Abstract
Background
There is a lack of evidence regarding whether combination therapy of hypomethylating agents (HMAs) has better outcomes than HMA monotherapy in patients with Philadelphia chromosome-negative accelerated or blast phase myeloproliferative neoplasms (MPN-AP/BP).
Materials and methods
Pubmed, Embase, Web of Science and Cochrane library databases were searched for studies from inception of each database until 31 December 2021. Data extraction and synthesis were conducted following the PRISMA reporting guideline.
Results
It was found that HMAs plus venetoclax therapy yielded a higher CR/CRi rate than HMAs alone [36% vs 19%, p = .0204] and a higher CR rate than HMAs plus ruxolitinib [22% vs 8%, p = .0313]. HMAs plus ruxolitinib combination showed a higher ORR than HMA monotherapy [45% vs 30%, p = .0395], but there was no improvement in CR/CRi. The one-year and two-year OS rate for patients treated with HMAs plus venetoclx/ruxolitinib demonstrated a trend towards prolonged survival than HMAs alone [HMAs plus venetoclax: 24% vs 11%, p = .1295 and 12% vs 3%, p = .2357; HMAs plus ruxolitinib: 25% vs 11%, p = .0774 and 33% vs 3%, p = .051].
Conclusion
It was confirmed that HMA in combination with venetoclax is an effective and well-tolerated option in MPN-AP/BP patients in pre- as well as post-haematopoietic stem cell transplantation settings. HMA plus ruxolitinib therapy was revealed to be effective in patients with MPN-AP.
Combination therapy with HMAs and venetoclax/ruxolitinib was associated with improved outcomes than HMAs alone in MPN-AP/BP patients.
Further large-scale randomized controlled trials are needed to confirm regarding to the optimal treatment for this patient population.
Key Messages
Introduction
The classical categories of Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) as listed in the 2016 World Health Organization (WHO) classification comprise a spectrum of disorders including primary myelofibrosis, essential thrombocythaemia and polycythaemia vera [Citation1]. In general, the natural pathological process of MPNs can be interrupted by disease transformation into the accelerated or blast phase (MPN-AP/BP) [Citation2,Citation3]. MPN-AP and MPN-BP (or post-MPN acute myeloid leukaemia [post-MPN AML]) are defined based on the percentage of blasts in the bone marrow or peripheral blood, which is ≥10–19% in MPN-AP and 20% or more in MPN-BP [Citation4]. MPN-AP/BP are portentous events and are likely emblematic of the late stages of the evolutionary history of MPNs.
At present, allogeneic haematopoietic stem cell transplantation (allo-HSCT) is the sole treatment modality with long-term survival data [Citation5,Citation6]. However, allo-HSCT is strongly discouraged in patients with MPN-AP/BP due to the poor treatment outcome [Citation7]. Moreover, allo-HSCT is not an optimal option for many patients owing to their advanced age, poor performance status, comorbidities, inability to achieve a reasonable response, or lack of a suitable donor [Citation6,Citation7].
Less intensive regimens, such as, hypomethylating agents (HMAs, including azacytidine and decitabine) combined with or without venetoclax/ruxolitinib, may be effective for post-MPN AML patients [Citation8,Citation9]. HMA-based combination therapy (HMAs plus venetoclax/ ruxolitinib) has significant anti-tumour activity and is being increasingly recognized as an alternative to AML-like therapy for unfit MPN-AP/BP patients, with the exception of higher-risk myelodysplastic syndrome (MDS) and AML [Citation10,Citation11]. Rampal et al. demonstrated an overall response rate (ORR) of 53% and a median overall survival (OS) of 7.9 months in MPN-AP/BP patients treated with combined decitabine and ruxolitinib [Citation12]. A multicentre retrospective study evaluated treatment outcomes of HMAs combined with venetoclax therapy among 32 MPN-BP patients [Citation13]. Complete remission (CR) or CR with incomplete count recovery (CRi) was achieved in 44% of patients, and the median OS was higher compared to controls treated with HMAs alone (8 vs. 5.5 months), but the difference was not statistically significant (p = .3) [Citation13]. However, due to the retrospective nature of the above reports and the limited number of participants, these findings cannot be considered conclusive. To our knowledge, no meta-analysis has been performed to compare the efficacy and safety of combination therapy vs monotherapy of HMAs in MPN-AP/BP patients.
The aim of this meta-analysis was to compare the efficacy and safety of HMAs combined with ruxolitinib or venetoclax to HMAs alone in patients with MPN-AP/BP.
Methods
Literature search strategy
We conducted a literature search for publications written in any language, by screening the Pubmed, Web of Science, Embase and Cochrane library databases, following a literature search strategy of keywords and Boolean operators presented in the Supplement. We also searched abstracts from the American Society of Hematology conferences, American Society of Clinical Oncology, European Hematology Association and European Society of Medical Oncology. A systematic search was performed from inception of each database until 31 December 2021. After removal of duplicates, two authors (J.C. and K.F.W.) independently screened all abstracts and titles against eligibility and exclusion criteria. Thereafter both reviewers assessed the full text of potentially relevant articles and decided whether or not to include them in the meta-analysis. The objectives and methods were predefined in a protocol registered at PROSPERO (CRD42022313523). This study followed the principles outlined in the Declaration of Helsinki and was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-analyses) guidelines [Citation14].
Selection of studies and data extraction
Studies were selected based on the following inclusion criteria: (1) clinical trials and retrospective studies; (2) patients with BCR-ABL negative MPN-AP/BP, or post-MPN AML (no sex or age restrictions); (3) patients treated with HMA monotherapy or combined HMA with ruxolitinib/venetoclax; (4) providing outcome measurements, such as ORR, CR, CRi, partial response (PR), median OS or adverse events (AEs). All selected clinical trials were registered online. Two authors (J.C. and K.F.W.) independently extracted data following a standard format. Any disagreement was resolved through discussion with a third reviewer (Z.F.X.). Extracted information included study characteristics, patient characteristics, interventions, survival data and AEs.
Quality assessment and bias risk
The results of quality assessment and bias risk are presented in the Supplemental information. The Methodological Index for Non-Randomized Studies (MINORS) scale [Citation15] was used to evaluated the quality of all evidence (Supplemental Figure 1) and a heat map (Supplemental Figure 2) was plotted by using the R programming software. According to the MINORS scale, eight methodologic items (1–8) were assigned to the noncomparative studies, and four items (9–12) were designated for the comparative studies. These items were scored as 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). Publication bias of studies (more than 10 studies) was evaluated by symmetry of the funnel plot and Egger’s test (Supplemental Figure 3).
Definition of response and endpoint
The primary outcome of interest was the ORR, defined as a composite of CR, CRi and PR. Given the wide range of publication dates of the included studies and the lack of validated response criteria for MPN-AP/BP [Citation16], definitions used by the individual publications varied slightly but were primarily based on the revised Cheson criteria [Citation17]. Key secondary endpoint included OS and rate of allo-HSCT.
Statistical analysis
The meta-analysis was conducted by using a common (fixed)-effects model according to the assumption of the similar effect size among studies, and the random-effects model was constructed to achieve consistency. All extracted outcome data underwent logarithmic transformation prior to pooling by inverse variance weighting method to improve the reliability of the combined results. As most of the selected studies were single-arm tests, we calculated the single ratio and then the integrated ratio with 95% confidence interval (95% CI) using the R package meta. Statistical heterogeneity between summary results was determined using Cochran’s Q test and I2 indices. The summary odds ratio (OR) and 95% CIs were calculated from the studies that contained data for the control group. Furthermore, we performed the planned subgroup analysis and univariate meta regression analysis to compare effect measurements of different studies based on the type of intervention. Sensitivity analysis was performed for the significant heterogeneity (defined as I2>60%; ≥3 studies) by using leave-one-out method. All analyses were performed using the R statistical software, version 4.1.2.
Results
Literature search
Our literature search strategy identified 637 publications after removal of the duplicates. Based on title and abstract reviews, studies reporting results on disorders other than accelerated or blast phase of MPN or post-MPN AML, review articles, use of HMAs in combination with drugs other than ruxolitinib and venetoclax, case reports, and those presenting insufficient data (less than five patients) were excluded from the analysis. Finally, 27 studies were included in the qualitative synthesis (3 studies are included both in and because they reported the results of combination therapy as well as monotherapy with HMAs). depicts the flow diagram outlining the study selection process. The general characteristics of the patients included in the individual studies selected for this meta-analysis are provided in and .
Figure 1. Flow diagram for selection of studies included in the meta-analysis. *Three studies (Lancman et al. [Citation22] USA, 2018; Yoon et al. [Citation29] USA, 2021; Zhou et al. [Citation10] USA, 2021) reported the results of combination therapy as well as monotherapy of HMAs simultaneously.
![Figure 1. Flow diagram for selection of studies included in the meta-analysis. *Three studies (Lancman et al. [Citation22] USA, 2018; Yoon et al. [Citation29] USA, 2021; Zhou et al. [Citation10] USA, 2021) reported the results of combination therapy as well as monotherapy of HMAs simultaneously.](/cms/asset/26aebd4f-cec0-4c9c-8cc9-a691d4688c9e/iann_a_2164611_f0001_b.jpg)
Table 1. Overview of the efficacy and safety of combination therapy with HMAs in Philadelphia negative MPN-AP/BP.
Table 2. Overview of the efficacy and safety of HMAs monotherapy in Philadelphia negative MPN-AP/BP.
Response to HMAs plus venetoclax vs HMAs alone in MPN-AP/BP
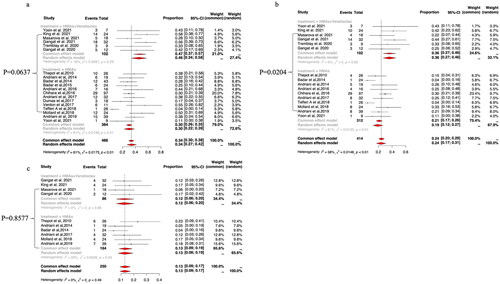
The pooled ORR was higher with HMAs plus venetoclax (47%, 95% CI: 37–57%; I2=18%) than with HMA monotherapy (30%, 95% CI: 22–39%; I2=61%), but the difference was not statistically significant in meta-regression analysis (p = .0637; ). CR/CRi rates were reported by six studies of HMAs plus venetoclax therapy. The pooled CR/CRi rate was 36% (95% CI: 27–46%; I2=0%) for HMAs plus venetoclax and 19% (95% CI: 12–27%; I2=61%) for HMA monotherapy, with the difference reaching statistical significance in meta-regression analysis (p = .0204; ). PR rates were reported by four studies on HMAs plus venetoclax combination therapy. The pooled PR rate was 12% (95% CI: 6–20%; I2=0%) for HMAs plus venetoclax and 13% (95% CI: 9–19%; I2=26%) for HMA monotherapy; the difference was not statistically significant in meta-regression analysis (p = .8577; ).
Figure 2. The pooled ORR (a), CR/CRi (b) and PR (c) rates of HMAs plus venetoclax vs HMAs alone in patients with MPN-AP/BP.
A pre-specified subgroup analysis of patients with MPN-BP revealed that the pooled ORR and PR rates of HMAs plus venetoclax vs HMAs alone did not reach a statistically significant difference in meta-regression analysis (p = .0667 and p = .6190, respectively, Supplemental Figure 4 C a/c). The pooled CR/CRi rate was 37% (95% CI: 27–48%; I2=33%) for HMAs plus venetoclax and 19% (95% CI: 12–27%; I2=58%) for HMAs alone, which reached statistically significant difference in meta-regression analysis (p = .0286; Supplemental Figure 4C b). The data on treatment response in the enrolled studies was insufficient to conduct subgroup meta-analysis in MPN-AP patients.
Response to HMAs plus ruxolitinib vs HMAs alone in MPN-AP/BP
The ORR of pooled studies was 45% (95% CI: 36–54%; I2=0%) for HMAs plus ruxolitinib and 30% (95% CI: 22–39%; I2=61%) for HMAs alone, which reached a statistically significant difference in meta-regression analysis (p = .0395; ). The pooled CR/CRi rate was 31% (95% CI: 22–40%; I2=0%) for HMAs plus ruxolitinib and 19% (95% CI: 12–27%; I2=61%) for HMA monotherapy, but there was no statistically significant difference in meta-regression analysis (p = .1020; ). The pooled PR rate was 18% (95% CI: 8–32%; I2=65%) for HMAs plus ruxolitinib and 13% (95% CI: 9–19%; I2=26%) for HMAs monotherapy, with the difference not reaching a statistical significance in meta-regression analysis (p = .4113; ).
Figure 3. The pooled ORR (a), CR/CRi (b) and PR (c) rates of HMAs plus ruxolitinib (Rux) vs HMAs monotherapy in patients with MPN-AP/BP.
A pre-specified subgroup analysis in patients with MPN-AP revealed a pooled ORR of 56% (95% CI: 32–79%; I2 = 0%) for HMAs plus ruxolitinib and 20% (95% CI: 3–47%; I2 = 63%) for HMAs alone, which reached a statistically significant difference in meta-regression analysis (p = .0464; Supplemental Figure 4A). With respect to MPN-BP, the pooled ORR, CR/CRi and PR rates for HMAs plus ruxolitinib did not show statistically significant differences in meta-regression analysis when compared with HMA monotherapy (Supplemental Figure 4B).
Response to HMAs plus venetoclax vs HMAs plus ruxolitinib in MPN-AP/BP
We conducted subgroup and meta-regression analyses of the efficacy of HMAs plus venetoclax vs HMAs plus ruxolitinib. ORR, CRi and PR rates were similar in studies reporting treatment with HMAs plus venetoclax vs HMAs plus ruxolitinib [47% (95% CI: 37–57%) vs 45% (95% CI: 36–54%), p = .7872, 16% (95% CI: 9–26%) vs 23% (95% CI: 16–31%), p = .2879 and 12% (95% CI: 6–20%) vs 18% (95% CI: 8–32%), p = .4436, respectively; Supplemental Figures 5I, III, IV]. However, the pooled CR rate appeared to be more favourable in patients treated with HMAs plus venetoclax than HMAs plus ruxolitinib [22% (95% CI: 11–35%) vs 8% (95% CI: 3–15%), p = .0313; Supplemental Figure 5II].
Response to azacytidine vs decitabine in MPN-AP/BP
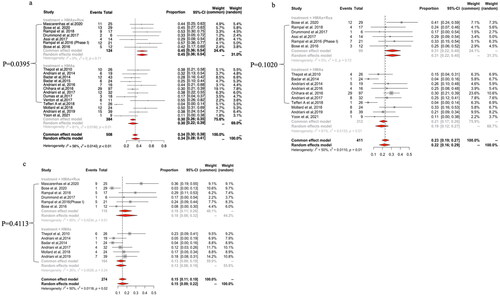
For patients treated with azacytidine or decitabine monotherapy (Supplemental Figure 6), the ORR for all studies was 38% with significant heterogeneity among the studies (Cochran’s Q statistic = 21.1, p = .01; I2 = 57.3%). The ORR was reported by eight studies for azacytidine and two studies for decitabine. The pooled ORR did not show a statistically significantly difference (p = .1362) between azacytidine [42% (95% CI: 31–52%), I2=55%] and decitabine [27% (95% CI: 17–38%), I2=0%].
Overall survival rates at one-year and two-years and rate of allo-HSCT
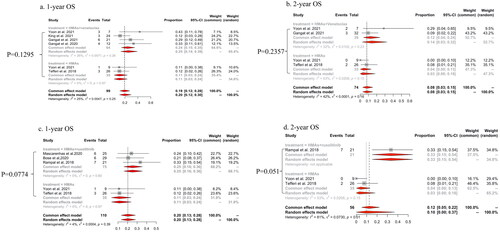
Patients treated with HMAs plus venetoclax demonstrated a trend towards prolonged survival compared to those treated with HMAs alone [pooled one-year OS rates were 24% (95% CI: 15–35%; I2=26%) and 11% (95% CI: 3–24%; I2=0%) respectively; p = .1295, ; pooled two-year OS rates were 12% (95% CI: 4–24%; I2=32%) and 3% (95% CI: 0–18%; I2=53%) respectively; p = .2357; ]. Similarly, patients treated with HMAs plus ruxolitinib showed a trend towards longer survival than HMAs alone [one-year OS rate: 25% (95%CI: 16–36%; I2=68.2%) and 11% (95% CI: 3–24%; I2=31.8%) respectively, p = .0774; two-year OS rate: 33% (95% CI: 15–54%; I2=34.8%) and 3% (95% CI: 0–18%; I2=65.2%), p = .051; ].
Figure 4. The pooled rates of one-year OS and two-year OS of HMAs plus venetoclax vs HMAs alone (a, b) and HMAs plus ruxolitinib vs HMAs alone (c, d) in patients with MPN-AP/BP.
Next, we summarized the proportion of patients with CR/Cri, who underwent allo-HSCT, in each treatment group (Supplemental Figure 7). There was a significant heterogeneity among the various studies, with a Cochran’s Q statistic of 33.45 (p < .001) and an I2 statistic of 72.9%. The rates of received allo-HSCT were 70% (95% CI: 34–95%; I2=62%), 25% (95% CI: 8–48%; I2=0%) and 54% (95% CI: 19–87%; I2=54%) for patients who received HMAs plus venetoclax, HMAs plus ruxolitinib and HMAs alone, respectively, with no statistically significant difference among groups in meta-regression analysis (Supplemental Figure 7).
Adverse events
No data on haematologic AEs were reported by studies in patients treated with HMAs plus venetoclax or HMAs alone. However, three studies had reported haematological AEs in patients treated with HMAs plus ruxolitinib (Supplemental Figure 8). Pooled analysis of the three studies revealed that the most common haematologic AE was neutropenia (30%, 95% CI: 6–64%; I2=82%), followed by thrombocytopenia (25%, 95% CI: 0–70%; I2=90%), lymphopenia (19%, 95% CI: 6–38%; I2=NA) and anaemia (15%, 95% CI: 6–27%; I2=0%). The commonly reported non-haematologic AEs were infection/fever, haemorrhage and thrombus formation (Supplemental Figure 9). We were unable to compare AEs across different treatment groups owing to the significant heterogeneity and limited data availability. In addition, systematic evaluation of whether the AEs were treatment-related or secondary to any underlying comorbidity was not feasible.
Sensitivity analysis
Separate sensitivity analyses for the pooled results of ORR, CR/CRi and PR with significant heterogeneity revealed that omitting any one study did not change the overall effect direction but did lead to a reduction in heterogeneity.
For ORR, the study by Tefferi et al. had the largest influence on heterogeneity [Citation39], in the group examining the use of HMA monotherapy. Removal of this study changed the ORR by 3% (from 32% to 35%), and the higher degree of heterogeneity significantly decreased (Cochran’s Q statistic = 26.63, p = .0052; I2 = 58.7%). Furthermore, removal of this study led to a loss of heterogeneity in the subgroup analysis of studies examining the use of HMA monotherapy in MPN-BP patients (Cochran’s Q statistic = 8.08, p = .3255; I2 = 58.7%). Removal of this study also reduced the CR/CRi by 2% (from 19% to 21%) and led to a loss of heterogeneity among studies examining the use of HMA monotherapy (Cochran’s Q statistic = 14.26, p = .0753; I2 = 43.9%).
Discussion
The outcomes of patients with MPN-AP/BP are generally poor, and therapy for this patient population represents a major unmet need. In the absence of direct comparisons of different treatment strategies due to a lack of randomized controlled trials in this area, it is not possible to suggest an optimal regimen. To the best of our knowledge, this is the first systematic review and meta-analysis on combination therapy vs monotherapy of HMAs in patients with MPN-AP/BP.
This meta-analysis summarizes the currently available evidence on the various therapy regimens in patients with MPN-AP/BP. The available evidence, albeit with low certainty and moderate risk of bias, suggests that the combination of HMAs and venetoclax is associated with a higher rate of CR/CRi than HMAs alone. HMAs plus ruxolitinib showed better ORR than HMA monotherapy, but subgroup analyses demonstrated a significantly lower CR rate than HMAs plus venetoclax. Patients treated with combination therapy showed a trend towards longer survival compared with HMAs alone. These results clearly support HMA-based combination therapy in in patients with MPN-AP/BP.
Data pertaining to efficacy in MPN-AP/BP patients treated with HMAs alone were inconsistent, and evidence of efficacy in comparison to HMAs plus venetoclax therapy remains insufficient. A retrospective study that enrolled 19 MPN-BP patients treated with azacytidine alone, reported an ORR of 26% [Citation30]. However, another retrospective study conducted by Mollard et al. demonstrated an ORR of 50% in patients treated with azacytidine alone [Citation38]. Only a small cohort study has demonstrated that MPN-BP patients receiving HMAs plus venetoclax achieved a higher CR rate compared to HMAs alone (25% vs 4%, p = .048) [Citation41]. In the current analysis, HMAs plus venetoclax therapy was associated with improved efficacy when compared with HMA monotherapy, suggesting that combination therapy has an advantage over monotherapy and should be routinely recommended for MPN-AP/BP patients.
Rampal et al. demonstrated that combination treatment with HMAs and ruxolitinib was well tolerated by MPN-AP/BP patients, with an ORR of 53% [Citation12]. In a multicentre, phase II clinical trial, a 44% ORR was achieved in 25 MPN-AP/BP patients treated with ruxolitinib in combination with decitabine, although response was not associated with improved survival [Citation25]. However, it was unclear whether HMAs plus ruxolitinib therapy was more effective than using HMAs alone. In the meta-regression analysis of the present study, ORR was found to be significantly different between the patients treated with HMAs plus ruxolitinib and those treated with HMAs alone. Our results also revealed that HMAs plus ruxolitinib combination achieved a higher ORR than HMAs alone in patients with MPN-AP. However, it is important to note that only two previous studies have reported ORR in MPN-AP patients treated with HMAs plus ruxolitinib. Therefore, the effect of this combination in patients with MPN-AP needs further investigation.
Two independent, nonrandomized trials demonstrated acceptable safety and high efficacy of HMAs plus venetoclax therapy in AML patients, who were ineligible for intensive chemotherapy [Citation42,Citation43]. To further compare the efficacy of HMAs plus venetoclax and HMAs plus ruxolitinib regimens in patients with MPN-AP/BP, we performed a subgroup analysis which revealed that the venetoclax-based combination had significantly better efficacy compared to HMAs plus ruxolitinib (CR: 22% vs 8%, p = .0313). These results suggest the possibility of a significant association between HMAs plus venetoclax combination treatment and blast count reduction in MPN-AP/BP. Further randomized head-to-head trials are needed to confirm our findings.
Azacytidine and decitabine are widely used in clinical practice, and both have beneficial effects in unfit AML or higher-risk MDS patients [Citation44]. However, only a few trials and retrospective studies have been conducted to directly compare the efficacy of azacytidine and decitabine in patients with MPN-AP/BP. Our meta-analysis included eight studies with 185 patients receiving azacytidine alone, and two studies with 67 patients receiving decitabine alone. The difference in the pooled ORR between azacytidine and decitabine was not statistically significant. Because all of the studies included in this meta-analysis were single-arm tests, there was a low certainty of evidence when comparing azacytidine and decitabine. Therefore, more head-to-head trials are needed in the future.
The goal of care for patients with MPN-AP/BP may still be haematopoietic cell transplantation (HCT) consolidation. Pooled analyses of OS rates in our study showed that patients treated with HMAs plus venetoclax or ruxolitinib tended to survive longer than patients treated with HMAs alone; however, the difference was not statistically significant. This may be explained by the fact that nearly half of the patients who responded to monotherapy with HMAs received allo-HSCT. However, only 25% of the patients who responded to HMAs plus ruxolitinib received transplantation, possibly due to the limited number of enrolled studies on combination therapy. Findings from recent studies corroborate our results that HMAs in combination with venetoclax prolong OS in secondary AML patients [Citation45,Citation46]. Based on the results of our study, the therapeutic option of HMAs combined with venetoclax increases the possibility of allo-HSCT and should be considered as a potential treatment bridge to HCT in a subset of patients.
There was significant heterogeneity in the present meta-analysis due to the small sample size, single-arm nature of most studies and the variety of reported treatment options. However, the heterogeneity did not significantly affect the results of the meta-analysis by sensitivity analyses after accounting for the 'leave-one-out’ method. In comparison to other studies, Tefferi et al. [Citation39] found a lower ORR and CR rate with HMA monotherapy in MPN-AP/BP patients. This can be explained by the fact that the PR rate and number of cycles of HMA treatment were not reported in this study [Citation39]. In addition, most patients (81%) of the cohort had an abnormal karyotype which has been validated as a poor prognostic factor. Excluding the study by Tefferi et al. [Citation39], heterogeneity in the analysis of ORR or CR/CRi rate decreased significantly with HMA monotherapy. This indicates that response to treatment with HMAs alone can be assessed more accurately by strict selection of MPN-AP/BP patients.
Limitations
This meta-analysis had several limitations. First, we had to rely on the response definitions provided by the researchers of the original studies. The degree of heterogeneity decreased but remained in our sensitivity analysis, which argues against a systematic effect of response definitions on our results. Second, since medical nomenclature has evolved over time, we cannot exclude the possibility that studies using terms likes ‘myeloproliferative disorders (MPD)’ instead of ‘myeloproliferative neoplasms or MPN’ might have been left out. However, the term ‘myeloproliferative disorders (MPD)’ has been officially renamed as ‘myeloproliferative neoplasms (MPN)’ since 2008, and HMAs combined with ruxolitinib or venetoclax have gained popularity in the treatment of unfit patients with MPN-AP/BP or AML only in recent years. Thus, it is unlikely that a more extensive search strategy would have changed the overall conclusions of this study.
Conclusions
Our data reflect that combination therapy of HMAs with venetoclax is an effective and well-tolerated option in MPN-AP/BP patients, which is suitable for use both before and after HSCT. Our findings also support the routine use of HMAs plus ruxolitinib in MPN-AP patients. Randomized clinical trials and head-to-head studies of HMA-based combinations are warranted to provide evidence for optimal combination therapy.
Ethical approval
Ethical approval was nor required as this study did not require use of patient identifiers.
Author contributions
J.C. collected, analysed the data and drafted the manuscript; Z.F.X. was the principal investigator and took primary responsibility for the paper. K.F.W., Z.J.X. and Z.F.X. contributed to the data analysis. All authors declare no competing interests.
Supplemental Material
Download MS Word (8.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed during this study are included in this article and its supplementary information files. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Yogarajah M, Tefferi A. Leukemic transformation in myeloproliferative neoplasms: a literature review on risk, characteristics, and outcome. Mayo Clin Proc. 2017;92(7):1118–1128.
- Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood. 2008;112(5):1628–1637.
- Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res. 2007;31(6):737–740.
- Palandri F, Breccia M, Tiribelli M, et al. Risk factors for progression to blast phase and outcome in 589 patients with myelofibrosis treated with ruxolitinib: real-world data. Hematol Oncol. 2020;38(3):372–380.
- Takagi S, Masuoka K, Uchida N, et al. Allogeneic hematopoietic cell transplantation for leukemic transformation preceded by Philadelphia chromosome-negative myeloproliferative neoplasms: a nationwide survey by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(12):2208–2213.
- Gowin K, Ballen K, Ahn KW, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020;4(9):1965–1973.
- Andrew H, Wei PM, Ivanov V, et al. Venetoclax plus LDAC for patients with untreated AML ineligible for intensive chemotherapy- phase 3 randomized placebo controlled trial. Blood. 2020;135(24):2137–2145.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Zhou S, Tremblay D, Hoffman R, et al. Clinical benefit derived from decitabine therapy for advanced phases of myeloproliferative neoplasms. Acta Haematol. 2021;144(1):48–57.
- Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood. 2010;116(19):3735–3742.
- Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in accelerated and blast-phase myeloproliferative neoplasms. Blood Adv. 2018;2(24):3572–3580.
- Gangat N, Guglielmelli P, Szuber N, et al. Venetoclax with azacitidine or decitabine in blast-phase myeloproliferative neoplasm: a multicenter series of 32 consecutive cases. Am J Hematol. 2021;96(7):781–789.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Mascarenhas J, Heaney ML, Najfeld V, et al. Proposed criteria for response assessment in patients treated in clinical trials for myeloproliferative neoplasms in blast phase (MPN-BP): formal recommendations from the post-myeloproliferative neoplasm acute myeloid leukemia consortium. Leuk Res. 2012;36(12):1500–1504.
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642–4649.
- Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with Post-Myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood. 2016;128(22):4262–4262.
- Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with Blast-Phase MPN and Post-MPN AML: results of a phase I study (myeloproliferative disorders research consortium 109 trial) [abstract]. Blood. 2016;128(22):1124–1124.
- Assi R, Bose P, Verstovsek S, et al. The combination of ruxolitinib (RUX) with decitabine (DAC) in patients (pts) with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): interim report of a phase I/II trial [abstract]. Blood. 2017;130(Suppl_1):1379.
- Drummond MW, Harrison CN, Vicente SM, et al. A phase Ib study to assess the safety and tolerability of ruxolitinib in combination with azacitidine in patients with advanced phase myeloproliferative neoplasms (MPN), including myelodysplastic syndromes (MDS) or ACUTE MYELOID LEUKAEMIA (AML) arising from MPN (the bloodwise/TAP PHAZAR study on behalf of the UK MPN CSG) [conference abstract]. Blood. 2017;130(Suppl_1):1649.
- Lancman G, Brunner A, Hoffman R, et al. Outcomes and predictors of survival in blast phase myeloproliferative neoplasms. Leuk Res. 2018;70:49–55.
- Bose P, Verstovsek S, Cortes JE, et al. A phase 1/2 study of ruxolitinib and decitabine in patients with post-myeloproliferative neoplasm acute myeloid leukemia. Leukemia. 2020;34(9):2489–2492.
- Gangat N, Morsia E, Foran JM, et al. Venetoclax plus hypomethylating agent in blast-phase myeloproliferative neoplasm: preliminary experience with 12 patients. Br J Haematol. 2020;191(5):e120–e124.
- Mascarenhas JO, Rampal RK, Kosiorek HE, et al. Phase 2 study of ruxolitinib and decitabine in patients with myeloproliferative neoplasm in accelerated and blast phase. Blood Adv. 2020;4(20):5246–5256.
- Tremblay D, Feld J, Dougherty M, et al. Venetoclax and hypomethylating agent combination therapy in acute myeloid leukemia secondary to a myeloproliferative neoplasm. Leuk Res. 2020;98:106456.
- Masarova L, DiNardo CD, Bose P, et al. Single-center experience with venetoclax combinations in patients with newly diagnosed and relapsed AML evolving from MPNs. Blood Adv. 2021;5(8):2156–2164.
- King AC, Weis TM, Derkach A, et al. Multicenter evaluation of efficacy and toxicity of venetoclax-based combinations in patients with accelerated and blast phase myeloproliferative neoplasms. Am J Hematol. 2022;97(1):E7–E10.
- Yoon JJ, Benitez LL, Bixby DL, et al. Efficacy of HMA +/- venetoclax or intensive chemotherapy in blast-phase myeloproliferative neoplasms [conference abstract]. Blood. 2021;138(Supplement 1):2569–2569.
- Andriani A, Montanaro M, Voso MT, et al. The experience of Gruppo Laziale for the study of Ph-, chronic myeloproliferative syndromes in the treatment of myeloproliferative neoplasms in blastic phase with azacytidine. Blood. 2014;124(21):3164–3164.
- Badar T, Kantarjian HM, Ravandi F, et al. Decitabine therapy for myeloproliferative neoplasm in accelerated (MPN-AP) or mastic (acute myeloid leukemia; MPN-AML) phase. J Clin Oncol. 2014;32(15_suppl):e18006.
- Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res. 2015;39(9):950–956.
- Andriani A, Latagliata R, Voso MT, et al. Azacitidine in the treatment of PH-myeloproliferative neoplasms in blastic phase - the experience of Gruppo Laziale for the study of PH-SMPC [abstract]. Blood. 2016;122(21):5270.
- Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood. 2016;128(22):1940–1940.
- Andriani A, Montanaro M, Breccia M, et al. Treatment of myeloproliferative neoplasms in blastic phase with azacytidine. Clinical results and identification of prognostic factors [abstract]. Blood. 2017;130(Suppl_1):1635.
- Dumas PY, Bertoli S, Bérard E, et al. Azacitidine or intensive chemotherapy for older patients with secondary or therapy-related acute myeloid leukemia. Oncotarget. 2017;8(45):79126–79136.
- Venton G, Courtier F, Charbonnier A, et al. Impact of gene mutations on treatment response and prognosis of acute myeloid leukemia secondary to myeloproliferative neoplasms. Am J Hematol. 2018;93(3):330–338.
- Mollard LM, Chauveau A, Boyer-Perrard F, et al. Outcome of Ph negative myeloproliferative neoplasms transforming to accelerated or leukemic phase. Leuk Lymphoma. 2018;59(12):2812–2820.
- Tefferi A, Mudireddy M, Mannelli F, et al. Blast phase myeloproliferative neoplasm: mayo-AGIMM study of 410 patients from two separate cohorts. Leukemia. 2018;32(5):1200–1210.
- Andriani A, Elli E, Trapè G, et al. Treatment of Philadelphia-negative myeloproliferative neoplasms in accelerated/blastic phase with azacytidine. Clinical results and identification of prognostic factors. Hematol Oncol. 2019;37(3):291–295.
- Morsia E, Gangat N, Foran JM, et al. Efficacy of venetoclax plus hypomethylating agent in blast phase myeloproliferative neoplasm [conference abstract]. Blood. 2020;136(Supplement 1):21.
- Wei AH, Strickland SA Jr, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- Ma J, Ge Z. Comparison between decitabine and azacitidine for patients with acute myeloid leukemia and higher-risk myelodysplastic syndrome: a systematic review and network meta-analysis. Front Pharmacol. 2021;12:701690.
- Maiti A, Qiao W, Sasaki K, et al. Venetoclax with decitabine vs intensive chemotherapy in acute myeloid leukemia: a propensity score matched analysis stratified by risk of treatment-related mortality. Am J Hematol. 2021;96(3):282–291.
- Apel A, Moshe Y, Ofran Y, et al. Venetoclax combinations induce high response rates in newly diagnosed acute myeloid leukemia patients ineligible for intensive chemotherapy in routine practice. Am J Hematol. 2021;96(7):790–795.
