Abstract
Objective
To evaluate the serotype distribution and antibiotic resistance in pneumococcal infections in adults and to provide a perspective regarding serotype coverage of both current and future pneumococcal vaccines.
Patients and methods
This passive surveillance study was conducted with the Streptococcus pneumoniae strains isolated from the specimens of patients with pneumonia (materials isolated from bronchoalveolar lavage), bacteraemia, meningitis, pleuritis and peritonitis between 2015 and 2018. Serogrouping and serotyping were performed by latex particle agglutination and by conventional Quellung reaction using commercial type-specific antisera, respectively. The strains were analysed for penicillin, cefotaxime, erythromycin and moxifloxacin susceptibilities by E-test.
Results
In the whole study group (410 samples from adults aged ≥18 years), the most frequent serotypes were 3 (14.1%), 19 F (12%) and 1 (9.3%). The vaccine coverage for PCV13, PCV15, PCV20 and PPV23 was 63.9%, 66.6%, 74.1% and 75.9%, respectively, in all isolates. Penicillin non-susceptibility in invasive pneumococcal disease (IPD) was 70.8% and 57.1% in the patients aged <65 and ≥65 years, respectively. About 21.1% and 4.3% of the patients with and without IPD had cefotaxime resistance. Non-susceptibility to erythromycin and moxifloxacin was 38.2% and 1.2%, respectively.
Conclusions
The results revealed that novel PCV vaccines may provide improved coverage as compared with the currently available vaccine, PCV13. The significant antibiotic resistance rates imply the need to extend the serotype coverage of the vaccines. Continuing the surveillance in pneumococcal diseases is critical to explore the serotype distribution and incidence changes of IPD cases in the population and to inform policy makers to make necessary improvements in the national immunization programmes.
This multicentre study demonstrated the most recent serotype distribution and antibiotic resistance in adult population in Turkey.
Shifting from PCV13 to novel conjugated vaccines will significantly increase the coverage.
Continuing the surveillance in pneumococcal diseases is critical to explore the serotype distribution changes and the incidence of cases with invasive pneumococcal disease in the population.
Key messages
Introduction
Streptococcus pneumoniae is the causative agent for the commonly seen noninvasive infections such as pneumonia, otitis and sinusitis, and the most common cause of community-acquired pneumonia (CAP) [Citation1]. Invasive pneumococcal disease (IPD) refers to the spread of S. pneumoniae to sterile sites like the joints, brain or blood. IPD is an aggressive disease with a significant burden of morbidity and mortality, especially among children younger than 2 years of age, individuals older than 65 years of age and special vulnerable groups like patients with malignancies or human immunodeficiency virus (HIV) infections [Citation2–4]. Although the incidence and prevalence data of IPD in Turkey is not currently available in adult patients, there are ongoing efforts to determine this information by national surveillance studies [Citation5]. Nevertheless, varying incidence rates were reported in different countries, i.e. 6 per 100,000 in the European Union countries in 2007 and 12.9 per 100,000 in the United States in 2009, and when considered for age groups separately, these incidences can reach to 18 and 60 per 100,000 in age groups <5 years and ≥65 years, respectively [Citation6,Citation7]. Moreover, IPD has a significant case-fatality rate, which was previously reported as high as 35% among adults older than 65 years of age [Citation8].
Another concern associated with S. pneumoniae is the antibacterial resistance to several antimicrobial drugs, including beta-lactams and macrolides [Citation9]. The primary risk factor for the progression of resistance is previous antimicrobial therapies. Other risk factors include being younger than 5 years old, living in vulnerable places like kindergarten or nursery where resistant strains can disseminate quickly, recent hospitalization, having a chronic pulmonary disease or underlying immune disorder and a previous history of an IPD [Citation10]. The previously published 2005–2015 data on adults of this current surveillance study revealed that oral and parenteral penicillin resistance in IPD was 21.7% and 0.6%, respectively, and parenteral penicillin resistance in meningitis was 52.6% [Citation11].
Pneumococcal vaccines can efficiently protect individuals from pneumococcal diseases and further antibiotic resistance. These vaccines are also recommended by the World Health Organization, especially for vulnerable groups like children and elderly. Currently, the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal conjugate vaccine (PCV13) are available in Turkey. The National Immunization Programme (NIP) incorporated the 7-valent PCV for children in November 2008, which was replaced with PCV13 in April 2011 [Citation5,Citation12]. Childhood vaccination programme is conducted by the family practitioners and followed by negative performance system by the Ministry of Health [Citation13]. Moreover, risk groups among individuals between 18 and 65 years of age and all adults over 65 years of age have been included in the immunization programme since 2016; all receive PCV13 free-of-charge at immunization centres, and high-risk groups receive PPV23 by reimbursement [Citation14]. The adult vaccination programme is not part of the family physician performance system. As a result, childhood vaccination rate was reported as 97% [Citation15], while the rate of adult vaccination was below 9.91% [Citation16].
The serotype coverage of pneumococcal vaccines is critical to prevent asymptomatic carriage and further noninvasive and invasive diseases. In addition, serotypes included in the pneumococcal vaccines are also important because the nonvaccine serotypes are responsible for the majority of further cases. Thus, increasing the serotype coverage of pneumococcal vaccines is an ongoing effort to minimize the disease risk associated with S. pneumoniae in the populations. In addition to serotype coverage, type of the vaccine is also important for outcomes. Although not indicated for children below 2 years of age, unconjugated polysaccharide vaccines induce an independent T-cell response without establishing B-cell memory that causes a negligible immune response in those children [Citation17]. However, conjugated vaccines include polysaccharides of each serotype covalently bound to a carrier protein, which presents an antigen complex to stimulate specific CD4+ T-helper cells. This stimulation also results in B-cell memory that forms a long-term serotype-specific immunological response. As a consequence, conjugate vaccines provide T-cell dependent immune response, mucosal response, protective immune memory and IgG-dependent long-term immune response [Citation5]. The efficacy of PCV13 against pneumonia was evaluated in adults over 65 years of age in the Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA) study [Citation18]. The CAPITA study showed a 46% reduction in first vaccine-type pneumococcal CAP, 45% reduction in first-vaccine type non-bacteraemic pneumococcal pneumonia and 75% reduction in first vaccine-type IPD in adults over 65 years of age. There is no study on the efficacy of PPV23 in mucosal diseases. The clinical trials of 15- and 20-valent conjugate vaccines (PCV15 and PCV20) promise to establish enhanced vaccine coverage for S. pneumoniae [Citation19,Citation20].
Based on this background, this study aimed to evaluate the serotype distribution in pneumococcal infections (using bronchoalveolar lavage [BAL] specimens) and in IPD cases in adult patients. It was also aimed to assess the serotype coverage rates of both existing and novel pneumococcal vaccines that are likely to improve results if used in practice. Moreover, this study was conducted as a routine surveillance of pneumococcal diseases to provide current epidemiological data on the serotype distribution and antibiotic resistance rates, which shall direct the efforts to decrease the burden of these diseases.
Patients and methods
This passive surveillance study was conducted with the S. pneumoniae strains isolated from the specimens of patients (aged ≥18 years) with pneumonia (materials isolated from BAL), bacteraemia, meningitis, pleuritis and peritonitis between 2015 and 2018. The samples were collected from 21 centres in Turkey (, ). The patients with a diagnosis of any of the conditions mentioned above were informed about this surveillance study, and their consents were taken to use the biological samples for the analyses. The study was approved by the Clinical Research Ethics Committee of Istanbul University Istanbul Medical Faculty (approval date: 30 November 2012 and approval number: 20).
Figure 1. The map of Turkey that geographically represents the study centres and the included isolates from each centre.
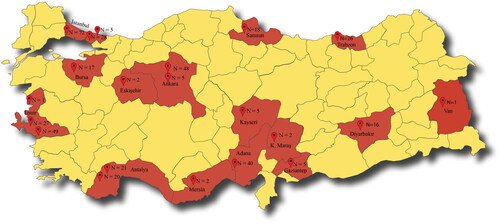
Table 1. Year-wise number of isolates obtained from 21 centres included in the study.
Laboratory analyses
Microbiological analyses were conducted at the Microbiology Laboratory of Hacettepe University Faculty of Medicine. All collected samples were frozen and kept at -800C in glycerol before analysis. Strains were identified by the presence of alpha haemolysis, optochin susceptibility test, lysis by sodium deoxycholate and latex tests (BD Directigen, Becton Dickinson and Company, USA) to detect pneumococcal antigen and MALDI-TOF for the differentiation of S. pneumoniae from other mitis group streptococci [Citation21]. Serogrouping was performed using latex particle agglutination, and serotyping was performed by the conventional Quellung reaction using commercial type-specific antisera (Statens Serum Institut, Copenhagen, Denmark). In addition, S. pneumoniae strains were analysed for penicillin, cefotaxime, erythromycin and moxifloxacin susceptibilities by E-test (AB Biodisk, Sweden). Results were evaluated according to the Clinical Laboratory Standards Institute (CLSI−2021) standards. According to CLSI-2021 minimum inhibitory concentration (MIC) breakpoints for parenteral penicillin were ≤2 susceptible, 4 intermediate, ≥8 resistant for non-meningitis specimens and ≤0.06 susceptible, ≥0.12 resistant for meningitis specimens. MIC break points for cefotaxime were ≤1 susceptible. 2 intermediate, ≥4 resistant for non-meningitis specimens and ≤0.5 susceptible, 1 intermediate, ≥2 resistant for meningitis specimens [Citation22]. Penicillin and cefotaxime susceptibilities were explicitly interpreted for the strains isolated from either cerebrospinal fluid (CSF) or other specimens.
Statistical analyses
All analyses were performed using the IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were expressed as median, interquartile range (IQR – 25th-75th percentiles) and range for continuous variables and as frequency and percentages for categorical variables. The serotypes covered by PCV13, PCV15, PCV20 and PPV23 are presented in , and the comparison of the coverage rates of pneumococcal vaccines were analysed using the McNemar test. A p value of <.05 was considered statistically significant.
Results
The study included 410 adult patients (males, 72.7%) with a median age of 60 years (IQR 49–68 years, range: 18–98 years). Of the patients, 34.4% were older than 65 years of age. The biological specimens were collected from blood (n = 191, 46.6%), BAL (n = 158, 38.5%), CSF (n = 38, 9.3%), pleural fluid (n = 17, 4.2%), peritoneal fluid (n = 4, 1%), paracentesis fluid (n = 1, 0.2%) and vitreous humour (n = 1, 0.2%). The samples were collected in 2015 (30.7%), 2016 (25.9%), 2017 (23.4%) and 2018 (20%).
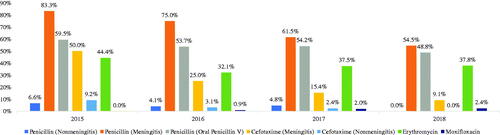
Antibiotic non-susceptibility in age groups is presented in . The resistance to penicillin in non-meningitis was 4.3% among all patients. The resistance rates in meningitis were 70.8% and 57.1% in the patients below 65 years of age and in those aged 65 years and above, respectively. Non-susceptibility to oral penicillin V was 54.6% among all patients. About 21.1% of the patients with meningitis and 4.3% of the patients with non-meningitis had cefotaxime resistance. Non-susceptibility to erythromycin and moxifloxacin was 38.2% and 1.2%, respectively. The changes in non-susceptibility over the years are presented in . There was a decrease in non-susceptibility to penicillin and cefotaxime but a modest increase against moxifloxacin.
Table 2. Antibiotic non-susceptibility according to age groups.
The distribution of the isolated serotypes according to age groups is presented in . Accordingly, the most frequent isolates were 19 F (13%), 3 (11.9%) and 1 (9.7%) among patients below 65 years of age. The most frequent isolates in the patients aged 65 years and above were 3 (18.4%), 19 F (9.9%) and 1 (8.5%). When the distribution of non-vaccine isolates was evaluated, it was found that the most frequent strains were 35 F (4.1%), 15 A (1.9%) and 18 F (1.5%) among patients below 65 years of age; 15 A (2.1%), 35 F (2.1%) and 11 C (1.4%) among patients aged 65 years and above; and, 35 F (3.4%), 15 A (2%), 18 F and 35B (1%, each) in the entire study population.
Table 3. Distribution of isolated serotypes according to age groups.
The proportion of strains covered by pneumococcal vaccines (PCV13, PCV15, PCV20, PPV23) in specimens from BAL and IPD cases is presented in . Accordingly, the coverage rates ranged between 60.8% (PCV13) and 74.7% (PPV23) for the BAL specimens and between 65.9% (PCV13) and 76.6% (PPV23) for the samples from IPD cases. When the age groups were considered, the coverage was higher among the patients aged 65 years and above for each vaccine.
Table 4. Coverage of pneumococcal vaccines according to age groups.
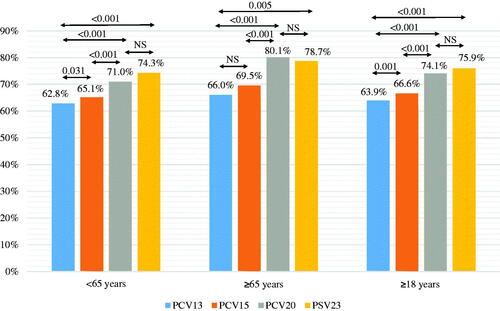
The comparison of pneumococcal vaccine coverage rates are presented in . For the patients aged 65 years and above, the coverage for PCV13, PCV15, PCV20 and PPV23 were 66%, 69.5%, 80.1% and 78.7%, respectively. Comparisons showed that the coverage rates of PCV13 and PCV15 were similar; however, the coverage rate of PCV20 was significantly higher than those of PCV13 and PCV15 (p < .001 for both). For the patients below 65 years of age, the coverage rates of PCV13, PCV15, PCV20 and PPV23 were 62.8%, 65.1%, 71% and 74.3%, respectively. Analyses revealed that the coverage rate of PCV15 was significantly higher than that of PCV13 (p = .031) and that the coverage rate of PCV20 was significantly higher than those of PCV13 and PCV15 (p < .001 for both). When all patients were considered, the coverage rates for PCV13, PCV15, PCV20 and PPV23 were 63.9%, 66.6%, 74.1% and 75.9%, respectively. PCV15 had a significantly higher coverage rate as compared with PCV13 (p = .001), and the coverage rate of PCV20 was higher than those of PCV13 and PCV15 (p < .001 for both). For all age groups, the coverage rate of PPV23 was similar to that of PCV20 and significantly higher when compared with that of PCV13. Additionally, coverage rate was higher among patients aged 65 years and above for all vaccines.
Discussion
This study evaluated the distribution of S. pneumoniae serotypes, the coverage rates of different vaccine types and the non-susceptibility to antibiotics in specimens collected from 410 patients with pneumococcal diseases between 2015 and 2018. We found that PCV13 serotypes were identified in 59.8% of the BAL samples from patients younger than 65 years old and 62.5% in older patients. When coverage of PCV13 was evaluated in samples from IPD cases, 64.7% and 68.2% of the samples from the patients below 65 years and those aged 65 years and above included PCV13 serotypes, respectively. Previous studies reported varying results regarding the coverage rates of the vaccines, in which the differences were primarily associated with the geographical region, age groups included in the studies, and the vaccination programmes conducted in the relevant populations. For example, previously published results from the paediatric population of our study (in which the paediatric and adult populations were studied with the same study protocol with a single ethical committee approval) revealed that the PCV13 coverage rate in the paediatric age group was 56.2% in the same period of our study [Citation23]. On the other hand, older studies reported that PCV13 serotypes were determined in 52.1% of pneumococcal diseases in the paediatric age group between 2008 and 2010 [Citation24]. Although the age groups are different from our study, the time trend in nonvaccine serotypes in clinical pneumococcal diseases suggests an increase in childhood, which can be accounted for the efficiency of vaccines that prevents the disease associated with vaccine serotypes. In addition, the proportion of vaccine serotypes in adults found in our study was higher in comparison to that in childhood, which might underline the importance of successful vaccination in childhood and the low rates of vaccination in adults.
Serotype surveillance in pneumococcal disease has particular importance. Identifying the most prevalent serotypes causing the disease provides robust data to policymakers to choose the most appropriate vaccine for immunization programmes and guidelines on a local scale. The polysaccharide capsule of S. pneumoniae is the primary virulence factor responsible for bacterial evasion, which is also the target used for serotyping the strains [Citation25]. Up to now, approximately 100 capsular serotypes of S. pneumoniae have been identified [Citation26]. The pneumococcal vaccines include the most prevalent and pathogenic serotypes, and the distribution of these serotypes has changed in communities after introducing the inactivated pneumococcal conjugate vaccines into vaccination practices. Previous studies have reported that the widespread use of pneumococcal vaccines increases the infection rates with nonvaccine serotypes, decreases the infection rates with serotypes included in the vaccines and alters the incidence and serotype distribution of the infections due to antibiotic-resistant serotypes [Citation27–30]. We found that the most common serotypes in adult population were 19F, 3 and 1 between 2015 and 2018 in Turkey. The European Centre for Disease Prevention and Control (ECDC) reported in 2016 that the most common serotypes in adults over 25 years of age were 3, 8 and 12F [Citation31]. The distribution of these serotypes is closely associated with the vaccination programmes utilized in the countries. Our data have revealed that vaccine serotypes are still the cause of disease in the adult age group in our country. It is important to increase the rates of adult vaccination in order to reduce the disease burden caused by the vaccine serotypes. The differences between countries might be associated with many factors such as vaccination rates, epidemiological characteristics of populations and antibiotic resistance conditions. Moreover, PCV13 serotypes are still effective in adults in Turkey. Thus, continuous surveillance is needed to follow the changes over time in the population. The previous paediatric data from our study in the same period reported the same serotypes; the 19F, 3 and 1 were the most common serotypes identified in childhood IPD in Turkey [Citation23]. This suggests similar epidemiological characteristics of pneumococcal diseases in all age groups in our country.
The current NIP in Turkey includes routine PCV13 for all children and consecutive PCV13 and PPV23 in high-risk groups, which are also being used in many countries worldwide. Nevertheless, the unavailability of vaccines including more serotypes cannot protect against nonvaccine serotypes and unencapsulated S. pneumoniae, which also increases antibiotic resistance, particularly in nonvaccine serotypes. Our results showed that the most frequent nonvaccine serotypes were 35F, 15A, 18F, 35B and 11C. A recent review reported that the most frequent nonvaccine serotypes in adult IPD cases were 38, 6C and 16F in the world [Citation32]. Apart from the vaccine coverage, our results showed that antibiotic non-susceptibility was significantly prominent in meningitis cases, 65.8% for penicillin and 21.1% for cefotaxime. The resistance for these drugs in non-meningitis disease was 4.3%. The non-susceptibility to erythromycin and oral penicillin V were 38.2% and 54.6%, respectively. These high rates also underline the importance of effective vaccination with modernized vaccines covering more serotypes that cause pneumococcal diseases.
The epidemiological data about the serotype distribution and antibiotic resistance of S. pneumoniae necessitates developing novel vaccines that should cover an increased number of serotypes that can meet the expectations to decrease pneumococcal diseases. PCV15 and PCV20 are among these promising vaccines that are approved in adults by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2021 and 2022, respectively [Citation33,Citation34].
Although PPV23 includes all serotypes except 6A covered by the PCVs, it cannot generate immunological memory and decrease the asymptomatic carriage in children. Thus, it is ineffective in children below 2 years of age [Citation35]. However, novel PCVs are promising to provide enhanced outcomes compared to PCV13. The PCV15 includes serotypes 22F and 33F, and the PCV20 includes 8, 10A, 11A, 12F, 15B, 22F and 33F, in addition to PCV13 serotypes [Citation19,Citation20]. Our results showed that, in IPD, PCV15 covered 68.7%, and PCV20 covered 75.4% of strains identified from the specimens in the entire study population. Likewise, the coverage was higher among patients aged 65 years and older. However, for PPV23, the coverage was similar for all age groups and was about 76.6%.
This study has several limitations. First, the number of biological samples included in the study was limited and may not reveal the actual serotype distribution in the population. Second, although we included 21 centres from 15 provinces in Turkey, this study’s wide geographical range may not represent the entire population and should be evaluated cautiously. Third, since the study was a passive surveillance, there may be missing cases and biological samples analysed, resulting in biased outcomes. Despite these limitations, this study revealed the most recent serotype distribution and antibiotic resistance in Turkey and implied the importance of enhancing the vaccines’ serotype coverage regarding disease burden. This study also provided valuable data for further academic and regulatory assessments to both researchers and policymakers.
Conclusions
Comparison of the coverage of vaccines revealed that shifting from PCV13 to novel conjugated vaccines will significantly increase the coverage. Thus, future amendments in the NIP to include these vaccines will be an effective intervention to decrease the burden of pneumococcal diseases. Continuing the surveillance in pneumococcal diseases is critical to explore the serotype distribution changes and incidence of IPD cases in the population for informing policy makers to make necessary improvements.
Author contributions
Gulsen Hascelik contributed to the conception and design of the work and the acquisition, analysis and interpretation of data, drafted the Manuscript and revised it critically for important intellectual content. Nezahat Gurler contributed to the conception and design of the work and the acquisition of data, drafted the Manuscript and revised it critically for important intellectual content. Mehmet Ceyhan contributed to the project administration and funding acquisition, acquisition and interpretation of data for the work and revised the Manuscript critically for important intellectual content. All the remaining authors– Guner Soyletir, Zeynep Gulay, Banu Sancak, Akgun Yaman, Sabire Sohret Aydemir, Gulcin Bayramoglu, Faruk Aydin, Yesim Cekin, Asuman Birinci, Cuneyt Ozakin, Nezahat Akpolat, Betil Ozhak Baysan, Meral Gultekin, Yasemin Zer, Laser Sanal, Cigdem Arabaci, Yasemin Ay Altintop and Candan Ozturk– contributed to the acquisition of data for the work and revised the Manuscript critically for important intellectual content. All authors provided final approval of the version to be published. All authors have agreed to be accountable for all aspects of the work.
Disclosure statement
Mehmet Ceyhan received consulting fees and research funding from Pfizer Pharmaceuticals, Istanbul, Turkey and all the remaining authors report no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Gulsen Hascelik, upon reasonable request.
Additional information
Funding
References
- Wasserman M, Chapman R, Lapidot R, et al. Twenty-year public health impact of 7- and 13-valent pneumococcal conjugate vaccines in US children. Emerg Infect Dis. 2021;27(6):1627–1636.
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20:45–51.
- O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902.
- Curcio D, Cané A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis. 2015;37:30–35.
- Senol E, Azap A, Erbay A, et al. Pneumococcal vaccine as one of the immunization coverage targets for adulthood vaccines: a consensus report of the study group for adult immunization of the Turkish Society of Clinical Microbiology and Infectious Diseases. Klimik Dergisi. 2018;31(1):2–18. Turkish.
- Vila-Corcoles A, Ochoa-Gondar O. Preventing pneumococcal disease in the elderly: recent advances in vaccines and implications for clinical practice. Drugs Aging. 2013;30(5):263–276.
- Sanford M. Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed): in older adults. Drugs. 2012;72(9):1243–1255.
- Jayaraman R, Varghese R, Kumar JL, et al. Invasive pneumococcal disease in Indian adults: 11 years’ experience. J Microbiol Immunol Infect. 2019;52(5):736–742.
- Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1–35.
- Lynch JP 3rd, Zhanel GG. Streptococcus pneumoniae: does antimicrobial resistance matter? Semin Respir Crit Care Med. 2009;30(02):210–238.
- Hasçelik G, Gürler N, Ceyhan M, et al. Serotype distribution and antibiotic resistance among isolates of Streptococcus pneumoniae causing invasive pneumococcal disease in adults in Turkey: 2005-2015. Int J Infect Dis. 2016;45:91.
- T.C. Sağlık Bakanlığı. Genişletilmiş Bağışıklama Programı Genelgesi 2009 [accessed 2022 Oct 23]. Available from: https://www.saglik.gov.tr/TR,11137/genisletilmis-bagisiklama-programi-genelgesi-2009.html
- Aile Hekimliği Sözleşme ve Ödeme Yönetmeliği 2021 [accessed 2022 May 25]. Available from: https://www.mevzuat.gov.tr/MevzuatMetin/21.5.4198.pdf
- T.C. Sağlık Bakanlığı Türkiye Halk Sağlığı Kurumu. Risk Grubu Aşılamaları; [accessed 2022 May 25]. Available from: https://asirehberi.saglik.gov.tr/uploads/2017-genelgeler/risk/2-risk-grubu-asilamalari-ek-risk-grubu-asilamalari-1-2.html
- T.C. Sağlık Bakanlığı. Sağlık İstatistikleri Yıllığı 2018. Haber Bülteni [accessed 2022 May 25]. Available from: https://sbsgm.saglik.gov.tr/Eklenti/33116/0/haber-bulteni–-2018-30092019pdf.pdf
- Mutlu HH, Coşkun FO, Sargın M. The incidence and awareness of vaccination among people aged 65 and over applied to a family medicine outpatient clinic. Ankara Med J. 2018;18:1–13.
- Daniels CC, Rogers PD, Shelton CM. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther. 2016;21(1):27–35.
- Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125.
- Thompson A, Lamberth E, Severs J, et al. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2019;37(42):6201–6207.
- Greenberg D, Hoover PA, Vesikari T, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine. 2018;36(45):6883–6891.
- Slotved HC, Facklam RR, Fuursted K. Assessment of a novel bile solubility test and MALDI-TOF for the differentiation of Streptococcus pneumoniae from other mitis group streptococci. Sci Rep. 2017;7(1):7167.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 27th ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2017.
- Ceyhan M, Aykac K, Gurler N, et al. Serotype distribution of Streptococcus pneumonia in children with invasive disease in Turkey: 2015-2018. Hum Vaccin Immunother. 2020;16(11):2773–2778. Erratum in: Hum Vaccin Immunother. 2021;17(7):2352.
- Ceyhan M, Ozsurekci Y, Gürler N, et al. Serotype distribution of Streptococcus pneumoniae in children with invasive diseases in Turkey: 2008-2014. Hum Vaccin Immunother. 2016;12(2):308–313.
- Geno KA, Gilbert GL, Song JY, et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28(3):871–899. Erratum in: Clin Microbiol Rev. 2020;34(2).
- Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10d, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio. 2020;11(3):e00937–e01020.
- Liñares J, Ardanuy C, Pallares R, et al. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. 2010;16(5):402–410.
- Furuya Y, Yamagishi Y, Okade H, et al. Impact of the pneumococcal conjugate vaccine on serotype distribution of adult non-invasive Streptococcus pneumoniae isolates in Tokai region, Japan, 2008-2016. J Infect Chemother. 2017;23(6):394–399.
- Stanek RJ, Norton NB, Mufson MA. A 32-year study of the effect of pneumococcal vaccines on invasive Streptococcus pneumoniae disease. Am J Med Sci. 2016;352(6):563–573.
- Hauser C, Kronenberg A, Allemann A, et al. Serotype/serogroup-specific antibiotic non-susceptibility of invasive and non-invasive Streptococcus pneumoniae, Switzerland, 2004 to 2014. Euro Surveill. 2016;21(21):1–10.
- European Centre for Disease Prevention and Control (ECDC). Invasive pneumococcal disease - annual epidemiological report for 2016; 2016 [accessed 2022 May 25]. Available from: https://www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2016
- Cui YA, Patel H, O'Neil WM, et al. Pneumococcal serotype distribution: a snapshot of recent data in pediatric and adult populations around the world. Hum Vaccin Immunother. 2017;13:1–13.
- U.S. Food and Drug Administration; [accessed 2022 May 25]. Available from: https://www.fda.gov/
- European Medicines Agency; [accessed 2022 May 25]. Available from: https://www.ema.europa.eu/en
- de Roux A, Schmöle-Thoma B, Siber GR, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis. 2008;46(7):1015–1023. Erratum in: Clin Infect Dis. 2008;46:1488.