehydrocholesterol is converted into pro-VitD3 by exposure to UVR. Next, the pro-VitD3 is thermally restructured into VitD3. After binding to the VitD binding protein (VDBP), it is transported to the liver [Citation2]. 25-hydroxyVitD3 (25(OH)D3) is hydroxylated by cytochrome P-450 enzymes (CYP2R1 and CYP27A) in the liver, their gene mutations may result in VitD deficiency [Citation3]. The concentration of 25(OH)D3 is a marker of VitD status in vivo, the bloodstream transports it into other organs, such as the brain, intestines, kidneys, lungs and heart [Citation4]. It is mainly the kidneys that activate VitD in the second step. In the kidneys, 25(OH)D3 is converted to 1,25-dihydroxy VitD3 (calcitriol, 1,25-(OH)2D3) by the 1α-hydroxylase [Citation5]. Since other tissues also contain 1α-hydroxylase, 1,25-(OH)2D3 could also be synthesized in them () [Citation6]. In the human brain, 1,25-(OH)2D3 acts upon the prefrontal cortex, hippocampus, cingulate gyrus, thalamus, hypothalamus, and substantia nigra [Citation7]. The role of 1,25-(OH)2D3 in neuroprotection is attributed to its abilities to inhibit oxidative stress and inflammation and regulate many neurotrophic factors [Citation8].
Figure 1. VitD metabolism. The synthesis of VitD3 in the skin is triggered by ultraviolet radiation (UVR) or by food consumption. By combining with VitD-binding protein (VDBP), VitD3 is transported into the liver. Cytochrome P-450 enzymes (CYP2R1 and CYP27A) are responsible for converting VitD3 to 25-hydroxyVitD (25(OH)D3) in the liver. The 25(OH)D3 is converted into 1,25-dihydroxyVitD3 (1,25-(OH)2D3) in the kidneys, liver, lungs, brain, intestines and heart by 1α-hydroxylase.
In the VitD family, VitD3 (cholecalciferol) and VitD2 (ergocalciferol) are essential for human health. VitD3 is the initial product of VitD metabolism in liver cells. In a double-blind experiment, adults with insufficient VitD took oral VitD3 stool samples for eight weeks. The results showed that serum 25(OH)D increased, beneficial bacteria in the intestine increased, and pathogenic bacteria decreased [Citation9]. Recent studies have shown that VitD3 levels of 40-60 ng/ml may reduce the risk of various types of cancer [Citation10]. Furthermore, researchers found that VitD3 inhibited inflammation and oxidative stress in cognitive impairment rats, alleviated the symptoms of memory dysfunction, and improved learning abilities [Citation11]. In natural world, VitD2 is a steroid derived chiefly from ergosterol, which is commonly present in fungi and certain plants [Citation12]. In recent years, people gradually paid more attention to the researches on VitD2. Studies have shown that oral administration of VitD2 in rats had anti-anxiety, anti-depressant and memory-enhancing effects after noise stimulation [Citation13]. According to Balachandar, VitD3 had higher efficacy in improving total 25(OH)D and reducing serum parathyroid level, comparing to VitD2 [Citation14].
Correlation of VitD deficiency with paediatric diseases
VitD is measured by the level of 25(OH)D in serum in the body. If the 25(OH)D concentrations are below 75 nmol/L (or 30 ng/ml), it is considered VitD deficiency. While concentrations are below 25 or 30 nmol/L (or 10/12 ng/ml), it is considered severe VitD deficiency [Citation15]. While standards vary from different countries, the Institute of Medicine (United States) and the Endocrine Society VitD Working Group offer 50 nmol/L as a critical threshold [Citation16].
The distribution of VitD levels in children aged 0–4 years in Yunnan Province, China, showed that children’s VitD levels were highest in summer and lowest in winter, and VitD deficiency was more common in girls than boys [Citation17]. Over the last two decades, global VitD deficiency increased. It is estimated that VitD deficiency ranged from 6.9% to 81.8% in European countries and from 2.0% to 87.5% in Asian countries [Citation18]. The majority of these surveys were conducted in cities. In mountainous areas of the northern Persian Gulf, VitD deficiency was prevalent at 78% [Citation19]. Important underlying factors associated with VitD deficiency in this population included dark skin, domestic and sedentary periods, insufficient sun exposure, air pollution, clothing style, obesity, sunscreen using, and lack of VitD supplementation [Citation20]. In addition, taking antiepileptic drugs (carbamazepine) and anticonvulsant drugs (levetiracetam) would lead to decreasing of VitD in vivo [Citation21]. The atrophy of the intestinal villi patients with coeliac disease led to nutrient absorption, reduced the absorption of VitD, calcium and magnesium [Citation22].
It has become apparent that VitD deficiency contributes to the occurrence or aggravation of many paediatric diseases. VitD deficiency could cause osteomalacia and rickets in children [Citation23]. Children’s Hospital in the United Arab Emirates showed 35% of patients with VitD deficiency [Citation24]. VitD deficiency was common in Egyptian children with allergic rhinitis [Citation25]. Evidences suggested that VitD supplementation could treat related childhood illnesses. Inflammatory markers IL-12, IL-17, IL-23, and TNF-α were significantly reduced after VitD supplementation in children with inflammatory bowel disease, and the frequency of hospitalizations and the number of emergency department visits were significantly lower [Citation26]. Clinical data showed that VitD supplementing effectively relieved osteomalacia in children and adolescents [Citation27]. However, there were also conflicting findings on whether VitD deficiency was associated with paediatric diseases. Thus, the primary purpose of this review was to summarize the current status of researches on VitD metabolism and to clarify its roles in paediatric diseases.
VitD and childhood autism
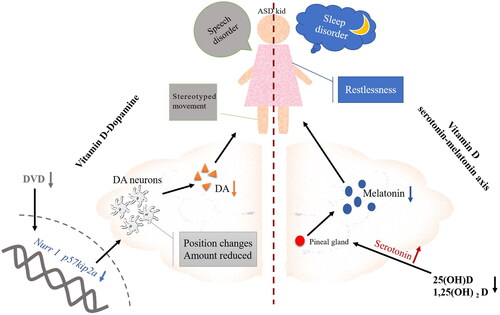
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by impaired social communication and interaction, and repetitive and stereotyped behaviours and interests [Citation28]. Autism is a multifactorial disease caused by the interaction of genetic and environmental factors. Hundreds of autism risk genes and various environmental factors (neonatal hypoxia and gestational diabetes) have been found [Citation29]. ASD children are known to exhibit various behaviour problems, including aggression, self-harm, attention deficit hyperactivity disorder, sleep disorders, and obsessive-compulsive disorder, which may prevent them from getting the same life and educational opportunities as other children have [Citation30]. VitD plays an important role in the regulation of gene expression in ASD. One study showed that 223 ASD risk genes in the SFARI database were VitD3-sensitive genes [Citation31]. A study by Wang et al. showed that the VitD levels of ASD children were lower than those of healthy children [Citation29]. It has been demonstrated that VitD levels in ASD children were lower also when compared with children with other neuropsychiatric disorders [Citation32]. In a randomized controlled trial, allergy and hyperactivity behaviours were improved after VitD and omega-3 long-chain polyunsaturated fatty acids were used to treat ASD children [Citation33]. Some studies observed that VitD supplementation significantly reduced the severity of ASD [Citation34]. But others showed that this effect was not consistently different between treatment and control groups [Citation35]. VitD supplementation was beneficial for hyperactivity but not core symptoms of ASD or other coexisting behaviours and conditions [Citation36]. Many factors could affect the experiments, including the small sample size of children with ASD, and the possible influence, such as sun exposure, race and skin colour, dietary habits and nutritional status [Citation37]. The impact of VitD on ASD is still under discussion. In addition to theoretical support that the effects of VitD on ASD may be related to signalling pathways involving whole blood arginine and nitric oxide, researchers also found that ASD is associated with disorders of the dopamine system and abnormalities of the serotonin-melatonin axis, which are caused by insufficient amounts of VitD () [Citation38].
Figure 2. ASD children’s behaviours may be affected by VitD regulating the secretion of dopamine and serotonin/melatonin. DVD: development VitD; DA: dopamine neurons.
VitD-dopamine hypothesis
A significant number of VDR have been found in the cortex and hippocampus [Citation39]. A study by Harms found that mice lacking developmental VitD (DVD) severely reduced hippocampus size [Citation40]. And insufficient DVD reduced the synthesis of dopamine (DA). In the embryonic brains of individuals with DVD deficiency, the gene expressions of Nurr1 and P57kip2a were reduced, the maturation of DA neurons and DA distribution within the midbrain were inhibited [Citation41]. The study found that 28 gene polymorphisms were associated with ASD, including the dopamine receptor D1 [Citation42]. Among 2084 children with ASD (age under six-year-old) in Vineland, 35% were found to have obvious movement difficulties. 44% displayed poor skills with sports stereotypes (hand slap, rotation, body shaking) and non-verbal behaviour [Citation43]. VitD and omega-3 long-chain polyunsaturated fatty acids reduced symptoms of dysphoria, and VitD also relieved symptoms of hyperactivity in ASD children 33. Brandenburg discovered that changes in the basal ganglia were associated with alterations in biochemistry or cellular processes, causing impaired language development and stereotyped movement patterns in ASD patients [Citation44]. Additionally, the dopamine receptors within the basal ganglia circuit were crucial to motor control [Citation45]. Consequently, ASD patients might suffer from a severely damaged dopaminergic system and neurochemical imbalance. Due to the lack of DVD, DA neurons were being damaged, the level of DA was reduced, and the growth of the striatum and the basal nucleus were abnormal, leading to speech disorder and stereotyped movements in ASD children.
VitD-serotonin-melatonin axis
Melatonin is an indoleamine produced by the pineal gland mostly found in the suprachiasmatic nucleus of the hypothalamus and the cerebrospinal fluid. Melatonin is essential for regulating sleep/wake cycles and biological rhythms [Citation46]. According to literature, the prevalence of sleep disorders in ASD children ranged from 40 to 93%, as recently reported by Petruzzelli MG [Citation47]. Melatonin is clinically used to treat sleep disorders. In the clinical trial, 104 weeks of melatonin therapy were administered to ASD children and adolescents. Melatonin therapy significantly improved their sleep disorders and sleep quality [Citation48]. Researchers proposed that VitD inhibited serotonin production by regulating melatonin levels outside the blood-brain barrier [Citation49]. In the evening, the levels of 25(OH)D and 1,25(OH)2D in serum and peripheral TPH1 gene expression increased, which stimulated melatonin production [Citation50]. ASD children with sleeping issues may benefit from low-dose melatonin due to its addictive properties. Therefore, it is still necessary to investigate the effects of melatonin combined with VitD in treating ASD children on sleeping problems.
VitD and childhood obesity
Children’s diets are full of high sugar and fatty junk food. Reduced exercise also contributed to children’s obesity. There were 14 million obese children in the United States [Citation51]. Childhood obesity has been associated with type 2 diabetes, orthopaedic diseases and psychological problems such as anxiety and autism [Citation52]. The meta-analysis showed that overweight children and adolescents had a higher prevalence of VitD deficiency, which they believed was due to the excessive absorption of fat soluble VitD by adipose tissue [Citation53]. Serum 25(OH)D of 80.4% children aged 4 to 14 years in Chile were too low [Citation54]; 64% morbidly obese children and adolescents were VitD deficient [Citation55]. Akter revealed that childhood obesity associated with VitD deficiency and VDR gene polymorphisms such as single nucleotide polymorphisms (SNPs) TaqI, BsmI, ApaI, FokI and Cdx2 [Citation56]. VDR genes’ polymorphisms were associated in body weight, insulin sensitivity, and susceptibility to type 1 or type 2 diabetes [Citation57].
Obesity inhibited CYP2R1 in the liver and consequently reduced VitD levels. In The research of Mahamoud et al. they found that obesity affected CYP2R1 gene expression in liver and kidneys, and the plasma 25(OH)D was decreased in mice [Citation58]. They also found that obesity repressed expression of the VDR in brown adipose tissue in mice. However, no effect was observed in the human subcutaneous adipose tissue. A study on 44 Brazilian children aged 4 to 11 years old with hypertriglyceridaemia who were supplemented with cholecalciferol observed that the blood lipid level in the children’s body was improved, but the change in weight was not significant [Citation59]. In another study of 73 obese children taking oral VitD3, 50% improved their VitD levels and BMI level, all had significantly lower percentages of fat mass, but they were still obese [Citation60]. Based on the above results, the therapeutic effect of simply supplementing VitD in the treatment of childhood obesity is not significant.
VitD and childhood asthma
Asthma is a chronic respiratory condition characterized by airway inflammation and hyperreactivity [Citation61]. Asthma is further complicated by smoke and chemical gas particles [Citation62]. It is the most frequent cause of acute admissions in children and a major source of morbidity for adults with asthma, causing mild wheezing or life-threatening airway obstruction [Citation63]. VitD had the ability to prevent airway inflammation in asthmatic mice by promoting Th17/Treg balance and inhibiting NF-κB-mediated inflammation [Citation64]. By activating VDR, VitD could influence various immune cells such as dendritic cells, monocytes, macrophages and T cells [Citation65]. A meta-analysis showed that asthmatic children had significantly lower 25(OH)D levels than non-asthmatic children [Citation66]. In a cross-sectional study in southern Jordan, 76.5% of 98 children with bronchial asthma were VitD deficient [Citation67]. VitD deficiency was associated with increased asthma exacerbations per year and length of stay per admission [Citation68]. According to Fergeson et al., the anti-inflammatory drugs used for the treatment of asthma were corticosteroids and β2-adrenergic receptor agonists [Citation69]. Taking steroid drugs would also reduce the VitD content in asthma children.
There are four types of gene polymorphisms in VDBP: GC1, GC2, GC1F and GC1S. Some studies showed that GC2 was closely related to asthma risk, and GC1 had therapeutic effect on asthma [Citation70]. VDR gene polymorphism is also a part of asthma susceptibility genes. A meta-analysis found that VDR FokI and VDR TaqI might be risk factors for childhood asthma [Citation71]. The VDR FokI polymorphism would result in a new isomer of VDR: VDRA. VDR TaqI will not alter the structure of VDR. However, it will affect the stability of VDR mRNA and directly affect protein transcription [Citation72]. The polymorphisms of the VDBP gene and VDR gene might have a role in the etiopathogenesis of childhood asthma.
Diverging opinions exist regarding the effects of VitD on asthma children. Some researchers suggested that VitD supplementation may not be protective against asthma. A clinical trial conducted by Chirag Thakur reported that VitD levels increased during 12 weeks of VitD supplementation, but asthma indicators such as the Childhood Asthma Control Test (C-ACT) and the number of asthma exacerbations did not improve [Citation73]. To determine the effectiveness of VitD supplementation, Forno’s clinical study showed that VitD3 supplementation in chronic asthmatic children with low VitD levels failed to significantly improve the time to severe asthma exacerbations [Citation74]. Systematic reviews and meta-analyses concluded that VitD supplementation had a small effect size and a low level of certainty. It was ineffective in reducing asthma attacks or systemic steroid levels, nor did it significantly improve emergency room visits or hospitalizations of asthma children [Citation75]. We couldn’t find consistent evidence that supplementing VitD reduces asthma exacerbations in a paediatric population. Therefore, more extensive clinical trials and preclinical medical experiment are necessary to determine the effectiveness of VitD in children with asthma.
VitD and rickets
Skeletal development problems occur in children and adolescents during their growth and development. In children with rickets, low calcium levels resulted in skeletal deformities, including pectus excavatum, X-legs and O-legs [Citation76]. Vitamin D is a key regulator of calcium and bone homeostasis. Low calcium levels symptoms were proved to be related to VitD deficiency. VitD deficiency rickets and other severe VitD deficiency manifestations, such as cardiomyopathy and hypocalcemic seizures, continued to be diagnosed in Canada [Citation77]. This is due to the disruption of calcium metabolism caused by low VitD levels. There are three main steps involved in the active transport of calcium across the cell membrane of the proximal intestine with VitD [Citation78] ():
Figure 3. Calcium is absorbed in the proximal intestine and transported to bones via paracellular transport and transcellular transport. VDR: VitD receptor; TRPV6: transient receptor potential vanilloid type 6; NCX1: Na+-Ca2+ exchanger 1.
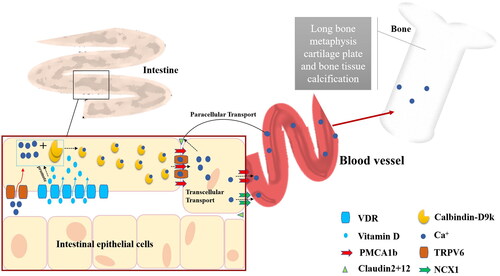
Luminal calcium enters the intestinal epithelial cells through transient receptor potential vanilloid type 6 (TRPV6);
In intestinal epithelial cells, VDR and VitD increase calcium binding to the calbindin-D9k protein and diffusing through the cytoplasm;
Calcium is transported from the basolateral membrane to blood vessels and bones through blood ATPase (PMCA1b) and Na+-Ca2+ exchanger 1 (NCX1) in a translational transport, or through claudin 2 and claudin 12 protein in a paracellular transport [Citation79].
In , the VitD and VDR enhanced calcium transport by paracellular transport and translational transport in the epithelial cells of villi and crypts, respectively [Citation80].
VitD is an essential medium for calcium intake in multiple organs. Studies have shown that lacking adequate amounts of VitD suffered a 75% reduction in intestinal calcium absorption [Citation81]. The kidney is also the main excretion pathway for phosphorus. Phosphate and calcium are simultaneously regulated by VitD3, parathyroid hormone (PTH) and calcitonin [Citation82]. PTH increased the activity of 1-hydroxylase, promoted the conversion of calcidiol into calcitriol, and increased the calcium secretion of calcitriol [Citation83]. Casselbrant et al. found, that heat shock protein in the bile could also regulate calcium transport via the VDR [Citation84]. Therefore, some researchers found that the prevalence of VitD deficiency in healthy children was also common, and suggested timely supplementation of VitD for early prevention of related diseases [Citation85].
Conclusion
It is important to underline that despite it is well known that VitD deficiency affects a big number of children, and could be associated with important childhood health problems, with VitD playing a potential role in their etiopathogenesis, nowadays data about the role of prevention and vitamin D supplementation are inconclusive.
Author contributions
ZL did the writing, reviewing and editing of this review. YNL did the conceptualization, methodology and literature search. XLY and YW assembled the data and performed the analysis. JZ and SMH revised it critically for intellectual content and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Acknowledgments
All the authors read and approved the final article. The authors would like to thank Zhixia Jiang, Professor, the president of Guizhou Nursing Vocational College, for her invaluable assistance with the literature search strategy, and Ming Li, MD, PhD, Associate Professor, Hunan University of Medicine, for his thoughtful comments and constructive advises during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Additional information
Funding
References
- Cardwell G, Bornman JF, James AP, et al. A review of mushrooms as a potential source of dietary vitamin D. Nutrients. 2018;10(10)):1498.
- Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–28.
- Thacher TD, Levine MA. CYP2R1 mutations causing vitamin D-deficiency rickets. J Steroid Biochem Mol Biol. 2017;173:333–336.
- Taskapan H, Wei M, Oreopoulos DG. 25(OH) vitamin D3 in patients with chronic kidney disease and those on dialysis: rediscovering its importance. Int Urol Nephrol. 2006;38(2):323–329.
- Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408.
- (a) Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30; (b) Latic N, Erben RG, Vitamin D. Vitamin D and cardiovascular disease, with emphasis on hypertension, atherosclerosis, and heart failure. Int J Mol Sci. 2020;21(18):6483.
- Lang F, Ma K, Leibrock CB. 1,25(OH)(2)D(3) in brain function and neuropsychiatric disease. Neurosignals. 2019;27(1):40–49.
- Lin CI, Chang YC, Kao NJ, et al. 1,25(OH)(2)D(3) alleviates Aβ(25-35)-induced tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line-derived neurotrophic factor signaling in SH-SY5Y cells. IJMS. 2020;21(12):4215.
- Charoenngam N, Shirvani A, Kalajian TA, et al. The effect of various doses of oral vitamin D(3) supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40(1):551–556.
- Grant WB, Moukayed M. Vitamin D(3) from ultraviolet-B exposure or oral intake in relation to cancer incidence and mortality. Curr Nutr Rep. 2019;8(3):203–211.
- Mokhtari-Zaer A, Hosseini M, Salmani H, et al. Vitamin D(3) attenuates lipopolysaccharide-induced cognitive impairment in rats by inhibiting inflammation and oxidative stress. Life Sci. 2020;253:117703.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–1364.
- Samad N, Imran A, Bhatti SA, et al. Vitamin D2 protects acute and repeated noise stress induced behavioral, biochemical, and histopathological alterations: possible antioxidant effect. Saudi J Biol Sci. 2022;29(1):601–609.
- Balachandar R, Pullakhandam R, Kulkarni B, et al. Relative efficacy of vitamin D(2) and vitamin D(3) in improving vitamin D status: systematic review and meta-analysis. Nutrients. 2021;13(10):3328.
- (a) Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine Committee to review dietary reference intakes for vitamin, D.; calcium, the national academies collection: reports funded by National Institutes of Health. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press (US); 2011. (b) Martineau AR, Jolliffe DA, Hooper RL, et al. Supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. (c) Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394–415.
- (a) Holick MF, Binkley NC, Bischoff-Ferrari HA, Endocrine Society, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930; (b) Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74(11):1498–1513.
- Wu Y, Yang Y, Xiao X, et al. The pattern of vitamin D levels in children 0-4 years of age in Yunnan province. Trop Pediatr. 2021;67(5):fmab093.
- Sooriyaarachchi P, Jeyakumar DT, King N, et al. Impact of vitamin D deficiency on COVID-19. Clin Nutr ESPEN. 2021;44:372–378.
- Marzban M, Kalantarhormozi M, Mahmudpour M, et al. Prevalence of vitamin D deficiency and its associated risk factors among rural population of the Northern part of the Persian Gulf. BMC Endocr Disord. 2021;21(1):219.
- Islam MZ, Bhuiyan NH, Akhtaruzzaman M, et al. Vitamin D deficiency in Bangladesh: a review of prevalence, causes and recommendations for mitigation. Asia Pac J Clin Nutr. 2022;31(2):167–180.
- (a) Vijayakumar M, Bk A, George B, et al. Vitamin D status in children on anticonvulsant therapy. Indian J Pediatr. 2022;89(6):541–545. (b) LoPinto-Khoury C, Brennan L, Mintzer S. Impact of carbamazepine on vitamin D levels: a meta-analysis. Epilepsy Res. 2021;178:106829.
- Cardo A, Churruca I, Lasa A, et al. Nutritional imbalances in adult celiac patients following a Gluten-Free diet. Nutrients. 2021;13(8):2877.
- Sizar O, Khare S, Goyal A, et al. Vitamin D deficiency. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- Alawadhi F, Yavuz L. Signs and symptoms of vitamin D deficiency in children: a cross-sectional study in a tertiary pediatric hospital in the United Arab Emirates. Cureus. 2021;13(10):e18998.
- Saad K, Abdelmoghny A, Aboul-Khair MD, et al. Vitamin D status in Egyptian children with allergic rhinitis. Ear Nose Throat J. 2020;99(8):508–512.
- El Amrousy D, El Ashry H, Hodeib H, et al. Vitamin D in children with inflammatory bowel disease: a randomized controlled clinical trial. J Clin Gastroenterol. 2021;55(9):815–820.
- Sahni SS, Kakkar S, Kumar R, et al. Osteomalacic myopathy in children and adolescents with vitamin-D deficiency. Neurol India. 2021;69(6):1650–1654.
- Lord C, Elsabbagh M, Baird G, et al. Autism spectrum disorder. Lancet. 2018;392(10146):508–520.
- Wang Z, Ding R, Wang J. The association between vitamin D status and autism spectrum disorder (ASD): a systematic review and meta-analysis. Nutrients. 2020;13(1):86.
- Lai MC, Kassee C, Besney R, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(10):819–829.
- Trifonova EA, Klimenko AI, Mustafin ZS, et al. The mTOR signaling pathway activity and vitamin D availability control the expression of most autism predisposition genes. Int J Mol Sci. 2019;20(24):6332.
- Petruzzelli MG, Marzulli L, Margari F, et al. Vitamin D deficiency in autism spectrum disorder: a cross-sectional study. Dis Markers. 2020;2020:9292560.
- Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9–16.
- (a) Javadfar Z, Abdollahzad H, Moludi J, et al. Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: a randomized clinical trial. Nutrition. 2020;79-80:110986. (b) Saad K, Abdel-Rahman AA, Elserogy YM, et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr Neurosci. 2016;19(8):346–351.
- (a) Kerley CP, Power C, Gallagher L, et al. Lack of effect of vitamin D(3) supplementation in autism: a 20-week, placebo-controlled RCT. Arch Dis Child. 2017;102(11):1030–1036. (b) Principi N, Esposito S. Vitamin D deficiency during pregnancy and autism spectrum disorders development. Front Psychiatry. 2019;10:987.
- Li B, Xu Y, Zhang X, et al. The effect of vitamin D supplementation in treatment of children with autism spectrum disorder: a systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci. 2022;25(4):835–845.
- Kittana M, Ahmadani A, Stojanovska L, et al. The role of vitamin D supplementation in children with autism spectrum disorder: a narrative review. Nutrients. 2021;14(1):26.
- Wang B, Dong H, Li H, et al. A probable way vitamin D affects autism spectrum disorder: the nitric oxide signaling pathway. Front Psychiatry. 2022;13:908895.
- Bivona G, Gambino CM, Iacolino G, et al. Vitamin D and the nervous system. Neurol Res. 2019;41(9):827–835.
- Harms LR, Cowin G, Eyles DW, et al. Neuroanatomy and psychomimetic-induced locomotion in C57BL/6J and 129/X1SvJ mice exposed to developmental vitamin D deficiency. Behav Brain Res. 2012;230(1):125–131.
- (a) Cui X, Pelekanos M, Burne TH, et al. Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci Lett. 2010;486(3):220–223. (b) Luan W, Hammond LA, Cotter E, et al. Developmental vitamin D (DVD) deficiency reduces Nurr1 and TH expression in post-mitotic dopamine neurons in rat mesencephalon. Mol Neurobiol. 2018;55(3):2443–2453.
- Mpoulimari I, Zintzaras E. Synthesis of genetic association studies on autism spectrum disorders using a genetic model-free approach. Psychiatr Genet. 2022;32(3):91–104.
- Licari MK, Alvares GA, Varcin K, et al. Prevalence of motor difficulties in autism spectrum disorder: analysis of a population-based cohort. Autism Res. 2020;13(2):298–306.
- Brandenburg C, Soghomonian JJ, Zhang K, et al. Increased dopamine type 2 gene expression in the dorsal striatum in individuals with autism spectrum disorder suggests alterations in indirect pathway signaling and circuitry. Front Cell Neurosci. 2020;14:577858.
- Saito N, Sasaoka T. [Dopamine and NMDA receptors in basal ganglia circuits and their roles regarding motor control and learning]. Brain Nerve. 2020;72(11):1135–1142.
- Filali S, Bergamelli C, Lamine Tall M, et al. Formulation, stability testing, and analytical characterization of melatonin-based preparation for clinical trial. J Pharm Anal. 2017;7(4):237–243.
- Petruzzelli MG, Matera E, Giambersio D, et al. Subjective and electroencephalographic sleep parameters in children and adolescents with autism spectrum disorder: a systematic review. J Clin Med. 2021;10(17):3893.
- Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after 2 years of Prolonged-Release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252–261.e3.
- Huiberts LM, Smolders K. Effects of vitamin D on mood and sleep in the healthy population: interpretations from the serotonergic pathway. Sleep Med Rev. 2021;55:101379.
- Jones KS, Redmond J, Fulford AJ, et al. Diurnal rhythms of vitamin D binding protein and total and free vitamin D metabolites. J Steroid Biochem Mol Biol. 2017;172:130–135.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. 2017;(288):1–8.
- (a) Valaiyapathi B, Gower B, Ashraf AP. Pathophysiology of type 2 diabetes in children and adolescents. Curr Diabetes Rev. 2020;16(3):220–229; (b) Deal BJ, Huffman MD, Binns H, et al. Perspective: childhood obesity requires new strategies for prevention. Adv Nutr. 2020;11(5):1071–1078; (c) Dhaliwal KK, Orsso CE, Richard C, et al. Risk factors for unhealthy weight gain and obesity among children with autism spectrum disorder. Int J Mol Sci. 2019;20(13):3285.
- Fiamenghi VI, Mello ED. Vitamin D deficiency in children and adolescents with obesity: a meta-analysis. J Pediatr. 2021;97(3):273–279.
- Pérez-Bravo F, Duarte L, Arredondo-Olguín M, et al. Vitamin D status and obesity in children from Chile. Eur J Clin Nutr. 2022;76(6):899–901.
- Soheilipour F, Hamidabad NM. Vitamin D and calcium status among adolescents with morbid obesity undergoing bariatric surgery. Obes Surg. 2022;32(3):738–741.
- Akter R, Afrose A, Sharmin S, et al. A comprehensive look into the association of vitamin D levels and vitamin D receptor gene polymorphism with obesity in children. Biomed Pharmacother. 2022;153:113285.
- (a) Wang D, Su K, Ding Z, et al. Association of vitamin D receptor gene polymorphisms with metabolic syndrome in Chinese children. Int J Gen Med. 2021;14:57–66. (b) Tangjittipokin W, Umjai P, Khemaprasit K, et al. Vitamin D pathway gene polymorphisms, vitamin D level, and cytokines in children with type 1 diabetes. Gene. 2021;791:145691.
- Elkhwanky MS, Kummu O, Piltonen TT, et al. Obesity represses CYP2R1, the vitamin D 25-hydroxylase, in the liver and extrahepatic tissues. JBMR Plus. 2020;4(11):e10397.
- Alves AGP, Cruvinel B, Schincaglia AC, et al. Vitamin D supplementation reduces serum lipids of children with hypertriacylglycerolemia: a randomized, triple-masked, placebo-controlled crossover trial. Nutrition. 2021;89:111296.
- De Cosmi V, Mazzocchi A, D'Oria V, et al. Effect of vitamin D and docosahexaenoic acid co-supplementation on vitamin D status, body composition, and metabolic markers in obese children: a randomized, double blind, controlled study. Nutrients. 2022;14(7):1397.
- Mims JW. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol. 2015;5(Suppl 1):S2–S6.
- Bontinck A, Maes T, Joos G. Asthma and air pollution: recent insights in pathogenesis and clinical implications. Curr Opin Pulm Med. 2020;26(1):10–19.
- (a) Haktanir Abul M, Phipatanakul W. Severe asthma in children: evaluation and management. Allergol Int. 2019;68(2):150–157. (b) Ramsahai JM, Hansbro PM, Wark PAB. Mechanisms and management of asthma exacerbations. Am J Respir Crit Care Med. 2019;199(4):423–432.
- Ma JG, Wu GJ, Xiao HL, et al. Vitamin D has an effect on airway inflammation and Th17/treg balance in asthmatic mice. Kaohsiung J Med Sci. 2021;37(12):1113–1121.
- Ahmad S, Arora S, Khan S, et al. Vitamin D and its therapeutic relevance in pulmonary diseases. J Nutr Biochem. 2021;90:108571.
- Wang Q, Ying Q, Zhu W, et al. Vitamin D and asthma occurrence in children: a systematic review and meta-analysis. J Pediatr Nurs. 2022;62:e60–e68.
- Al-Zayadneh E, Alnawaiseh NA, Ajarmeh S, et al. Vitamin D deficiency in children with bronchial asthma in Southern Jordan: a cross-sectional study. J Int Med Res. 2020;48(12):300060520974242.
- Aziz DA, Abbas A, Viquar W, et al. Association of vitamin D levels and asthma exacerbations in children and adolescents: experience from a tertiary care center. Monaldi Arch Chest Dis. 2022.[published online ahead of print, 2022 May 24]. doi:10.4081/monaldi.2022.2230
- Fergeson JE, Patel SS, Lockey RF. Acute asthma, prognosis, and treatment. J Allergy Clin Immunol. 2017;139(2):438–447.
- Rozmus D, Ciesielska A, Płomiński J, et al. Vitamin D binding protein (VDBP) and its gene polymorphisms-the risk of malignant tumors and other diseases. Int J Mol Sci. 2020;21(21):7822.
- (a) Ruan Z, Shi Z, Zhang G, et al. Asthma susceptible genes in children: a meta-analysis. Medicine. 2020;99(45): e23051; (b) Zhou Y, Li S. Meta-analysis of vitamin D receptor gene polymorphisms in childhood asthma. Front Pediatr. 2022;10:843691.
- Ruiz-Ballesteros AI, Meza-Meza MR, Vizmanos-Lamotte B, et al. Association of vitamin D metabolism gene polymorphisms with autoimmunity: evidence in population genetic studies. Int J Mol Sci. 2020;21(24):9626.
- Thakur C, Kumar J, Kumar P, et al. Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: a randomized controlled trial (ViDASTA trial). Pediatr Pulmonol. 2021;56(6):1427–1433.
- Forno E, Bacharier LB, Phipatanakul W, et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA. 2020;324(8):752–760.
- Kumar J, Kumar P, Goyal JP, et al. Vitamin D supplementation in childhood asthma: a systematic review and meta-analysis of randomised controlled trials. ERJ Open Res. 2022;8(1):00662–2021.
- Uday S, Högler W. Nutritional rickets & osteomalacia: a practical approach to management. Indian J Med Res. 2020;152(4):356–367.
- Irvine J, Ward LM. Preventing symptomatic vitamin D deficiency and rickets among indigenous infants and children in Canada. Paediatr Child Health. 2022;27(2):127–128.
- (a) Christakos S, Dhawan P, Porta A, et al. Intestinal calcium absorption. Mol Cell Endocrinol. 2011;347 (1-2):25–29. (b) Christakos S. Vitamin D: a critical regulator of intestinal physiology. JBMR Plus. 2021;5(12):e10554.
- Fleet JC. Vitamin D-Mediated regulation of intestinal calcium absorption. Nutrients. 2022;14(16):3351.
- Christakos S, Li S, De La Cruz J, et al. Vitamin D and the intestine: review and update. J Steroid Biochem Mol Biol. 2020;196:105501.
- Pansu D, Bellaton C, Roche C, et al. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol. 1983;244(6):G695–700.
- Shrimanker I, Bhattarai S. Electrolytes. In StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- Atapattu N, Shaw N, Högler W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr Res. 2013;74(5):552–556.
- Casselbrant A, Fändriks L, Wallenius V. Glycocholic acid and butyrate synergistically increase vitamin D-induced calcium uptake in caco-2 intestinal epithelial cell monolayers. Bone Rep. 2020;13:100294.
- Martínez Redondo I, García Romero R, Calmarza P, et al. [Vitamin D insufficiency in a healthy pediatric population. The importance of early prophylaxis]. Nutr Hosp. 2021;38(6):1155–1161.
