Abstract
Rheumatoid arthritis (RA) is a systemic and autoimmune disease that is mainly featured abnormal fibroblast-like synoviocyte (FLS) proliferation and inflammatory cell infiltration. Abnormal expression or function of long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) are closely related to human diseases, including RA. There has been increasing evidence showing that in the competitive endogenous RNA (ceRNA) networks, both lncRNA and circRNA are vital in the biological functions of cells. Nevertheless, the exact mechanism of ceRNA in RA remains to be investigated. Herein, we summarized the molecular potencies of lncRNA/circRNA-mediated ceRNA networks in RA, with emphasis on the phenotypic regulation of ceRNA in the progression of RA, including regulation of proliferation, invasion, inflammation and apoptosis, as well as the role of ceRNA in traditional Chinese medicine (TCM) in the treatment of RA. In addition, we also discussed the future direction and potential clinical value of ceRNA in the treatment of RA, which may provide potential reference value for clinical trials of TCM therapy for the treatment of RA.
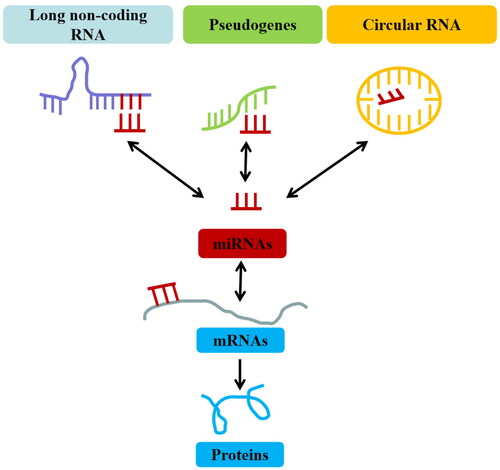
Long noncoding RNA/circular RNA can work as the competitive endogenous RNA sponge and participate in the pathogenesis of rheumatoid arthritis.
Traditional Chinese medicine and its agents have shown potential roles in the prevention and treatment of rheumatoid arthritis via competitive endogenous RNA.
Key messages
Introduction
Rheumatoid arthritis (RA) is an inflammatory and destructive disease with clinical manifestations of primarily symmetrical polyarthritis and extra-articular injury [Citation1,Citation2]. It eventually contributes to joint deformity and disability, seriously reduces the patient’s quality of life and affects social participation, which is considered as ‘Deathless cancer’ [Citation3–6]. The onset of RA has no age limitation with a high incidence, and the age of incidence is mainly 40–60 years, and the number of female RA patients is two to three times of male RA patients [Citation3,Citation7]. Currently, the exact pathogenesis of RA remains unknown. This chronic autoimmune disease with a high disability rate, is easy to attack repeatedly and is difficult to cure [Citation8]. Meanwhile, the long treatment cycle of this disease brings a huge financial burden to the patient’s families and society. The current treatment approaches for RA are based on glucocorticoids, non-steroidal anti-inflammatory drugs, traditional anti-rheumatic drugs, as well as biological agents [Citation9,Citation10]. Nevertheless, improper drug administration may cause cardiovascular system damage, liver and kidney dysfunction, gastrointestinal discomfort and other adverse reactions [Citation11]. Moreover, the expensive biologic agents weaken the immune system and thus may increase the risk of infections [Citation12].
Fibroblast-like synoviocytes (FLSs) are key effector cells in RA and are regarded as possible therapeutic targets for RA [Citation13,Citation14]. FLS is a crucial player in RA pathogenesis, exhibiting diverse invasive features, including apoptosis resistance, hyperproliferation, enhanced invasiveness, as well as secretion of inflammatory mediators [Citation15]. On the one hand, RA-FLS can synthesize and secrete matrix metalloproteinases to erode the cartilage, causing inflammatory cell infiltration into the involved joints and chronic cartilage destruction [Citation16]. On the other hand, activated FLSs secrete many chemokines, growth factors and pro-inflammatory cytokines to promote disease severity [Citation17]. In addition, the defective apoptosis of FLSs can result in synovial excessive proliferation, pannus formation and progressive joint destruction with irreversible loss of articular function [Citation18]. Moreover, oxidative stress exhibits a positive relation with inflammation and promoted joint destruction in RA patients [Citation19,Citation20]. It is suggested that the proliferation, invasion, inflammation and apoptosis of RA-FLS are potential mechanisms in RA.
Noncoding RNAs (ncRNAs) account for more than 98% of the human genome and play an important role in gene expression and regulation, including Long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), microRNAs (miRNAs), transcribed pseudogenes [Citation21]. miRNAs (20–200 nucleotides) function by binding to complementary sequences in the 3′-untranslated region (UTR) of their target mRNAs, thereby triggering translational repression of transcripts or mRNA degradation [Citation22]. lncRNAs (more than 200 nucleotides) usually do not encode proteins and act as transcriptional regulators [Citation21]. circRNAs are endogenous ncRNAs lacking the 5′ and 3′ ends, and their loop-like structure gives them a higher stability [Citation23]. The sequence of a pseudogene is usually similar to the corresponding gene, but is at least partially lost, such as not encoding a protein or encoding a protein without function [Citation24]. Long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), microRNAs (miRNAs), as well as transcribed pseudogenes, correlate with many diseases, including RA belonging to autoimmune diseases [Citation25–27]. Although many studies have provided evidence that both lncRNAs and circRNAs become a research hotspot in RA through their functions in many life activities [Citation28,Citation29]. However, at the sequence level, lncRNAs and circRNAs are poorly conserved across species.
In 2011, Salmena et al. proposed a competitive endogenous RNA (ceRNA) hypothesis describing that lncRNA/circRNA competes with protein-coding mRNA to bind miRNA [Citation30]. It emphasizes that lncRNAs/circRNAs act as miRNA sponges that protect target mRNAs from inhibition by sequestering specific miRNAs [Citation30] (). However, due to the limitation of current experimental approaches, this challenging area of ceRNA research is still in its infancy, and there are still many aspects to be improved and some issues to be solved. Fortunately, recent research has shown that ceRNAs participate in several diseases, including cardiovascular and cerebrovascular diseases [Citation31], nervous system diseases [Citation32], respiratory diseases [Citation33], immune system diseases [Citation34,Citation35] and malignant tumours [Citation36,Citation37].
Given the important role of ceRNAs in RA, this review concentrated on the lncRNAs/circRNAs-mediated ceRNA networks to figure out the potential functions of lncRNAs/circRNAs in modulating RA. Besides, we systematically summarized the functions of the lncRNA/circRNA-miRNA-mRNA axis in proliferation, invasion, inflammation and apoptosis in different cell lines and traditional Chinese medicine (TCM) intervention effect of ceRNAs in RA treatment (). Moreover, we probed into the challenges and therapeutic implications of ceRNAs in RA.
Table 1. Research involving the long noncoding RNA/circular RNA (lncRNA/circRNA)-mediated the competitive endogenous RNA (ceRNA) networks in rheumatoid arthritis (RA).
lncRNAs and circRNAs as latent diagnostic and prognostic biomarkers
The advancement of high-throughput sequencing and bioinformatics has contributed to the discovery of lncRNAs and circRNAs in RA. Quantities of lncRNAs and circRNAs that are dysregulated in RA cells have been identified, which are utilized as key clinical diagnostic biomarkers in RA.
For example, 5045 differentially expressed lncRNAs were identified via a genome-wide microarray analysis of 10 RA patients and 10 healthy controls, among which 2410 lncRNAs were upregulated and 2635 lncRNAs were downregulated [Citation86]. Through transcriptome sequencing (RNA-seq), Long et al. found 341 differentially expressed lncRNAs in peripheral blood mononuclear cells (PBMCs) in three RA patients and normal people [Citation87]. Zhang et al. identified 135 lncRNAs differentially expressed in FLSs of RA patients and normal people [Citation88]. Of those, ENST00000483588 was upregulated; uc004afb.1, ENST00000438399 and ENST00000452247 were downregulated in RA-FLSs. Receiver operating characteristic (ROC) curve analysis was performed to show that these lncRNAs can act as a suitable biomarker for RA diagnosis. Moreover, microarray analysis was used to identify disease activity-associated 683 upregulated and 1,416 downregulated lncRNAs in PBMCs from RA patients, which showed that ENST00000456270 could be a biomarker of RA risk and severity [Citation89]. In another study, Wen et al. analysed the difference between apoptosis- and autophagy-related lncRNAs in PBMCs of three RA patients by high-throughput lncRNA sequencing [Citation90]. After biological validation in 20 RA patients and 20 healthy controls, seven lncRNAs (C5orf17, LINC01189, LINC01006, MAPKAPK5-AS1, DSCR9, MIR22HG and ENST00000619282) were validated as the most significantly differentially expressed lncRNAs, which were correlated with clinical indicators and self-cognitive ability of patients [Citation90].
In 2017, a total of 584 circRNAs (255 upregulated and 329 downregulated circRNAs) differentially expressed were observed in RA patients through the circRNA microarray analysis [Citation91]. Moreover, the differentially expressed circRNAs in RA were screened and validated by the high-throughput analysis and bioinformatics analyses, underscoring the capacity of hsa_circ_0003353 in immunity and inflammation of RA [Citation92]. Through gene microarray technology, Lu et al. obtained 149 upregulated and 250 downregulated circRNAs in PBMCs from RA patients, which showed that lncRNA hsa_circ_101328 has a significant positive correlation with C-reactive protein (CRP) and may be a new marker of RA diagnosis [Citation93]. Alternatively, a recent study by Wen et al. found 165 differentially expressed circRNAs via RNA-seq of three RA patients and three healthy controls, further clinical study revealed that hsa circ 0001200, hsa circ 0001566, hsa circ 0003972, as well as hsa circ 0008360 expression levels were in accord with the RNA-seq, which could act as potent biomarkers for RA diagnosis [Citation94]. In comparison, 71 dysregulated circRNAs were identified in RA, and both hsa_circ_0000396 and hsa_circ_0130438 may exhibit a better diagnostic value in RA [Citation95].
In general, there are a large number of aberrantly expressed lncRNAs and circRNAs in RA patients in contrast to normal people. In most studies, the number of lncRNAs and circRNAs was closely related to RA disease activity. Due to the variable abundance and interactions and crosstalk of individual ncRNAs, combinatorially variable series of ncRNAs may be more promising as biomarkers than individual ncRNAs.
Regulatory roles of ceRNAs in RA
As the vital regulatory mode of gene expression, the lncRNA/circRNA-mediated ceRNA networks are expected to exhibit pleiotropic effects in autoimmune diseases such as RA. ceRNAs have both beneficial (such as suppressing the malignant subtype of RA-FLS) and detrimental (such as promoting inflammatory responses) roles in RA pathogenesis [Citation51,Citation74]. In fact, the lncRNA/circRNA-mediated ceRNA network regulates multiple cellular processes in RA, including proliferation, invasion, inflammation and apoptosis.
Proliferation, migration and invasion capabilities
Like tumour cells, RA-FLSs also share the properties of biological functions and enhanced resistance to apoptosis. Wang et al. found that lncRNA LINC-PINT is upregulated in TNF-α-induced RA-FLSs, which elevates SOCS1 expression through sponging miR-155-5p, leading to the promotion of the proliferation, migration and invasion [Citation38]. Another study revealed that Linc0238 exacerbates the tumour-like phenotype of FLSs in RA through the miR-590-5p/MAP2K3 axis [Citation42]. In addition, lncRNA HAND2-AS1 binds with miR-143-3p to elevate the levels of TNFAIP3/NF-κB, thus strengthening proliferation, migration and invasion of RA-FLS [Citation45].
As reported, LOC100912373 sponges miR-17-5p to enhance PDK1 expression, thereby facilitating proliferation, migration and invasion of RA-FLS [Citation45]. Also, lncRNA NEAT1 has been revealed to bind with miR-410-3p, thus mediating RA-FLS proliferation, migration and invasion [Citation52]. Furthermore, lncRNA NR-133666 promotes RA progression by acting as a miR-133c sponge and thereby reducing the inhibition of MAPK1 by miR-133c [Citation53]. Wnt signalling is a crucial mediator of cellular activities [Citation55]. lnc RNAS56464.1 promotes RA-FLS malignant subtype by sponging miR-152-3p, thereby activating the Wnt signalling pathway. lncRNAS56464.1 interference inhibits FLS proliferation and reduces the expression of Wnt1, β-catenin, c-Myc, cyclin D1, p-GSK-3β/GSK-3β [Citation55]. Other publications have elucidated the elevation of lncRNA GAS5 [Citation67], lncRNA NEAT1_1 [Citation67] and lncRNA FOXD2-AS1 [Citation68] in RA-FLS. To conclude, the downregulation of these lncRNAs may alleviate the RA-FLS dysfunction, thereby exerting biological activities by serving as ceRNAs.
circRNAs are also implicated in the RA-FLSs biological activities. Hsa_circ_0088036 mediates the RA-FLS biological activities through regulating SIRT1 [Citation70]. Circ_0088194 contributes to RA progression by enhancing MMP2 expression and reducing miR-766-3p [Citation73]. Circ-PTTG1IP suppresses RA-FLS malignant subtypes via the miR-671-5p/TLR4 axis [Citation74]. Circ_0008360 plays a protective effect in RA by acting as a miR-135b-5p sponge and downregulating HDAC4 [Citation75].
Inhibiting RA-FLS proliferation, migration and invasion are potential therapeutic strategies for RA. Li et al. proposed that the downregulation of circ_0130438 ameliorates RA by restricting RA-FLS functional properties via the miR-130a-3p/KLF9 axis [Citation77]. Also, the downregulation of circASH2L represses RA-FLS tumour-like properties via the miR-129-5p/HIPK2 axis [Citation80]. Luo et al. supported that the reduced circMAPK9 retards RA progression via the miR-140-3p/PPM1A axis [Citation81]. Similarly, many other ceRNAs, such as circ_0025908/miR-137/HIPK2, circ_0004712/miR-633/TRAF6, circPTTG1IP/miR-431-5p/FSTL1 as well as circ_0088194/miR-30a-3p/ADAM10, have been validated to attenuate RA progression [Citation82,Citation84,Citation85,Citation96].
Proliferation and apoptosis
Certain ceRNA networks are linked to the viability and apoptosis of RA. Wang et al. in their work suggested that the LINC00152/miR-1270/FOXM1 axis influences the pathogenesis of RA [Citation47]. Depletion of lncRNA ZFAS leads to an inhibition in viability and an enhancement in apoptosis of RA-FLSs via the miR-2682-5p/ADAMTS9 axis [Citation48]. Zheng et al. also stated that lncRNA RNA ZFAS1 results in the suppression of the RA process through the miR-296-5/MMP-15 axis [Citation97]. Downregulation of lncRNA GAS5 results in suppressed proliferation and induced apoptosis via the modulation of the miR-361-5p/PDK4 axis [Citation62]. CircFADS2 and mTOR were upregulated but miR-498 was downregulated in TNF-α-induced RA-FLS. Moreover, CircFADS2 could mediate mTOR expression via binding to miR-498, and CircFADS2 stimulated mitophagy via the miR-498/mTOR axis [Citation71]. Furthermore, lncRNA OIP5-AS1/miR-448/TLR3/NF-κB, lncRNA BZRAP1-AS1/miR-1286/COL5A2 and LncRNA XIST/miR-126-3p/NF-κB have been found to strengthen proliferation and restrict apoptosis of RA-FLS [Citation49,Citation54,Citation58].
Proliferation and inflammation
Some lncRNAs and circRNAs exert proinflammatory and proliferative functions in RA-FLS. LncRNA GAS5 and lncRNA MEG3 were upregulated in RA-FLS. LncRNA GAS5 can sponge miR-222-3p, thus elevating Sirt1 levels [Citation98]. LncRNA MEG3 can act as a ceRNA of miR‐141, thereby activating the AKT/mTOR pathway [Citation41]. Similarly, lncRNA GAPLINC exhibits pro-inflammatory and proliferative effects via the miR-382-5p/miR-575 axis [Citation51]. Another study showed that lncRNA OSER1-AS1 is markedly upregulated RA-FLS. Knockdown of lncRNA OSER1-AS1 prevents anti-inflammatory and anti-proliferative capacities by sponging miR-1298-5p [Citation57]. LINC00665 can impede RA-FLS viability and inflammation via the miR-122-3p-3p/EIF2AK1 signalling axis [Citation59]. Additionally, lncRNA-H19 acts as a miR-124a sponge and subsequently downregulates CDK2 and MCP-1 levels to facilitate RA-FLS viability and inflammation, leading to RA progression [Citation60]. LncRNA ZNF667-AS1 facilitates RA-FLS viability and inflammation through the miR-523-3p/JAK/STAT axis [Citation61]. LncRNA HOTTIP influences RA-FLS proliferation and inflammation through the miR-1908-5p/STAT3 axis [Citation63]. Moreover, lncRNA NEAT1 in exosomes of RA stimulates proliferation and inflammation via the miR-23a/MDM2/SIRT6 axis [Citation50].
Zhi et al. recently revealed that the reduction of circ_AFF2 mediates the miR-375/TAB2 axis to impede RA-FLS proliferation and inflammation [Citation72]. Geng et al. proposed that circ_0088036 contributes to an amelioration of RA progression through blocking miR-326 activity and elevating FZD4 expression [Citation76]. Wang et al. pointed out that circ_0088036 results in an exacerbation of RA via the downregulation of miR-1263 and upregulation of REL [Citation78]. In addition, the NF-κB-regulated genes are also vital in the process of invasiveness and inflammation. For instance, Yang et al. first stressed that downregulation of circRNA_09505 attenuates RA progression via the miR-6089/AKT1 axis and the modulation of the NF-κB pathway [Citation27]. Additionally, many other ceRNAs, such as lncRNA SNHG14/miR-17-5p/MINK1/JNK [Citation65], lncRNA RNA XIST/let-7c-5p/STAT3 [Citation66] and circ-AFF2/miR-650/CNP [Citation79], have been validated to participate in the RA-FLS viability and inflammation.
Apoptosis and inflammation
Inflammation and apoptosis are critically important in RA pathogenesis. As described, lncRNA PVT1 was observed to mediate inflammatory responses in RA-FLS by serving as a miR-543 sponge and positively modulating SCUBE2 expression [Citation43]. TLR4, as the main receptor impacting the NF-κB activation, could influence RA progression. Yan et al. addressed that via the modulation of the TLR4/miR-6089 axis, lncRNA HIX003209 evoked an inflammatory response in RA [Citation44]. Except that, the signalling axis of LINC01197/miR-150/THBS2 [Citation39] and lncRNA CASC2/miR-18a-5p/BTG3 [Citation56] have been elucidated to intensify inflammation and diminish apoptosis of RA-FLS.
Methods for characterization of ceRNA interactions
Several bioinformatic tools and genomics databases can be utilized for the construction of a lncRNA/circRNA-miRNA-mRNA network. Different databases have their own prediction rules and characteristics, which leads to different prediction results, and therefore a combination of various databases is needed to give robust information.
Five main algorithms (TargetScan, RNA22, miRanda, PicTar and PITA) are adopted for the prediction of potential miRNA targets, which is helpful for the miRNA-sponge interactions and ceRNA relationships [Citation99–103]. Furthermore, the open-source database StarBase v2.0 (http://starbase.sysu.edu.cn/) provides the CLIP-Seq data to experimentally support the interaction networks of miRNA-mRNA and miRNA-lncRNA [Citation104]. This database incorporates the gene expression data (AGO-CLIP and small RNA-seq data), which increases the reliability of the lncRNA/circRNA-miRNA relationship predictions [Citation105]. Similarly, many databases can be implemented to probe into the interacting miRNAs of lncRNAs. LncCeRBase is a relatively small database that consists of 432 lncRNA-miRNA-mRNA interactions [Citation106]. LncACTdb 2.0 is a comprehensive database that offers comprehensive information on ceRNAs in varying species and diseases [Citation107].
In addition to conducting database predication, the functions of the lncRNA/circRNAs sponging with its target miRNA need to be verified by experiments. First, we need to identify the target lncRNA/circRNA and its functional phenotypes and the clinical diagnostic and prognostic value in diseases. Second, RNA-FISH and nuclear-cytoplasmic separation experiments were chosen to verify whether lncRNA/circRNA mainly located in the cytoplasm, was facilitated to determine whether it can regulate miRNAs at the posttranscriptional level [Citation108]. Also exciting is that in cells and tissues, the FISH assay also can evaluate the colocalization of lncRNA/circRNA with miRNAs. But, it is important to note that circRNA is conserved and stable, and formed through back-splicing events of precursor mRNA, which should avoid recognition of their cognate linear RNAs [Citation109]. Third, the dual-luciferase reporter gene assay has been widely applied to validate human miRNA targets [Citation110]. The luciferase reporter vector wild-type (WT)-lncRNA/circRNA is established by the WT and mutant type (MUT) sequences of lncRNA/circRNA containing the binding sites of miRNA inserting into the pGL3 vector (Promega Corporation) [Citation110]. Transfection with miRNA mimics diminished the WT luciferase reporter activity; yet, the transfection failed to diminish the MUT luciferase reporter activity [Citation110]. Forth, the co-immunoprecipitation of lncRNA/circRNA and miRNA with anti-AGO2 could suggest that lncRNA/circRNA is observed in RNA-induced silencing complexes containing AGO2, possibly through the interaction with miRNA, which further validates lncRNA/circRNA’s miRNA sequestering activity [Citation111]. More importantly, direct interaction between lncRNA/circRNA and miRNA was confirmed by luciferase activity and RIP assays, this finding strongly supported the ceRNA theory that lncRNA/circRNA could compete for miRNA [Citation112]. Finally, an RNA pull-down assay with biotinylated probes can be designed specifically for the lncRNA/circRNA-miRNA [Citation113]. After addressing the RNAs obtained after the enrichment, the interacting miRNA-lncRNA/circRNA molecules are elucidated by mass spectrometry analyses [Citation114].
ceRNA involved in TCM treatment of RA
TCM has attracted more and more attention owing to its advantages of safety and fewer adverse reactions [Citation75]. The use of herbs in treating RA has a history of thousands of years in many Asian countries, and curative effects have been proven by both clinical applications and experimental research [Citation18,Citation115]. As is well known, herbal medicine can function through multiple targets and multiple pathways [Citation116,Citation117]. Herb medicine is anti-rheumatic and possesses diverse pharmacological actions, such as regulation of anti-inflammatory, analgesic and immunomodulatory, inhibits hyperplasia of synovial cells, and suppresses angiogenesis [Citation118,Citation119]. It is still noteworthy that herbal medicines and their monomer show promising effects on the inhibition of synovial hyperplasia, and the specific mechanisms are primarily realized via the regulation of ceRNA. We summarized that TCM exerts its therapeutic effect in RA by regulation of ceRNAs, as detailed in .
Table 2. Traditional Chinese medicine (TCM) exerts its therapeutic effect on rheumatoid arthritis (RA) by regulating the competitive endogenous RNAs (ceRNAs).
lncRNA/circRNA involved in Chinese medicine monomer treatment of RA
Tripterygium wilfordii Hook.f., also called Leigongteng in TCM, is a commonly used anti-rheumatic herbal drug [Citation137]. Triptolide (TPL) is a diterpene lactone epoxide compound extracted from Tripterygium, and it possesses diverse biological profiles, such as anti-fertility, anti-tumour, anti-inflammatory, as well as immunosuppressive activities [Citation138,Citation139]. TPL inhibits RA-FLS proliferation, invasion and inflammation by suppressing the levels of TNF-α, IL-1β, IL-6, MMP-3 and MMP-9, exhibiting a therapeutic role in collagen-induced arthritis (CIA) rats [Citation129]. However, these effects were reversed by lncRNA RP11-83J16.1 overexpression [Citation129]. ENST00000619282 expression was elevated both in RA-PBMCs and RA-FLS, while ENST00000619282 was significantly decreased following treatment with TPL [Citation131]. In addition, ENST00000619282 showed a close clinical correlation with the disease activity. Furthermore, TPL exerts an anti-inflammatory and pro-apoptotic function that was reversed by overexpression ENST00000619282. The same conclusion could be drawn from another study. TPL exerts anti-inflammatory and anti-migratory effects in RA-FLS through the circRNA 0003353/JAK2/STAT3 signalling pathway [Citation133].
Other Chinese medicine monomers were also reported to exhibit a potential role in treating RA via lncRNAs. For instance, shikonins have been reported to confer an anti-inflammatory role against RA. In a study conducted by Yang et al. shikonin inhibits RA-FLS inflammatory reaction via lncRNA-NR024118 [Citation121]. Additionally, it has been found that tanshinone IIA promotes RA-FLS apoptosis by elevating lncRNA GAS5 [Citation128]. Moreover, Fang et al. also found that quercetin contributes to induction of RA-FLS apoptosis by upregulating lncRNA MALAT1 [Citation128].
lncRNA/circRNA involved in TCM compound treatment of RA
Xinfeng Capsule (XFC) is a TCM prescription that is commonly applied in RA therapy, and which is composed of four TCM components: Radix astragali, Coicis semen, T. wilfordii and Centipedes [Citation140]. A recent review demonstrates the effectiveness and safety of XFC for RA therapy via meta-analysis. A multicenter, parallel, placebo-controlled, double-blind and randomized controlled trial (RCT) involving 304 patients with RA from China showed that XFC can effectively reduce joint pain and improve laboratory indicators, which was found to be comparable to leflunomide [Citation141]. The level of lncRNA MAPKAPK5-AS1 in the RA-PBMCs and RA-FLS were down-regulated and can mediate RA-FLS inflammation and apoptosis [Citation135]. Interestingly, restored lncRNA MAPKAPK5-AS1 can reverse the effect of Xinfeng Capsules (XFC) on RA inflammation and apoptosis, which indicated that lncRNA MK5-AS1 participated in RA treatment with XFC [Citation135].
Huayu Qiangshen Tongbi formula (HQT) is utilized for RA treatment by dissipating blood stasis, activating blood circulation, as well as dispelling pathogenic cold, wind and wet. There has been a study indicating that lncRNA uc.477 has a direct regulatory effect on the expression of miR-19b in RA [Citation120]. Importantly, HQT treatment normalized the lncRNA uc.477 and miR-19b levels in RA-FLS and the CIA mice model. Thus, lncRNA uc.477 could be a latent therapeutic marker for HQT on RA and its therapeutic mechanism may be through the upregulation of miR-19b.
ceRNA involved in Chinese medicine monomer treatment of RA
Many ceRNA networks were linked to the treatment of T. wilfordii in RA. Zhang et al. suggested that the ceRNA network (lncRNA ENST0000494760/miR-654-5p/C1QC) is confirmed by the results obtained from microarray and was deemed to be a biomarker for RA response to Tripterysium Glycosides Tablets (TGT) by clinical cohort, in vitro and in vivo experiments [Citation123]. Circ0003353 was confirmed as a key circRNA that reacted to inflammation and immunity in RA. It was found that circ0003353 sponged miR-31-5p to upregulate CDK1 and thus promote RA-FLS proliferation and inflammation. Interestingly, circ0003353/miR-31-5p/CDK1 axis could reverse the effect of TPL on RA -FLS through a series of rescue and gain-of-function experiments [Citation130]. (5 R)-5-hydroxytriptolide (LLDT-8), as a novel analogue of TPL, is both qualified and optimized in structure, and possesses better immunosuppressive activities and lower toxicities than TPL. The LLDT-8-induced elevation of lncRNA WAKMAR2 induced was the most remarkable in RA-FLS and restored WAKMAR2 evoked cell viability, invasion, as well as inflammation in RA-FLS. Mechanistically, it has been proven that WAKMAR2 plays a ceRNA role in regulating E2F1 expression by competitively binding to miR-4478, which can be regulated by LLDT-8 [Citation142]. Thus, it is important to consider the ceRNA axis as a potential therapeutic target for T. wilfordii in RA.
Of course, other studies have unveiled the effect of Chinese medicine monomer treatment on RA through the ceRNA axis. For example, In a study conducted by Ma et al. paeoniforin mediated the circ-FAM120A/miR-671-5p/MDM4 pathway to impede RA-FLS viability and inflammation and trigger cell cycle arrest [Citation122]. Additionally, one study found that astragaloside regulates the LOC100912373/miR-17-5p/PDK1 axis for the suppressed FLS proliferation in rats with RA [Citation143]. Pan et al. also found that total saponins of radix clematis modulated RA-FLS via the OIP5-AS1/miR-410-3p/wnt7b axis [Citation134]. Furthermore, arsenic trioxide harbours a protective function on RA-FLS and CIA synovium through blocking the circHIPK3/miR-149-5p/FOXO1/VEGF module [Citation125]. Another publication by Duan et al. also elucidated that tetrandrine downregulates NEAT1 expression, mechanistically, NEAT1 exerts ‘sponge-like’ effects on specific miR-17-5p to affect miR-17-5p binding to target gene STAT3, causing restricted RA-FLS viability and proliferation [Citation144]. Thus, the above studies lay a basis for an effective treatment approach for RA.
ceRNA involved in TCM compound treatment of RA
Huangqin Qingre Chubi Capsule (HQC) is a prescription for RA therapy, which is currently used as an in-hospital preparation at the First Affiliated Hospital of Anhui University of Chinese medicine [Citation145]. The study on peripheral blood mononuclear cells of 24 patients with RA from China showed that HQC drug-containing serum could activate Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) and Forkhead box protein O3a (FoxO3a) proteins in PBMCs of patients with RA and improve the state of oxidative stress in patients with RA [Citation145]. Clinical studies have shown that HQC can significantly reduce indicators of disease activity and that it has a good therapeutic effect on decreasing joint pain and improving joint function [Citation146]. Circ_0015756 expression was upregulated both in RA-FLS, synovium of CIA mice, as well as CIA-FLS. As reported, circ_0015756 expedited the inflammation and viability of RA by modulating the miR-942-5p/CUL4B/Wnt axis. HQC can attenuate joint damage in CIA mice and inhibit inflammation and proliferation of RA-FLS, which is associated with its interference with the effects of the circ_0015756/miR-942-5p/CUL4B axis [Citation132]. The discovery of this axis offers a novel ceRNA mechanism for RA and provides a basis for HQC’s functions in RA through multimolecular, multitarget and multi-pathway.
Conclusion and prospects
In the last decade, substantial progress has been made in identifying the genetic basis of RA thanks to the generation of several molecular tools and experimental studies. However, the available clinical therapeutic strategies for RA are still unsatisfactory. ncRNAs are now considered a hot topic of scientific research due to their great potential. Available evidence suggests that ceRNAs can regulate inflammation and autoimmunity. It has been elucidated that lncRNA/circRNA, miRNA and mRNA can play an integral regulatory role in the pathological process of RA in a ceRNA pattern. Specifically, ceRNAs are involved in proliferation, invasion, inflammation and apoptosis phenotypes in RA. Altering lncRNA/circRNA levels to affect target gene levels to reverse RA is promising. In addition, low toxicity and multi-targeted herbal medicines affect ceRNAs at the epigenetic level, which may provide a new reference for the treatment of RA.
Although the role of ceRNAs in RA is becoming increasingly evident, their specific mechanisms in RA progression need to be further explored. Existing studies on the regulation of ceRNA by herbal medicine are still limited to preclinical investigations at the molecular, cellular and animal model levels, which are challenging and promising to translate into clinical practice. Meanwhile, how to ensure therapeutic efficacy and safety, and prevent off-target effects, need to be considered.
In the future, studies on the interactions of ncRNA, ceRNA and RA should lay much attention on the following aspects. Initially, the construction of complex regulatory ceRNA network models with single lncRNA or circRNA modifications in RA is required. Focus on the improvement of the new network model to obtain and develop novel targets or treatment strategies for RA is certainly warranted. Second, in future studies, the sample size must be expanded to improve the reliability of the findings. Third, further validation with in vivo studies is critical for the development of ceRNA-targeted therapy in RA. Forth, in combination with the cells-secreted exosomes or vesicles, the feasibility and safety of RNA-modifying factors wrapped in exosomes is the basis of RA progression. Fifth, it is currently unknown how to effectively control the lncRNA/circRNA levels in target cells. m6A modification was abundant in many circRNAs and lncRNAs, and this kind of methylation modification could drive circRNA and lncRNA translation. Therefore, it is also important to explore the relationship between epigenetic modification and ceRNAs. Finally, TCM is known as multi-compound and multitarget medicines for wide application in RA through multiple targets, pathways, as well as links. UPLC-Q/TOF-MS analysis is a fact and useful technology for identifying TCM complex chemical compounds, which contribute to identifying the main effective ingredient of TCM to exhibit a potential role in treating RA through ceRNA.
In summary, this paper reviews the regulation of RA progression by lncRNA/circRNA-mediated ceRNA patterns involving the regulation of multiple phenotypes of proliferation, migration, apoptosis and inflammation, which provides new avenues for the exploration of autoimmune diseases including RA. Likewise, this review highlights that herbal medicines and their components can treat RA via ceRNAs, pointing to some directions for the clinical application of the aforementioned herbal medicines as anti-RA agents.
Author contributions
All authors read and approved the final manuscript. Jianting Wen and Jian Liu conceptualized and designed the study. Jianting Wen and Fanfan Wang prepared original draft. Lei Wan analysed and interpreted the results. Jian Liu reviewed and edited the final draft.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.
- Coutant F, Miossec P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr Opin Rheumatol. 2020;32(1):57–63.
- Van Der Woude D, Van Der Helm-Van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(2):174–187.
- Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):Itc1–itc16.
- Khan A, Pooja V, Chaudhury S, et al. Assessment of depression, anxiety, stress, and quality of life in rheumatoid arthritis patients and comparison with healthy individuals. Ind Psychiatry J. 2021;30(Suppl 1):S195–s200.
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–1372.
- De Stefano L, D'onofrio B, Manzo A, et al. The genetic, environmental, and immunopathological complexity of autoantibody-negative rheumatoid arthritis. Int J Mol Sci. 2021;22(22). doi:10.3390/ijms222212386
- Conigliaro P, Triggianese P, De Martino E, et al. Challenges in the treatment of rheumatoid arthritis. Autoimmun Rev. 2019;18(7):706–713.
- Bermas BL. Non-steroidal anti inflammatory drugs, glucocorticoids and disease modifying anti-rheumatic drugs for the management of rheumatoid arthritis before and during pregnancy. Curr Opin Rheumatol. 2014;26(3):334–340.
- Kim MJ, Lee SK, Oh S, et al. Efficacy of abatacept versus tumor necrosis factor inhibitors in anti-citrullinated protein antibody-positive patients with rheumatoid arthritis: results from a Korean Nationwide Biologics Registry. Rheumatol Ther. 2022;9(4):1143–1155.
- Mcinnes IB, O’dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69(11):1898–1906.
- Beukelman T, Xie F, Chen L, et al. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64(8):2773–2780.
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255.
- Doody KM, Bottini N, Firestein GS. Epigenetic alterations in rheumatoid arthritis fibroblast-like synoviocytes. Epigenomics. 2017;9(4):479–492.
- Karami J, Aslani S, Tahmasebi MN, et al. Epigenetics in rheumatoid arthritis; fibroblast-like synoviocytes as an emerging paradigm in the pathogenesis of the disease. Immunol Cell Biol. 2020;98(3):171–186.
- Wang X, Tang K, Wang Y, et al. Elevated microRNA‑145‑5p increases matrix metalloproteinase‑9 by activating the nuclear factor‑κB pathway in rheumatoid arthritis. Mol Med Rep. 2019;20(3):2703–2711.
- Yoshida K, Hashimoto T, Sakai Y, et al. Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. J Immunol Res. 2014, 2014;2014:282495.
- Liu X, Wang Z, Qian H, et al. Natural medicines of targeted rheumatoid arthritis and its action mechanism. Front Immunol. 2022;13:945129.
- Miglioranza Scavuzzi B, Holoshitz J. Endoplasmic reticulum stress, oxidative stress, and rheumatic diseases. Antioxidants (Basel). 2022;7(11). doi:10.3390/antiox11071306
- Wang X, Fan D, Cao X, et al. The role of reactive oxygen species in the rheumatoid arthritis-associated synovial microenvironment. Antioxidants. 2022;11(6). doi:10.3390/antiox11061153
- Uszczynska-Ratajczak B, Lagarde J, Frankish A, et al. Towards a complete map of the human long non-coding RNA transcriptome. Nat Rev Genet. 2018;19(9):535–548.
- Gerstein MB, Kundaje A, Hariharan M, et al. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489(7414):91–100.
- Li X, Yang L, Chen L-L. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428–442.
- Chen X, Wan L, Wang W, et al. Re-recognition of pseudogenes: from molecular to clinical applications. Theranostics. 2020;10(4):1479–1499.
- Zhai K, Duan H, Wang W, et al. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm Sin B. 2021;11(11):3493–3507.
- Zhai KF, Duan H, Shi Y, et al. miRNAs from plasma extracellular vesicles are signatory noninvasive prognostic biomarkers against atherosclerosis in LDLr(-/-)mice. Oxid Med Cell Longev. 2022;2022:6887192.
- Yang J, Cheng M, Gu B, et al. CircRNA_09505 aggravates inflammation and joint damage in collagen-induced arthritis mice via miR-6089/AKT1/NF-κB axis. Cell Death Dis. 2020;11(10):833.
- Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18(1):206.
- Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388.
- Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language?. Cell. 2011;146(3):353–358.
- Zhang S, Zhu T, Li Q, et al. Long non-coding RNA-mediated competing endogenous RNA networks in ischemic stroke: molecular mechanisms, therapeutic implications, and challenges. Front Pharmacol. 2021;12:765075.
- Tehrani SS, Ebrahimi R, Al EAA, et al. Competing endogenous RNAs (CeRNAs): novel network in neurological disorders. Curr Med Chem. 2021;28(29):5983–6010.
- Chen X, Hu J, Pan Y, et al. Novel noncoding RNAs biomarkers in acute respiratory distress syndrome. Expert Rev Respir Med. 2020;14(3):299–306.
- Li LJ, Zhao W, Tao SS, et al. Competitive endogenous RNA network: potential implication for systemic lupus erythematosus. Expert Opin Ther Targets. 2017;21(6):639–648.
- Zhang C, Wang C, Jia Z, et al. Differentially expressed mRNAs, lncRNAs, and miRNAs with associated co-expression and ceRNA networks in ankylosing spondylitis. Oncotarget. 2017;8(69):113543–113557.
- Akhbari MH, Zafari Z, Sheykhhasan M. Competing Endogenous RNAs (ceRNAs) in colorectal cancer: a review. Expert Rev Mol Med. 2022;24:e27.
- Liu Y, Khan S, Li L, et al. Molecular mechanisms of thyroid cancer: a competing endogenous RNA (ceRNA) point of view. Biomed Pharmacother. 2022;146:112251.
- Wang J, Zhao Q. LncRNA LINC-PINT increases SOCS1 expression by sponging miR-155-5p to inhibit the activation of ERK signaling pathway in rheumatoid arthritis synovial fibroblasts induced by TNF-α. Int Immunopharmacol. 2020;84:106497.
- Zhao F, Dong J, Guo J, et al. Inhibiting role of long non-coding RNA LINC01197 in inflammation in rheumatoid arthritis through the microRNA-150/THBS2 axis. Exp Cell Res. 2020;394(2):112136.
- Yang Z, Lin S, Zhan F, et al. GAS5LncRNA alleviates rheumatoid arthritis through regulating miR-222-3p/Sirt1 signalling axis. Autoimmunity. 2021;54(1):13–22.
- Li G, Liu Y, Meng F, et al. LncRNA MEG3 inhibits rheumatoid arthritis through miR-141 and inactivation of AKT/mTOR signalling pathway. J Cell Mol Med. 2019;23(10):7116–7120.
- Wang J, Zhao Q. Linc02381 exacerbates rheumatoid arthritis through adsorbing miR-590-5p and activating the mitogen-activated protein kinase signaling pathway in rheumatoid arthritis-fibroblast-like synoviocytes. Cell Transplant. 2020;29:963689720938023.
- Wang J, Kong X, Hu H, et al. Knockdown of long non-coding RNA PVT1 induces apoptosis of fibroblast-like synoviocytes through modulating miR-543-dependent SCUBE2 in rheumatoid arthritis. J Orthop Surg Res. 2020;15(1):142.
- Yan S, Wang P, Wang J, et al. Long non-coding RNA HIX003209 promotes inflammation by sponging miR-6089 via TLR4/NF-κB signaling pathway in rheumatoid arthritis. Front Immunol. 2019;10:2218.
- Su Y, Liu Y, Ma C, et al. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-κB pathway. J Orthop Surg Res. 2021;16(1):116.
- Fan C, Cui X, Chen S, et al. LncRNA LOC100912373 modulates PDK1 expression by sponging miR-17-5p to promote the proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Am J Transl Res. 2020;12(12):7709–7723.
- Wang W, Guo P, Chen M, et al. FOXM1/LINC00152 feedback loop regulates proliferation and apoptosis in rheumatoid arthritis fibroblast-like synoviocytes via wnt/β-catenin signaling pathway. Biosci Rep. 2020;40(1). doi:10.1042/BSR20191900
- Yang S, Yin W, Ding Y, et al. Lnc RNA ZFAS1 regulates the proliferation, apoptosis, inflammatory response and autophagy of fibroblast-like synoviocytes via miR-2682-5p/ADAMTS9 axis in rheumatoid arthritis. Biosci Rep. 2020;40(8). doi:10.1042/BSR20201273
- Qing P, liu Y. Inhibitory role of long non-coding RNA OIP5-AS1 in rheumatoid arthritis progression through the microRNA-448-paraoxonase 1-toll-like receptor 3-nuclear factor κB axis. Exp Physiol. 2020;105(10):1708–1719.
- Rao Y, Fang Y, Tan W, et al. Delivery of long non-coding RNA NEAT1 by peripheral blood monouclear Cells-Derived exosomes promotes the occurrence of rheumatoid arthritis via the MicroRNA-23a/MDM2/SIRT6 axis. Front Cell Dev Biol. 2020;8:551681.
- Mo BY, Guo XH, Yang MR, et al. Long non-coding RNA GAPLINC promotes tumor-like biologic behaviors of fibroblast-like synoviocytes as microRNA sponging in rheumatoid arthritis patients. Front Immunol. 2018;9:702.
- Wang Y, Hou L, Yuan X, et al. LncRNA NEAT1 targets Fibroblast-Like synoviocytes in rheumatoid arthritis via the miR-410-3p/YY1 axis. Front Immunol. 2020;11:1975–1975.
- Zhang N, Zheng N, Luo D, et al. Long non-coding RNA NR-133666 promotes the proliferation and migration of fibroblast-like synoviocytes through regulating the miR-133c/MAPK1 axis. Front Pharmacol. 2022;13:887330.
- Zhu J, Tu S, Qu Q. lncRNA BZRAP1-AS1 alleviates rheumatoid arthritis by regulating miR-1286/COL5A2 axis. Immun Inflamm Dis. 2022;10(2):163–174.
- Jiang H, Liu J, Fan C, et al. lncRNAS56464.1 as a ceRNA promotes the proliferation of fibroblast‑like synoviocytes in experimental arthritis via the wnt signaling pathway and sponges miR‑152‑3p. Int J Mol Med. 2021;47(3). doi:10.3892/ijmm.2021.4850
- Ye Z, Wei L, Yin X, et al. Long non-coding RNA cancer susceptibility candidate 2 regulates the function of human fibroblast-like synoviocytes via the microRNA-18a-5p/B-cell translocation gene 3 signaling axis in rheumatoid arthritis. Bioengineered. 2022;13(2):3240–3250.
- Fu Q, Song MJ, Fang J. LncRNA OSER1-AS1 regulates the inflammation and apoptosis of rheumatoid arthritis fibroblast like synoviocytes via regulating miR-1298-5p/E2F1 axis. Bioengineered. 2022;13(3):4951–4963.
- Liu W, Song J, Feng X, et al. LncRNA XIST is involved in rheumatoid arthritis fibroblast-like synoviocytes by sponging miR-126-3p via the NF-κB pathway. Autoimmunity. 2021;54(6):326–335.
- Wang Z, Tian Q, Tian Y, et al. MicroRNA-122-3p plays as the target of long non-coding RNA LINC00665 in repressing the progress of arthritis. Bioengineered. 2022;13(5):13328–13340.
- Fu X, Song G, Ni R, et al. LncRNA-H19 silencing suppresses synoviocytes proliferation and attenuates collagen-induced arthritis progression by modulating miR-124a. Rheumatology. 2021;60(1):430–440.
- Zhuo Q, Wei L, Yin X, et al. LncRNA ZNF667-AS1 alleviates rheumatoid arthritis by sponging miR-523-3p and inactivating the JAK/STAT signalling pathway. Autoimmunity. 2021;54(7):406–414.
- Zhang W, Li B, Xia N, et al. lncRNA GAS5 suppresses rheumatoid arthritis by inhibiting miR-361-5p and increasing PDK4. Biochem Biophys Res Commun. 2021;583:7–13.
- Yao X, Wang Q, Zeng P, et al. LncRNA HOTTIP from synovial fibroblast-derived exosomes: a novel molecular target for rheumatoid arthritis through the miR-1908-5p/STAT3 axis. Exp Cell Res. 2021;409(2):112943.
- Peng T, Ji D, Jiang YJM. Long non-coding RNA GAS5 suppresses rheumatoid arthritis progression via miR-128-3p/HDAC4 axis. Mol Cell Biochem. 2021;476(6):2491–2501.
- Zhang J, Lei H, Li X. LncRNA SNHG14 contributes to proinflammatory cytokine production in rheumatoid arthritis via the regulation of the miR-17-5p/MINK1-JNK pathway. Environ Toxicol. 2021;36(12):2484–2492.
- Wang ZQ, Xiu DH, Jiang JL, et al. Long non-coding RNA XIST binding to let-7c-5p contributes to rheumatoid arthritis through its effects on proliferation and differentiation of osteoblasts via regulation of STAT3. J Clin Lab Anal. 2020;34(11):e23496.
- Wang M, Chen Y, Bi X, et al. LncRNA NEAT1_1 suppresses tumor-like biologic behaviors of fibroblast-like synoviocytes by targeting the miR-221-3p/uPAR axis in rheumatoid arthritis. J Leukoc Biol. 2022;111(3):641–653.
- Zhao Q, Zhao F, Liu C, et al. LncRNA FOXD2-AS1 promotes cell proliferation and invasion of fibroblast-like synoviocytes by regulation of miR-331-3p/PIAS3 pathway in rheumatoid arthritis. Autoimmunity. 2021;54(5):254–263.
- Zheng J, Zeng P, Zhang H, et al. Long noncoding RNA ZFAS1 silencing alleviates rheumatoid arthritis via blocking miR-296-5p-mediated down-regulation of MMP-15. Int Immunopharmacol. 2021;90:107061.
- Zhong S, Ouyang Q, Zhu D, et al. Hsa_circ_0088036 promotes the proliferation and migration of fibroblast-like synoviocytes by sponging miR-140-3p and upregulating SIRT 1 expression in rheumatoid arthritis. Mol Immunol. 2020;125:131–139.
- Li G, Tan W, Fang Y, et al. circFADS2 protects LPS-treated chondrocytes from apoptosis acting as an interceptor of miR-498/mTOR cross-talking. Aging. 2019;11(10):3348–3361.
- Zhi L, Liang J, Huang W, et al. Circ_AFF2 facilitates proliferation and inflammatory response of fibroblast-like synoviocytes in rheumatoid arthritis via the miR-375/TAB2 axis. Exp Mol Pathol. 2021;119:104617.
- Cai Y, Liang R, Xiao S, et al. Circ_0088194 promotes the invasion and migration of rheumatoid arthritis fibroblast-like synoviocytes via the miR-766-3p/MMP2 axis. Front Immunol. 2021;12:628654.
- Chen L, Huang H, Chen L, et al. Circ-PTTG1IP/miR-671-5p/TLR4 axis regulates proliferation, migration, invasion and inflammatory response of fibroblast-like synoviocytes in rheumatoid arthritis. Gen Physiol Biophys. 2021;40(3):207–219.
- Hao J, Chen Y, Yu Y. Circular RNA circ_0008360 inhibits the proliferation, migration, and inflammation and promotes apoptosis of fibroblast-like synoviocytes by regulating miR-135b-5p/HDAC4 axis in rheumatoid arthritis. Inflammation. 2022;45(1):196–211.
- Geng X, Zhao C, Zhang Z, et al. Circ_0088036 facilitates the proliferation and inflammation and inhibits the apoptosis of fibroblast-like synoviocytes through targeting miR-326/FZD4 axis in rheumatoid arthritis. Autoimmunity. 2022;55(3):157–167.
- Li L, Zhan M, Li M. Circular RNA circ_0130438 suppresses TNF-α-induced proliferation, migration, invasion and inflammation in human fibroblast-like MH7A synoviocytes by regulating miR-130a-3p/KLF9 axis. Transpl Immunol. 2022;72:101588.
- Wang X, Liu D, Cui G, et al. Circ_0088036 mediated progression and inflammation in fibroblast-like synoviocytes of rheumatoid arthritis by miR-1263/REL-activated NF-κB pathway. Transpl Immunol. 2022;73:101604.
- Qu W, Jiang L, Hou G. Circ-AFF2/miR-650/CNP axis promotes proliferation, inflammatory response, migration, and invasion of rheumatoid arthritis synovial fibroblasts. J Orthop Surg Res. 2021;16(1):165.
- Li X, Qu M, Zhang J, et al. CircASH2L facilitates tumor-like biologic behaviours and inflammation of fibroblast-like synoviocytes via miR-129-5p/HIPK2 axis in rheumatoid arthritis. J Orthop Surg Res. 2021;16(1):302.
- Luo Z, Chen S, Chen X. CircMAPK9 promotes the progression of fibroblast-like synoviocytes in rheumatoid arthritis via the miR-140-3p/PPM1A axis. J Orthop Surg Res. 2021;16(1):395.
- Wang X, Zhang Z, Liang H, et al. Circ_0025908 regulates cell vitality and proliferation via miR-137/HIPK2 axis of rheumatic arthritis. J Orthop Surg Res. 2021;16(1):472.
- Zhang S, Shen Z, Chao G, et al. Circ_0004712 silencing suppresses the aggressive changes of rheumatoid arthritis fibroblast-like synoviocytes by targeting miR-633/TRAF6 axis. Biochem Genet. 2022. doi:10.1007/s10528-022-10265-w
- Yang C, Liu Q, Jiang Z. CircPTTG1IP knockdown suppresses rheumatoid arthritis progression by targeting miR-431-5p/FSTL1 axis. Transpl Immunol. 2022;75:101685.
- Feng L, Jing W, Jin S, et al. Circ_0088194 regulates proliferation, migration, apoptosis, and inflammation by miR-30a-3p/ADAM10 axis in rheumatoid arthritis fibroblastic synovial cells. Inflammation. 2022. doi:10.1007/s10753-022-01719-9
- Luo Q, Xu C, Li X, et al. Comprehensive analysis of long non-coding RNA and mRNA expression profiles in rheumatoid arthritis. Exp Ther Med. 2017;14(6):5965–5973.
- Long Y, Liu J, Jiang H, et al. Network analysis and transcriptome profiling in peripheral blood mononuclear cells of patients with rheumatoid arthritis. Exp Ther Med. 2021;21(2):170.
- Zhang Y, Xu YZ, Sun N, et al. Long noncoding RNA expression profile in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res Ther. 2016;18(1):227.
- Yuan M, Wang S, Yu L, et al. Long noncoding RNA profiling revealed differentially expressed lncRNAs associated with disease activity in PBMCs from patients with rheumatoid arthritis. PLoS One. 2017;12(11):e0186795.
- Wen J, Liu J, Jiang H, et al. lncRNA expression profiles related to apoptosis and autophagy in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FEBS Open Bio. 2020;10(8):1642–1654.
- Zheng F, Yu X, Huang J, et al. Circular RNA expression profiles of peripheral blood mononuclear cells in rheumatoid arthritis patients, based on microarray chip technology. Mol Med Rep. 2017;16(6):8029–8036.
- Wen J, Liu J, Wang X, et al. Expression and clinical significance of circular RNAs related to immunity and inflammation in patients with rheumatoid arthritis. Int Immunopharmacol. 2021;92:107366.
- Lu H, Yang Y, Kuang D, et al. Expression profile of circRNA in peripheral blood mononuclear cells of patients with rheumatoid arthritis. BMC Med Genomics. 2022;15(1):77.
- Wen J, Liu J, Zhang P, et al. RNA-seq reveals the circular RNA and miRNA expression profile of peripheral blood mononuclear cells in patients with rheumatoid arthritis. Biosci Rep. 2020;40(4). doi:10.1042/BSR20193160
- Yang X, Li J, Wu Y, et al. Aberrant dysregulated circular RNAs in the peripheral blood mononuclear cells of patients with rheumatoid arthritis revealed by RNA sequencing: novel diagnostic markers for RA. Scand J Clin Lab Invest. 2019;79(8):551–559.
- Zhang S, Shen Z, Chao G, et al. Circ_0004712 silencing suppresses the aggressive changes of rheumatoid arthritis fibroblast-like synoviocytes by targeting miR-633/TRAF6 axis. Biochem Genet. 2022. doi:10.1007/s10528-022-10265-w
- Zheng J, Zeng P, Zhang H, et al. Long noncoding RNA ZFAS1 silencing alleviates rheumatoid arthritis via blocking miR-296-5p-mediated down-regulation of MMP-15. Int Immunopharmacol. 2021;90:107061.
- Yang Z, Lin SD, Zhan F, et al. LncRNA GAS5 alleviates rheumatoid arthritis through regulating miR-222-3p/Sirt1 signalling axis. Autoimmunity. 2021;54(1)
- Mcgeary SE, Lin KS, Shi CY, et al. The biochemical basis of microRNA targeting efficacy. Science. 2019;366(6472). doi:10.1126/science.aav1741
- Miranda KC, Huynh T, Tay Y, et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217.
- Betel D, Wilson M, Gabow A, et al. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36(Database issue):D149–53.
- Krek A, Grün D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37(5):495–500.
- Weingarten-Gabbay S, Elias-Kirma S, Nir R, et al. Comparative genetics. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science. 2016;351(6270). doi:10.1126/science.aad4939
- Li JH, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–D97.
- Grosswendt S, Filipchyk A, Manzano M, et al. Unambiguous identification of miRNA:target site interactions by different types of ligation reactions. Mol Cell. 2014;54(6):1042–1054.
- Pian C, Zhang G, Tu T, et al. LncCeRBase: a database of experimentally validated human competing endogenous long non-coding RNAs. Database. 2019;2019. doi:10.1093/database/baz090
- Wang P, Li X, Gao Y, et al. LncACTdb 2.0: an updated database of experimentally supported ceRNA interactions curated from low- and high-throughput experiments. Nucleic Acids Res. 2019;47(D1):D121–D127.
- Ren J, Ding L, Zhang D, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8(14):3932–3948.
- Qu S, Yang X, Li X, et al. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365(2):141–148.
- Clément T, Salone V, Rederstorff M. Dual luciferase gene reporter assays to study miRNA function. Methods Mol Biol. 2015;1296:187–198.
- Jia Y, Tian C, Wang H, et al. Long non-coding RNA NORAD/miR-224-3p/MTDH axis contributes to CDDP resistance of esophageal squamous cell carcinoma by promoting nuclear accumulation of β-catenin. Mol Cancer. 2021;20(1):162.
- Zhang ZY, Gao XH, Ma MY, et al. CircRNA_101237 promotes NSCLC progression via the miRNA-490-3p/MAPK1 axis. Sci Rep. 2020;10(1):9024.
- Kim SY, Hakoshima T. GST pull-down assay to measure complex formations. Methods Mol Biol. 2019;1893:273–280.
- Giessing AM, Kirpekar F. Mass spectrometry in the biology of RNA and its modifications. J Proteomics. 2012;75(12):3434–3449.
- Li TP, Zhang AH, Miao JH, et al. Applications and potential mechanisms of herbal medicines for rheumatoid arthritis treatment: a systematic review. RSC Adv. 2019;9(45):26381–26392.
- Ma Q, Jiang JG. Functional Components from Nature-Derived drugs for the treatment of rheumatoid arthritis. Curr Drug Targets. 2016;17(14):1673–1686.
- Keisuke I, Bian BL, Li XD, et al. Action mechanisms of complementary and alternative medicine therapies for rheumatoid arthritis. Chin J Integr Med. 2011;17(10):723–730.
- Lü S, Wang Q, Li G, et al. The treatment of rheumatoid arthritis using Chinese medicinal plants: from pharmacology to potential molecular mechanisms. J Ethnopharmacol. 2015;176:177–206.
- Yuan HY, Zhang XL, Zhang XH, et al. Analysis of patents on anti-rheumatoid arthritis therapies issued in China. Expert Opin Ther Pat. 2015;25(8):909–930.
- Wang M, Mei L, Liu Z, et al. The mechanism of Chinese herbal formula HQT in the treatment of rheumatoid arthritis is related to its regulation of lncRNA uc.477 and miR-19b. J Leukoc Biol. 2020;108(2):519–529.
- Yang KY, Chen DL. Shikonin inhibits inflammatory response in rheumatoid arthritis synovial fibroblasts via lncRNA-NR024118. Evid Based Complement Alternat Med. 2015;2015:631737.
- Ma J, Meng Q, Zhan J, et al. Paeoniflorin suppresses rheumatoid arthritis development via modulating the Circ-FAM120A/miR-671-5p/MDM4 axis. Inflammation. 2021;44(6):2309–2322.
- Zhang Y, Wang X, Li W, et al. Inferences of individual differences in response to tripterysium glycosides across patients with rheumatoid arthritis using a novel ceRNA regulatory axis. Clin Transl Med. 2020;10(6):e185.
- Zhou X, Xie D, Huang J, et al. Therapeutic effects of (5R)-5-hydroxytriptolide on fibroblast-like synoviocytes in rheumatoid arthritis via lncRNA WAKMAR2/miR-4478/E2F1/p53 axis. Front Immunol. 2021;12:605616.
- Zhang J, Ma Y, Zhang Y, et al. Angiogenesis is inhibited by arsenic trioxide through downregulation of the CircHIPK3/miR-149-5p/FOXO1/VEGF functional module in rheumatoid arthritis. Front Pharmacol. 2021;12:751667.
- Fan G, Pa N, Lih UA, et al. Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med. 2016;38(5):1507–1514.
- Jiang H, Fan C, Lu Y, et al. Astragaloside regulates lncRNA LOC100912373 and the miR‑17‑5p/PDK1 axis to inhibit the proliferation of fibroblast‑like synoviocytes in rats with rheumatoid arthritis. Int J Mol Med. 2021;48(1). doi:10.3892/ijmm.2021.4963
- Li G, Liu Y, Meng F, et al. Tanshinone IIA promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by up-regulating lncRNA GAS5. Biosci Rep. 2018;38(5). doi:10.1042/BSR20180626
- Piao X, Zhou J, Xue L. Triptolide decreases rheumatoid arthritis fibroblast-like synoviocyte proliferation, invasion, inflammation and presents a therapeutic effect in collagen-induced arthritis rats via inactivating lncRNA RP11-83J16.1 mediated URI1 and β-catenin signaling. Int Immunopharmacol. 2021;99:108010.
- Wen JT, Liu J, Wan L, et al. Triptolide inhibits cell growth and inflammatory response of fibroblast-like synoviocytes by modulating hsa-circ-0003353/microRNA-31-5p/CDK1 axis in rheumatoid arthritis. Int Immunopharmacol. 2022;106:108616.
- Wen J, Liu J, Wang X, et al. Triptolide promotes the apoptosis and attenuates the inflammation of fibroblast-like synoviocytes in rheumatoid arthritis by down-regulating lncRNA ENST00000619282. Phytother Res. 2021;35(8):4334–4346.
- Wang X, Chang J, Zhou G, et al. The Traditional Chinese medicine compound Huangqin Qingre Chubi capsule inhibits the pathogenesis of rheumatoid arthritis through the CUL4B/wnt pathway. Front Pharmacol. 2021;12:750233.
- Wang J, Liu J, Wen J, et al. [Triptolide inhibits inflammatory response and migration of fibroblast like synovial cells in rheumatoid arthritis through the circRNA 0003353/JAK2/STAT3 signaling pathway]. Zhongguo Zhong Yao Za Zhi. 2022;42(3):367–374.
- Pan L, Sun Y, Jiang H, et al. Total saponins of radix clematis regulate Fibroblast-Like synoviocyte proliferation in rheumatoid arthritis via the LncRNA OIP5-AS1/MiR-410-3p/Wnt7b signaling pathway. Evid Based Complement Alternat Med. 2022;2022:8393949.
- Wen JT, Liu J, Wang X, et al. [Xinfeng capsules promotes apoptosis of synovial fibroblasts and attenuates inflammation in rheumatoid arthritis by regulating lncRNA MAPKAPK5-AS1]. Zhongguo Zhong Yao Za Zhi. 2021;46(24):6542–6548.
- Duan B, Yu Z, Liu R, et al. Tetrandrine-induced downregulation of lncRNA NEAT1 inhibits rheumatoid arthritis progression through the STAT3/miR-17-5p pathway. Immunopharmacol Immunotoxicol. 2022:44(6):886–893.
- Wang HL, Zhao Q, Li W, et al. A systematic review and network meta-analysis about the efficacy and safety of Tripterygium wilfordii Hook F in rheumatoid arthritis. Evid Based Complement Alternat Med. 2022;2022:3181427.
- Zhang Y, Mao X, Li W, et al. Tripterygium wilfordii: an inspiring resource for rheumatoid arthritis treatment. Med Res Rev. 2021;41(3):1337–1374.
- Liu YF, Zhang Z, Zhang JJ, et al. The derivative of Tripterygium wilfordii Hook F-Kunxian capsule, attenuated rheumatoid arthritis: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:4178140.
- Wen JT, Liu J, Wang X, et al. [Effect of Xinfeng capsules-containing serum on TNF-α-induced apoptosis and inflammation of fibroblast-like synoviocytes in rheumatoid arthritis]. Zhongguo Zhong Yao Za Zhi. 2021;46(2):436–443.
- Liu J, Wang Y, Huang C, et al. Efficacy and safety of Xinfeng capsule in patients with rheumatoid arthritis: a multi-center parallel-group double-blind randomized controlled trial. J Tradit Chin Med. 2015;35(5):487–498.
- Zhou X, Xie D, Huang J, et al. Therapeutic effects of (5R)-5-hydroxytriptolide on fibroblast-like synoviocytes in rheumatoid arthritis via lncRNA WAKMAR2/miR-4478/E2F1/p53 axis. Front Immunol. 2021;12:605616.
- Jiang H, Fan C, Lu Y, et al. Astragaloside regulates lncRNA LOC100912373 and the miR‑17‑5p/PDK1 axis to inhibit the proliferation of fibroblast‑like synoviocytes in rats with rheumatoid arthritis. Int J Mol Med. 2021;48(1). doi:10.3892/ijmm.2021.4963
- Duan B, Yu Z, Liu R, et al. Tetrandrine-induced downregulation of lncRNA NEAT1 inhibits rheumatoid arthritis progression through the STAT3/miR-17-5p pathway. Immunopharmacol Immunotoxicol. 2022;44(6):886–893.
- Guo J-C, Liu J, Zhang X-J, et al. [Effect of Huangqin Qingre Chubi capsules containing serum on oxidative stress and protein expression of AMPK and FoxO3a in rheumatoid arthritis patients]. Zhongguo Zhong Yao Za Zhi. 2020;45(13):3228–3232.
- Huang D, Liu J, Zong RK, et al. [Huangqin Qingre Chubi capsules in improving oxidative stress of patients with ankylosing spondylitis via activating PPARγ mediated AMPK/FOXO3a pathway]. Zhongguo Zhong Yao Za Zhi. 2020;45(2):451–456.