Abstract
Background
Patients with walled-off necrosis (WON) are still challenging to treat safely and effectively. Recently, double-pigtail plastic stents (DPS), bi-flanged metallic stents (BFMS), and lumen-apposing metal stents (LAMS) have been employed with endoscopic ultrasound-guided (EUS-guided) drainage. However, there is little solid evidence to support the effectiveness and safety of using stents. This study aims to compare the outcomes of the LAMS and the PS.
Method
Till July 2022, a thorough database search was done, and studies that met the criteria were chosen. By using the RevMan software, the technical and clinical success and other secondary outcomes were calculated. Subgroup analysis was performed between the LAMS and the BFMS.
Results
Fifteen studies (two randomized controlled trials and thirteen observational) with 687 patients receiving metal stents and 771 patients receiving plastic stents were selected for final analysis. There was no significant risk of bias or publication bias. The odds ratios (OR) for technical and clinical success were 0.36 (95% confidence interval (95% CI) 0.08, 1.52) and 2.26 (95%CI 1.62, 3.15), respectively. The OR for overall adverse events was 0.74 (95% CI 0.41, 1.34). In subgroup analysis, the LAMS and the BFMS showed the same outcomes.
Conclusion
Compared to DPS, LAMS had better clinical outcomes and fewer side effects when treating patients with WON.
Key Messages
What is the best endoscopic treatment option for the walled-off necrosis (WON)?
A brief comparison of lumen-apposing metal stents (LAMS), bi-flanged metallic stents (BFMS) and double-pigtail plastic stents (DPS) for the treatment of WON patients.
How can we limit the adverse events and provide better treatment.
Introduction
Pancreatic fluid collection (PFC) is a major problem for patients suffering from chronic pancreatic inflammation. Pancreatic pseudocysts (PP) and walled-off necrosis (WON) are examples of PFC that have lasted longer than 4 weeks in a patient. On the other hand, examples of pancreatic fluid that is <4 weeks old include acute peri-pancreatic and acute post-necrotic fluid collections. WON has a death rate of between 8% and 39%, which can happen to about 20% of people with severe pancreatitis [Citation1,Citation2]. The majority of PFCs can dissolve naturally. Abdominal discomfort, an infection, or an obstruction of the biliary or gastric tract necessitates the drainage of pancreatic fluid [Citation3]. Therefore, a multidisciplinary approach, including surgery, radiology and endoscopy, is typically required to treat PFCs, especially WONs. Drainage of PFC utilizing transmural stents guided by endoscopic ultrasonography [Citation4] has been the standard treatment method since 1996 [Citation3,Citation5,Citation6]. For WON, a mature encapsulated collection of pancreatic necrosis that contains both liquid and solid components, transmural drainage is insufficient and endoscopic necrosectomy may be required [Citation7–10].
EUS-guided drainage can utilize a variety of stents, including double-pigtail plastic stents (DPS), self-expanding metallic stents, and lumen-apposing metal stents (LAMS). DPS has been the therapeutic choice for endoscopic treatment of WON to access and drain PF. But these stents have several drawbacks, such as ineffective drainage, secondary infection, bleeding, perforation and the requirement of tract balloon-dilation for endoscopic necrosectomy for the endoscope to enter the collection. Additionally, these stents increase the risk of bleeding during endoscopic necrosectomy [Citation11–15]. Since LAMS are so simple to use and offer obvious benefits, including a wide path for direct endoscopic necrosectomy and drainage, they have largely replaced traditional methods of treating PFCs [Citation16]. Multiple studies have shown that LAMS is either more effective than DPS or is equally effective. These trials support that LAMS is effective, showing similar adverse outcomes as DPS. However, there is limited information from RCT studies, and LAMS are more expensive.
This meta-analysis was conducted to determine whether LAMS is safe and effective for draining PFC in WON patients. The studies that compared the outcomes of both WON and PP or PP only were not included in this analysis.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines were followed in this systematic review and meta-analysis [Citation17].
Search strategy
We searched medical databases such as PubMed, Web of Science, and the Cochrane library using various word combinations such as ‘endoscopic ultrasound-guided drainage’, ‘stents’, ‘plastic stent’, ‘lumen-apposing metal stent’, ‘PFCs’ and ‘WON’. Our search was conducted up until July 2022. The investigation was restricted to only human subjects and the English language. Two different authors were responsible for collecting all of the relevant references, and disagreements were settled through discussion.
Studies selection
The following criteria were used to determine which studies should be included and which should be eliminated.
Inclusion criteria
Studies comparing the outcomes of metal stents and plastic stents in EUS-guided drainage.
Research involving only patients diagnosed with WON.
Full-text studies comparing technical and clinical success.
Human subject studies in the English language.
Exclusion criteria
Case reports, abstracts, reviews and letters were not included.
Studies comparing other PFCs, such as PP or a combination of PP and WON.
Single-arm studies such as reporting only metal stents or plastic stent outcomes.
Studies with incomplete results or missing technical and clinical success.
Articles are not published in the English language.
Research in the field of paediatrics.
Animal studies.
Data extraction
Two writers independently collected study data based on inclusion criteria. Each study’s name, year of publication, study design, place of study, total number of patients in each arm, mean age of patients, male and female percentage, mean size of PFC, technical and clinical success, mean number of procedure sessions, mean procedure time, hospital stay, total adverse events (AEs), bleeding, perforation, stent migration and occlusion and infection were collected. Different coefficients were rescaled. Each study had LAMS/BFMS and DPS arms.
Outcomes and definitions
Our primary outcomes were technical success (TS) and clinical success. TS is defined as successful stent installation and access to the PFC site. In contrast, clinical success is defined as a reduction in the volume of the PFC and an improvement in the clinical symptoms. The secondary outcomes were the average number of procedure sessions, mean procedure time, AEs (infection, bleeding, perforation and stent migration or occlusion), hospital stay, mortality and the requirement for surgical necrosectomy. In subgroup analysis, studies were divided into LAMS and BFMS subgroups.
Risk of bias assessment of included studies
We used Cochrane risk of bias assessment tools for RCT studies, whereas non-RCT studies were assessed by non-RCT studies’ risk of bias assessment tools. When a study had a low risk of bias, a ‘low’ grade was assigned; ‘some concern’ for a moderate risk of bias or information reported was insufficient to make a risk of bias decision; and ‘high’ for higher risk of bias [Citation18].
Publication bias and study effect
Using a funnel plot, this study identified publication bias. Data on the funnel plot appeared symmetrical, indicating no publishing bias. Each study’s effect on the overall result was evaluated by first deleting each study individually and then analysing how this changed the overall result.
Statistical analysis
We used the Cochrane Review Manager Software (version 5.4.1) to calculate odds ratios (ORs) and pooled mean differences for outcomes. We used the Mantel-Haenszel random effect model for dichotomous data to calculate OR with their 95% confidence interval (95% CI). On the other hand, we used an inverse variance statistical method and a random effect model to determine the pooled mean difference between the two groups for continuous data. For the heterogeneity calculation, chi-square tests and I2 statistics were used. I2 values between 0% and 25% were considered potentially insignificant, 25%–49% were considered lower heterogeneity, 50%–74% were regarded as moderate heterogeneity, and values over 75% were considered higher heterogeneity. A P-value <0.05 was considered statistically significant [Citation19].
Results
Study selection
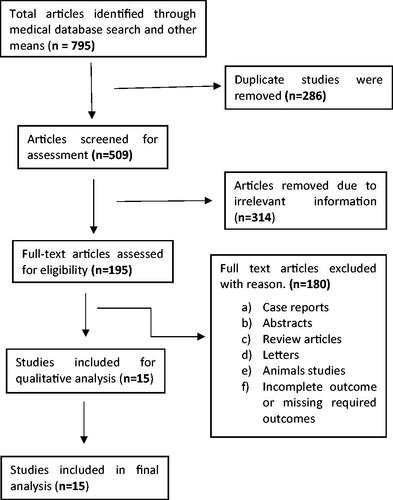
Medical databases and other resources yielded 795 articles. After reading topics and abstracts to remove duplicate and irrelevant articles, 195 articles were evaluated for eligibility. After removing abstracts, case reports, reviews, letters, animal studies, and incomplete or missing outcome studies, 15 studies were selected. The flow chart shows that 15 publications were chosen for the final meta-analysis ( flow chart).
Studies characteristics
A total of 1458 patients were included in the selected studies, of which 687 patients received metal stents (512 received LAMS and 175 received BFMS), while 771 patients underwent plastic stents. Two RCTs [Citation20,Citation21] and thirteen observational studies [Citation22–34] compared the LAMS/BFMS and DPS outcomes of patients with WON. Seven studies were carried out in the USA [Citation20,Citation22–24,Citation29,Citation30,Citation34], three in India [Citation27,Citation28,Citation33] and one each in Japan [Citation26], Saudi Arabia [Citation25], Sweden [Citation31], the Netherlands [Citation21] and China [Citation32]. and display all study characteristics. WON aetiologies include alcohol, gallstones, idiopathic diseases, hypertriglyceridaemia, post-ERCP, trauma and drugs; 7–10 Fr DPS were used. Most study utilized HOT AXIOS (Boston Scientific, USA) LAMS, SPAXUS (Taewoong Medical, Korea), NAGI (Niti-S, Taewoong Medical CO, Korea), and Micro-Tech (Micro-Tech CO, China).
Table 1. Characteristics of included studies.
Table 2. Characteristics of included studies.
Risk of bias and Publication bias
Two RCTs were low-risk, while 13 observational studies were moderate-risk (Table S1). All studies and outcomes had moderate bias. A funnel plot of outcome data was used to assess publication bias. Our study found symmetric funnel plots. Each study’s impact on the outcome was examined by eliminating it. All the studies had little effect, and the ultimate result was unaffected (supplementary Funnel plots). If a study’s outcome changed significantly, we would exclude it from the analysis.
Primary outcomes
Technical success
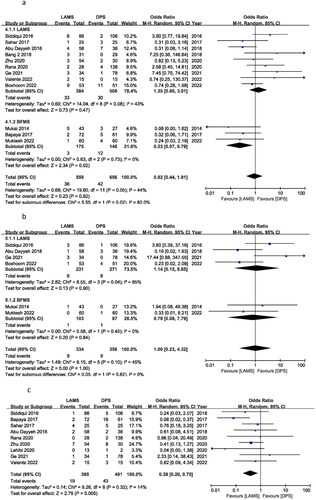
Ten out of fifteen studies reported 100% TS for both groups (as shown in the TS column). Only five studies reported different TS and were utilized to calculate the TS OR between LAMS and DPS. The OR of TS was 0.36 (95% CI 0.08–1.52), I2 = 20% and P = 0.16. This shows that OR of TS for LAMS and DPS was not significantly different with no heterogeneity between the two groups (). That means TS is almost same for both LAMS and DPS. This difference may be related to difficulty to implant the stent due to different location and anatomy of the patients.
Figure 2. Forest plot for (a) TS, (b) clinical success and (c) total AEs.
Clinical success
Clinical success OR was calculated to use all 15 studies. Clinical success OR was 2.26 (95% CI 1.62–3.15), I2 = 3%, P = 0.00001. There was a significant difference in clinical success between the LAMS and the DPS groups for treating WON patients. In a subgroup analysis of LAMS and BFMS, 12 studies reported an OR of 2.00 (95% CI 1.41, 2.85), I2 = 0%, P = 0.0001 and three studies reported an OR of 4.54 (95% CI 1.98, 10.41), I2 = 0%, P = 0.0004, respectively. There was no significant difference in TS between the BFMS and the LAMS ().
Secondary outcomes
Adverse events
The OR for overall AEs was 0.74 (95% CI 0.41, 1.34), I2 = 74%, P = 0.32. LAMS and DPS had the same number of AEs. In the subgroup, LAMS had an OR of 0.97 (95% CI 0.52, 1.82) I2 = 72%, P = 0.93, while BFMS had an OR of 0.24 (95% CI 0.09, 0.62) I2 = 39%, P = 0.003. Subgroup results show fewer AEs for BFMS. Only three trials were published for BFMS, and Bapaya et al. considerably affected the final result of the subgroup analysis ().
Bleeding
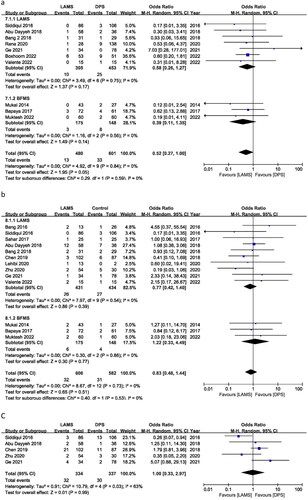
For PFC, bleeding is the most frequent AE. Twelve studies reported bleeding, with an OR of 0.92 (95% CI 0.44, 1.91) I2 = 44%, P = 0.82. For bleeding cases, there is no significant difference between DPS and LAMS. According to subgroup analysis, the ORs were 1.35 (95% CI 0.60, 3.01), I2 = 43%, P = 0.47 and 0.23 (95% CI 0.07, 0.79), I2 = 0%, P = 0.02 for LAMS and BFMS, respectively. LAMS has higher bleeding cases than BFMS. But maybe this is related to the drainage’s position, age and duration ().
Perforation
Six trials reported perforation incidents with OR = 1.00 (95% CI: 0.23, 4.32), I2 = 45%, and P = 1.00. LAMS and DPS perforation cases were similar. BFMS had an OR of 0.79 (95% CI 0.08, 7.79), I2 = 0%, P = 0.84, while LAMS had an OR of 1.14 (95% CI 0.15, 8.85), I2 = 65% and P = 0.90. LAMS and BFMS have similar perforation rates ().
Infection
Nine studies reported infection events and it showed an OR of 0.38 (95% CI 0.20, 0.75), I2 = 14%, P = 0.005. Infection cases are significantly lower in the LAMS group compared with BFMS. No subgroup analysis was performed between the LAMS and the BFMS ().
Mortality
During follow-up, 10 studies reported patient deaths. The OR was 0.52 (95% CI: 0.27, 1.00), I2 = 0%, and P = 0.05. The DPS group resulted in more patient deaths than the LAMS group. In contrast, BFMS showed an OR of 0.39 (95% CI 0.11, 1.35), I2 = 0% and P = 0.14, and LAMS showed an OR of 0.58 (95% CI 0.26, 1.27), I2 = 0% and P = 0.17. In subgroup analysis, there was no mortality difference between BFMS and LAMS ().
Stent migration
Stent migration occurrences were recorded in thirteen studies. Stent migration did not differ between the LAMS and the DPS groups according to the OR of 0.83 (95% CI 0.48, 1.44), I2 = 0%, and P = 0.51. In subgroup analysis, BFMS produced an OR of 1.22 (95% CI 0.33, 4.49), I2 = 0%, and P = 0.77, while LAMS showed an OR of 0.77 (95% CI 0.42, 1.40), I2 = 0%, and P = 0.39. Stent migration incidents between BFMS and LAMS were the same ().
Stent occlusion
Stent occlusion cases were recorded in five studies. The OR for stent occlusion was 1.00 (95% CI 0.33, 2.97), I2 = 63%, and P = 0.99. Between the LAMS and the DPS groups, there were no discernible differences in the number of stent occlusion occurrences ().
Recurrence of WON
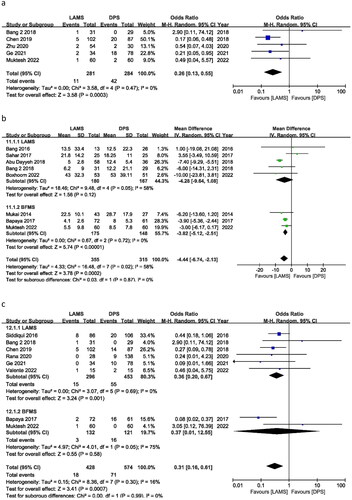
Five studies reported recurrences of WON events producing OR of 0.26 (95% CI 0.13, 0.55), I2 = 0% and P = 0.0003. OR indicated a significant difference in recurrences between the LAMS and the DPS groups in favour of LAMS ().
Hospital stay
Eight trials documented stent-related hospital stays. The pooled mean difference between LAMS and DPS was −5.11 days (CI: –7.09, −3.14), I2 = 0% and P = 0.00001. The LAMS subgroup had a shorter hospital stay. In the subgroup, LAMS exhibited a mean difference of −7.31 days (95% CI −9.13, −5.49), I2 = 0% and P = 0.00001; BFMS showed a mean difference of −3.82 days (95% CI − = 0.00001; BFMI2 = 0% and P = 0.00001. The LAMS and BFMS hospitalizations were very different. LAMS demonstrated shorter hospital stays than DPS and BFMS ().
Surgical necrosectomy
In eight trials, surgical necrosectomy was required; the OR was 0.31 (95% CI 0.16, 0.61), I2 = 16% and P = 0.0007. LAMS and DPS have different rates of necrosectomy. This is because most of patients in LAMS can perform endoscopic necrosectomies and there was no need for additional surgical necrosectomy whereas in DPS group unable to perform endoscopic necrosectomy, so they need surgical necrosectomy. That is why fewer patients in the LAMS group need surgical necrosectomy and OR was significantly in the favour of the LAMS group. Subgroup analyses of the LAMS and BFMS studies revealed substantially different ORs of 0.36 (95% CI 0.20, 0.67) I2 = 0% and P = 0.001 and 0.37 (95% CI 0.01, 12.55) with 75% I2 and P = 0.58. LAMS has fewer necrosectomies than BFMS and DPS ().
Mean number of procedures for PFC drainage
Ten investigations compared LAMS and DPS drainage processes. Seven studies reported a pooled mean difference of −0.41 (95% CI: −0.70, −0.12), I2 = 56% and P = 0.005. In subgroup analysis, the BFMS showed a pooled mean difference of −1.09, −0.30), I2 = 75% and P = 0.58, whereas the LAMS showed a mean difference of −0.25 (95% CI-0.51, 0.01), I2 = 20% and P = 0.06. LAMS has fewer drainage interventions than DPS and BFMS. Regarding the BFMS, data from only two studies were used, and the findings of Bapaya et al. have a higher influence on the outcome. When drawing any conclusions based on the findings of this investigation, need to keep this impact in mind (Fig. S1a).
Mean procedure time
Five studies reported the procedure’s average time for the implantation of the stents. The pooled mean difference was statistically significant in favour of the LAMS, −26.16 min (95% CI −28.68, −23.65) I2 = 0% and P < 0.00001. The LAMS group took less time than the DPS group to complete the stent implantation (Fig. S1b).
Discussion
This systematic review and meta-analysis compared the safety and efficacy of EUS-guided drainage using the LAMS/BFMS and the DPS for WON patients. The LAMS has a higher clinical success rate than the DPS, similar AEs, a shorter hospital stay, shorter procedure duration and fewer procedure sessions. Only two of the 15 studies were randomized controlled trials (RCTs). Thirteen were observational. This is the first meta-analysis comparing LAMS and DPS for WON drainage. It also compares BFMS and LAMS subgroups.
EUS-guided PFC drainage is the standard treatment for WON. In a RCT of patients with infected necrotizing pancreatitis, endoscopic necrosectomy was associated with reduced morbidity compared with surgical necrosectomy [Citation4]. A systematic review and meta-analysis of nine studies of WON comparing the outcomes of the LAMS and the DPS groups suggested that LAMS showed higher clinical success and comparable AEs. But they included two abstract studies in the meta-analysis [Citation35]. In contrast, we had our meta-analysis’s 15 most recent full-text articles and independently compared LAMS and BFMS outcomes in a subgroup analysis. Studies comparing BFMS and LAMS for WON treatment have shown that the two methods produce similar results with fewer AE [Citation36,Citation37]. Our subgroup analysis also showed the same outcome results for the LAMS and the DPS, but only three studies reporting the outcome of the BFMS compared with the DPS were included in our analysis. Such small data is not adequate for final results. Another meta-analysis by Mohan et al. [Citation38] The LAMS and the DPS showed 88.5% and 88.1% clinical success, respectively. According to the author, there was no distinction between the LAMS and the DPS regarding the clinical success or AEs when treating WON. However, our analysis revealed that the LAMS had superior clinical outcomes, shorter hospital stays, and similar AEs. In contrast to this meta-analysis, we only included WON studies that compared LAMS and DPS outcomes. We did not include any studies that used a single control group. Recent research made use of advanced and improved LAMS was included. Maybe these are the aspects that make our analysis different from other studies.
A number of meta-analyses have demonstrated the LAMS’s superiority over the PFC’s DPS. A recent meta-analysis comparing 13 studies reporting outcomes of the LAMS and DPS for PFC suggested that both groups have higher technical and clinical success, but LAMS showed lower AEs. Compared with our study, this meta-analysis included patients with pancreatic fluids [Citation39]. Another case series and meta-analysis by Li et al. [Citation40] also reported that for PFC, LAMS showed preferable clinical success and AEs. A meta-analysis of eleven studies comparing the data of 688 patients with PFC through the LAMS and the DPS indicated that the LAMS showed better efficacy in managing PFC [Citation41]. Another meta-analysis comparing 15 studies with PFC suggests that LAMS has higher clinical success, fewer recurrences and fewer additional interventions [Citation42]. Our analysis also confirmed same WON’s results. First, we compared LAMS and BFMS separately. In overall analysis, LAMS and BFMS were regarded the same kind, however in subgroup analysis, they were separated. Many studies have shown that the LAMS is safer and more effective for bile collection for biliary and gallbladder illnesses [Citation43–46]. Single-arm studies also showed LAMS are safe and effective. A recent study found no difference between 10, 15 and 20 mm LAMS for PFC [Citation47]. Another study also indicated that endoscopic drainage of WON patients is effective and less invasive than surgery [Citation48].
LAMS’s direct necrosectomy channel is its main advantage over DPS. The larger LAMS diameter allows for faster and more spontaneous direct draining into the stomach or duodenum, allowing necrosectomy without extra intervention. This improves patient comfort, speeds recovery, and shortens hospital stays. The improved LAMS has a simple and quick deployment system. Necrosectomy demands more time and effort for DPS and need to perform surgical necrosectomy. Only four studies reported data about the additional percutaneous drainage method but they did not explain any impact of this additional drainage method on the final outcome. To elaborate this impact more studies are needed more focussing this aspect. Our meta-analysis also showed that OR for surgical necrosectomy was in the favour of the LAMS that mean less patients in the LAMS group need surgical necrosectomy. Only two RCTs comparing WON treatments have been described. Surprisingly, the LAMS did not exhibit the expected clinical success advantage over the DPS. Both RCTs found different AEs. In meta-analyses, LAMS did better than DPS. Most of the studies included in meta-analyses are observational studies with various follow-up lengths, PFC sizes, stent counts and recording methods. RCT studies must follow a predetermined protocol to track outcomes and endpoints. More RCTs are needed to provide more precise and accurate data.
Stent related AEs were almost same for both the LAMS and DPS. The large lumen of LAMS made it possible to rapidly drain the liquid component but left the solid component in the cavity, where it eventually caused stent obstruction; and the larger number of side holes in PS meant that the stent could not be completely blocked. Stent obstruction can occur when a large quantity of solid debris is discharged through a large lumen LAMS and some of the solid components accumulate in the stent. So, PS placement in LAMS has been suggested as a way to reduce complications caused by stents. According to a study by Brimhall et al. [Citation49], postoperative complications of LAMS were found to account for 24.7% of cases, with stent-related haemorrhage accounting for 15.5% of these cases and pseudoaneurysm haemorrhage accounting for ∼8.2% of these cases. Further prospective studies are required to accurately characterize patients who have LAMS-associated bleeding. But it is possible that the presence of pseudoaneurysms could raise the risk of bleeding caused by LAMS.
Most of the included articles are observational studies, not RCTs. More RCTs are needed to assess WON treatments’ safety and efficacy. Different research defines therapy effectiveness differently, which could influence our real outcome and primary purpose. Only three BFMS-reporting trials were considered for subgroup analysis. These results do not prove the BFMS. More research is needed to compare the LAMS and BFMS’ efficacy and safety. The included studies have varying follow-up lengths. We didn’t assess how long researchers followed participants. Specific experiments used hydrogen peroxide irrigation, nasocystic drainage, percutaneous drainage and antibiotics, which may have altered results. So, more studies focussing these impacts are needed. Methodological restrictions must be considered.
Conclusion
When comparing the LAMS to the DPS for the treatment of WON, this meta-analysis of fifteen studies found that the LAMS is associated with superior clinical outcomes, shorter hospital stays and procedure times, fewer interventions, and equivalent AEs. Results from subgroup analysis showed no difference between the BFMS and LAMS.
Approval and consent to publish
All authors have approved to this submission to your esteemed journal. Its publication is also approved tacitly by the responsible authorities where the work was carried out.
Informed consent statement
Not applicable.
Institutional review board statement
Not applicable.
Author contributions
All authors contributed to study design, critically reviewed the first draft, approved the final version and agreed to be accountable for the work.
Khizar Hayat: Wrote the original Manuscript.
Software usage and Calculations: Le Chenyu, Bian Ying.
Study Search and Data Collection: Hu Yufei, Wu Yanhua.
Figure edition and manuscript Revision: Chen Wangyang, Huang Zhicheng, Kamran Ali.
Jianfeng Yang: Provided article ideas and article review.
Supplemental Material
Download MS Word (1.2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The supporting data of this study is available in supplementary materials and more information can be found on request from the corresponding author (YJF), upon reasonable request.
Additional information
Funding
References
- Zaheer A, Singh VK, Qureshi RO, et al. The revised atlanta classification for acute pancreatitis: updates in imaging terminology and guidelines. Abdom Imaging. 2013;38(1):125–136.
- Tenner S, Baillie J, DeWitt J, American College of Gastroenterology, et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–1415; 1416.
- Gerolami R, Giovannini M, Laugier R. Endoscopic drainage of pancreatic pseudocysts guided by endosonography. Endoscopy. 1997;29(2):106–108.
- Bakker OJ, van Santvoort HC, van Brunschot S, Dutch Pancreatitis Study Group, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307(10):1053–1061.
- Panamonta N, Ngamruengphong S, Kijsirichareanchai K, et al. Endoscopic ultrasound-guided versus conventional transmural techniques have comparable treatment outcomes in draining pancreatic pseudocysts. Eur J Gastroenterol Hepatol. 2012;24(12):1355–1362.
- Fabbri C, Luigiano C, Maimone A, et al. Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J Gastrointest Endosc. 2012;4(11):479–488.
- Gardner TB, Coelho-Prabhu N, Gordon SR, et al. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73(4):718–726.
- Banks PA, Bollen TL, Dervenis C, Acute Pancreatitis Classification Working Group, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111.
- Gardner TB, Chahal P, Papachristou GI, et al. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69(6):1085–1094.
- Baron TH, Thaggard WG, Morgan DE, et al. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111(3):755–764.
- Krüger M, Schneider AS, Manns MP, et al. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63(3):409–416.
- Lopes CV, Pesenti C, Bories E, et al. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42(4):524–529.
- Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video). Gastrointest Endosc. 2008;68(4):656–666.
- Seewald S, Ang TL, Kida M, EUS 2008 Working Group, et al. EUS 2008 working group document: evaluation of EUS-guided drainage of pancreatic-fluid collections (with video). Gastrointest Endosc. 2009;69(2 Suppl):S13–S21.
- Lang GD, Fritz C, Bhat T, et al. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc. 2018;87(1):150–157.
- Nayar M, Leeds JS, Oppong K, UK & Ireland LAMS Colloborative Lumen-apposing metal stents for drainage of pancreatic fluid collections: does timing of removal matter? Gut 2022;71(5):850–853.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10: ed000142.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- Bang JY, Navaneethan U, Hasan MK, et al. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut. 2019;68(7):1200–1209.
- Boxhoorn L, Verdonk RC, Voermans RP, et al. Comparison of lumen-apposing metal stents versus double-pigtail plastic stents for infected necrotising pancreatitis. Gut. 2023;72(1):66–72.
- Bang JY, Hasan MK, Navaneethan U, et al. Lumen-apposing metal stents for drainage of pancreatic fluid collections: when and for whom? Dig Endosc. 2017;29(1):83–90.
- Abu Dayyeh BK, Mukewar S, Majumder S, et al. Large-caliber metal stents versus plastic stents for the management of pancreatic walled-off necrosis. Gastrointest Endosc. 2018;87(1):141–149.
- Chen Y-I, Yang J, Friedland S, et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: a large international multicenter study. Endosc Int Open. 2019;7(3):E347–e354.
- Al Lehibi A, Al Jabri A, Abbarh S, et al. Peripancreatic fluid collections, plastic stents, and different Sub-types of metal stents: where does the evidence land? Saudi J Gastroenterol. 2021;27(2):85–90.
- Mukai S, Itoi T, Baron TH, et al. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: a retrospective single-center series. Endoscopy. 2015;47(1):47–55.
- Muktesh G, Samanta J, Dhar J, et al. Endoscopic ultrasound-guided drainage of patients with infected walled-off necrosis: which stent to choose? Surg Laparosc Endosc Percutan Tech. 2022;32(3):335–341.
- Rana SS, Sharma R, Dhalaria L, et al. Efficacy and safety of plastic versus lumen-apposing metal stents for transmural drainage of walled-off necrosis: a retrospective single-center study. Ann Gastroenterol. 2020;33(4):426–432.
- Siddiqui AA, Kowalski TE, Loren DE, et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc. 2017;85(4):758–765.
- Sahar N, Kozarek R, Kanji ZS, et al. Do lumen-apposing metal stents (LAMS) improve treatment outcomes of walled-off pancreatic necrosis over plastic stents using dual-modality drainage? Endosc Int Open. 2017;5(11):E1052–e1059.
- Valente R, Zarantonello L, DEL Chiaro M, et al. Lumen apposing metal stents vs double pigtail plastic stents for the drainage of pancreatic walled-off necrosis. Minerva Gastroenterol. 2022 (Online ahead of print).
- Zhu H, Xie P, Wang Y, et al. The role of solid debris in endoscopic ultrasound-guided drainage of walled-off necrosis: a large cohort study. J Gastroenterol Hepatol. 2020;35(12):2103–2108.
- Bapaye A, Dubale NA, Sheth KA, et al. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig Endosc. 2017;29(1):104–110.
- Ge PS, Young JY, Jirapinyo P, et al. Comparative study evaluating lumen apposing metal stents versus double pigtail plastic stents for treatment of Walled-Off necrosis. Pancreas. 2020;49(2):236–241.
- Calo NC, Bishay K, Yaghoobi M, et al. Comparative effectiveness of Lumen-Apposing metal stents and plastic stents for the treatment of pancreatic Walled-Off necrosis: a meta-analysis. J Can Assoc Gastroenterol. 2022;5(2):68–78.
- Bekkali NLH, Nayar MK, Leeds JS, et al. A comparison of outcomes between a lumen-apposing metal stent with electrocautery-enhanced delivery system and a bi-flanged metal stent for drainage of walled-off pancreatic necrosis. Endosc Int Open. 2017;5(12):E1189–e1196.
- Siddiqui A, Naveed M, Basha J, et al. International, multicenter retrospective trial comparing the efficacy and safety of bi-flanged versus lumen-apposing metal stents for endoscopic drainage of walled-off pancreatic necrosis. Ann Gastroenterol. 2021;34(2):273–281.
- Mohan BP, Jayaraj M, Asokkumar R, et al. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents? A systematic review and meta-analysis of studies published since the revised Atlanta classification of pancreatic fluid collections. Endosc Ultrasound. 2019;8(2):82–90.
- Guzmán-Calderón E, Chacaltana A, Díaz R, et al. Head-to-head comparison between endoscopic ultrasound guided lumen apposing metal stent and plastic stents for the treatment of pancreatic fluid collections: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2022;29(2):198–211.
- Li J, Zhang Q, Zhou A, et al. Comparative outcomes of endoscopic ultrasound-guided lumen-apposing mental stents drainage for pancreatic pseudocysts and walled-off necrosis: case series and meta-analysis. Chronic Dis Transl Med. 2021;7(3):157–168.
- Hammad T, Khan MA, Alastal Y, et al. Efficacy and safety of Lumen-Apposing metal stents in management of pancreatic fluid collections: are they better than plastic stents? A systematic review and Meta-Analysis. Dig Dis Sci. 2018;63(2):289–301.
- Chen L, Li T, Wang B, et al. Endoscopic versus percutaneous drainage for pancreatic fluid collection after pancreatic surgery: an up-to-date meta-analysis and systematic review. Asian J Surg. 2022;45(8):1519–1524.
- Hakim S, Khan Z, Shrivastava A, et al. Endoscopic gastrointestinal anastomosis using lumen-apposing metal stent (LAMS) for benign or malignant etiologies: a systematic review and Meta-Analysis. J Clin Gastroenterol. 2021;55(7):e56–e65.
- Pinto-Carta R, Solano J, Cabrera LF, et al. Endoscopic ultrasound (EUS) guided choledocoduodenostomy in a patient with irresecable pancreas cancer and biliar obstruction using a luminal apposing metal stent (LAMS). Int J Surg Case Rep. 2020;74:23–26.
- Krishnamoorthi R, Dasari CS, Thoguluva Chandrasekar V, et al. Effectiveness and safety of EUS-guided choledochoduodenostomy using lumen-apposing metal stents (LAMS): a systematic review and meta-analysis. Surg Endosc. 2020;34(7):2866–2877.
- Ginestet C, Sanglier F, Hummel V, et al. EUS-guided biliary drainage with electrocautery-enhanced lumen-apposing metal stent placement should replace PTBD after ERCP failure in patients with distal tumoral biliary obstruction: a large real-life study. Surg Endosc. 2022;36(5):3365–3373.
- Anderloni A, Fabbri C, Nieto J, et al. The safety and efficacy of a new 20-mm lumen apposing metal stent (lams) for the endoscopic treatment of pancreatic and peripancreatic fluid collections: a large international, multicenter study. Surg Endosc. 2021;35(4):1741–1748.
- Ebrahim M, Werge MP, Hadi A, et al. Clinical outcomes following endoscopic or video-assisted retroperitoneal management of acute pancreatitis with large (>15 cm) walled-off pancreatic necrosis: retrospective, single tertiary center cohort study. Dig Endosc. 2022;34(6):1245–1252.
- Brimhall B, Han S, Tatman PD, et al. Increased incidence of pseudoaneurysm bleeding with Lumen-Apposing metal stents compared to Double-Pigtail plastic stents in patients with peripancreatic fluid collections. Clin Gastroenterol Hepatol. 2018;16(9):1521–1528.