Abstract
Background
Among malignant neoplasm patients taking immune checkpoint inhibitors (ICIs), it remains unknown how the systemic immune-inflammation index (SII) affects their clinical prognosis. We therefore performed the present meta-analysis by collecting the most recent data, so that SII’s prognostic value among ICI-receiving carcinoma patients could be fully clarified.
Methods
For the prognostic significance evaluation of SII in ICI-receiving carcinoma patients, the combined hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated.
Results
The number of studies enrolled in the present meta-analysis totaled 17, where 1,990 patients were involved. Among the ICI-treated carcinoma patients, a high SII was linked significantly to inferior overall survival (OS) (HR = 2.62, 95% CI = 1.76–3.90), as well as progression-free survival (PFS) (HR = 2.09, 95% CI = 1.48–2.95) (p both <.001). Contrastively, SII was linked insignificantly to the age (OR = 1.08, 95% CI = 0.39–2.98, p = .881), gender (OR = 1.01, 95% CI = 0.59–1.73, p = .959), lymph node (LN) metastasis (OR = 1.41, 95% CI = 0.92–2.17, p = .117), or metastatic site quantity (OR = 1.49, 95% CI = 0.90–2.46, p = .119).
Conclusion
There are prominent associations of elevated SII with the poor survival outcomes (both short- and long-terms) among the ICIreceiving carcinoma patients. SII has potential as a reliable and cheap prognostic biomarker in the clinic for carcinoma patients receiving ICIs.
Key Messages
The current meta-analysis represents the first attempt to explore SII’s prognostic efficiency among carcinoma patients treated with ICIs.
There are prominent associations of elevated SII with the poor survival outcomes (both short- and long-terms) among the ICI-receiving carcinoma patients.
SII may serve as a reliable and cheap prognostic biomarker in the clinical settings.
Introduction
Being the leading cause of death, cancer crucially impedes prolongation of the anticipated life span on a global scale [Citation1]. According to the GLOBOCAN 2020 estimates in 185 countries around the world, there were 19,292,789 new cancer cases, as well as 9,958,133 cases died due to cancer in 2020 [Citation1]. Regarding treatment for patients with cancer, surgery, chemotherapy, and radiotherapy are mainstay in clinical practice. In recent years, immune checkpoint inhibitors (ICIs) have been more and more commonly applied and have dramatically improved survival in various malignancies [Citation2]. ICIs contain therapeutic antibodies that disrupt negative immune regulatory checkpoints and therefore enhance antitumour immune responses [Citation3]. ICIs such antibodies targeting programmed cell death 1 (PD-1), cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and PD1 ligand 1 (PD-L1) have showed promising therapeutic effect and have been applied for many cancer types in the clinic [Citation4,Citation5]. Novel and reliable prognostic biomarker identification is crucial to attaining individualized therapy among carcinoma patients receiving ICIs in clinical settings [Citation6].
Growing evidence in recent years has demonstrated the heavy implications of inflammatory and immunoresponses in the cancer development [Citation7]. For the carcinoma patients undergoing ICIs, their parameters derived from blood test have been explored as valid biomarkers for outcomes [Citation8]. The haematological markers include the neutrophil–lymphocyte ratio (NLR) [Citation8], the platelet–lymphocyte ratio (PLR) [Citation9], the modified Glasgow prognostic score (mGPS) [Citation10], as well as the systemic inflammation index (SII) [Citation11]. Computational equation for SII is: SII = platelet × neutrophil/lymphocyte [Citation12]. SII is presented as a combination of NLR and PLR. SII’s prognostic effects have been priorly reported for many tumours [Citation13,Citation14]. Growing studies also explored its prognostic role in a variety of ICI-receiving carcinoma patients; however, the results remained controversial [Citation11,Citation15–30]. For instance, some investigators regarded SII as a significant marker to suggest oncological results in cancer patients receiving ICIs [Citation19,Citation20], whereas some other investigators found the association was non-significant [Citation15,Citation30]. Hence, for a complete prognostic value assessment on SII among ICI-receiving carcinoma patients, we collected the most recent data and performed this meta-analysis. Additionally, we examined how SII was associated with the clinicopathological traits in cancer patients receiving ICIs as well.
Materials and methods
Study guideline
The present meta-analysis was carried out as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [Citation31].
Ethics statement
Since all the data in the present meta-analysis were retrieved from public databases, neither the institutional review board nor the ethics committee was required.
Search strategy
Literature was retrieved in a systematic and holistic manner by utilizing online databases like Web of Science, PubMed, Cochrane Library and Embase between the dates of inception and 1 January 2023. We registered this meta-analysis protocol in INPLASY. The registration number is INPLASY202310018.The search terms shown below were used: (SII or Systemic Immune-Inflammation Index) and (immune check point inhibitor or PD-L1 or PD-1 or avelumab or CTLA-4 or durvalumab or nivolumab or ipilimumab or atezolizumab or pembrolizumab or camrelizumab or tislelizumab or toripalimab or penpulimab or immune checkpoint blockade or immunotherapy). Only the articles published in English were retrieved. Possible inclusions were recognized also by prudently checking the references in qualified researches, as well as relevant reviews.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients were pathologically diagnosed with cancer and treated with ICIs; (2) available data of pretreatment SII; (3) studies investigating the relationship between SII and prognosis of patients with cancer undergoing ICIs; (4) a cut-off value was identified to stratify patients as high/low SII groups; (5) any survival outcomes were reported including overall survival (OS), progression-free survival (PFS), recurrence-free survival (RFS) etc., (6) studies published in English language. The exclusion criteria were: (1) reviews, letters, meeting abstracts, and case reports; (2) studies did not provide survival data; (3) duplicate studies and (4) non-human studies.
Data extraction and quality assessment
Relevant researches were collected by two independent researchers (Y.W. and Q.N.) through retrieval and scanning of identified titles and abstracts. All disagreements were addressed through negotiation until reaching consensus. Information extracted from qualified researches included: first author’s name, country, sample size, publication year, study design, age, gender, cancer type, study period, ICIs type, tumour-node-metastasis (TNM) stage, study centre, survival outcomes, cut-off value of SII, follow-up, hazard ratio (HR), survival assessment, as well as 95% confidence interval (CI). Pooling of HRs and 95% CIs was accomplished from multivariate assessments if provided. Otherwise, univariate assessments were the source of HRs and 95% CIs. The enrolled studies were subjected to quality evaluation via the Newcastle–Ottawa Scale (NOS) [Citation32]. This scale assesses the research quality regarding selection, comparability and prognosis. Studies scoring ≥6 on this nine-point NOS are considered to have high quality.
Statistical analysis
SII’s prognostic significance among the ICI-receiving carcinoma patients was evaluated by estimating the combined HRs and 95% CIs. Cochran’s Q test was employed to assess the inter-study heterogeneity by utilizing I2 statistics. In the case of insignificant heterogeneity (p > .05 for χ2 test or I2< 50%), a fixed-effect model was adopted. Otherwise, a random-effects model was selected. Heterogeneity source was identified by conducting subgroup analysis stratified by diverse variables. How SII was associated with the clinicopathological traits of cancer patients using ICIs was assessed by pooling odds ratios (ORs) and 95%CIs. Possible publication bias was evaluated through Begg’s test. Stata 12.0 (College Station, TX) was utilized to analyse the entire data. p < .05 was regarded as statistically significant.
Results
Search results
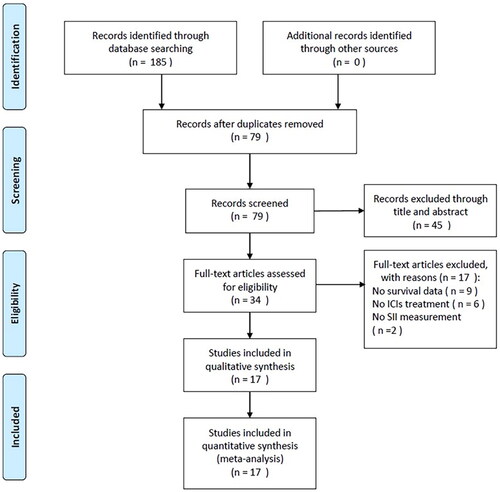
We identified 185 publications through the initial literature retrieval, as displayed in . After removal of duplicates, 79 studies remained for abstract and title scanning. Then 45 records were eliminated by title and abstract and 34 studies were assessed further via full-text reading. Thereafter, we eliminated 17 studies due to no survival data (n = 9), no ICIs treatment (n = 6), and no information on SII provided (n = 2). Finally, 17 studies were enrolled in total in the present meta-analysis, where 1990 patients were involved [Citation11,Citation15–30]. illustrates the flowchart for screening the studies.
Characteristics of included studies
details the baseline features of the enrolled studies [Citation11,Citation15–30], T whose publication years ranged from 2019 to 2022. Among them, nine studies were carried out in China [Citation15,Citation16,Citation18,Citation21,Citation25–27,Citation29,Citation30], three studies were carried out in Italy [Citation11,Citation17,Citation20], two studies were performed in Germany [Citation23,Citation28], and one each in Japan [Citation22], Korea [Citation24], and Turkey [Citation19], respectively. English was the language of publication for all included studies. The scope of sample sizes was 29–313, and the median value was 106. Four studies were prospective clinical trials [Citation11,Citation17,Citation20,Citation23] and 13 studies were of retrospective design [Citation15,Citation16,Citation18,Citation19,Citation21,Citation22,Citation24–30]. Three studies recruited non-small cell lung cancer (NSCLC) patients [Citation11,Citation23,Citation25], three studies enrolled renal cell carcinoma (RCC) patients [Citation17,Citation22,Citation28], two studies included patients with oesophageal cancer [Citation15,Citation29], two studies enrolled patients with gastric cancer [Citation16,Citation26], and one each study recruited patients with biliary tract cancer (BTC) [Citation18], hepatocellular carcinoma (HCC) [Citation21], melanoma [Citation24], pancreatic cancer [Citation27], small cell lung cancer (SCLC) [Citation30], urothelial carcinoma [Citation20], and various cancers [Citation19], respectively. Nine studies included patients with TNM stage IV [Citation11,Citation17–19,Citation22–24,Citation26,Citation28], seven studies enrolled patients with stage III-IV [Citation15,Citation16,Citation20,Citation21,Citation25,Citation27,Citation29], and one study enrolled stage I-IV SCLC patients [Citation30]. Regarding ICIs type, ten studies used anti-PD-1 antibodies [Citation11,Citation15,Citation17,Citation18,Citation21,Citation23–26,Citation29], two studies applied anti-PD-1/anti-PD-L1 antibodies [Citation16,Citation30], two studies used anti-PD-1 plus anti-CTLA-4 antibodies [Citation22,Citation28], two studies selected anti-PD-1/PD-L1 + anti-CTLA-4 antibodies [Citation19,Citation27] and one study applied anti-PD-L1 antibodies [Citation20]. The SII threshold scope was 197.2–1375, with a median of 829. Thirteen studies [Citation11,Citation15–21,Citation24–28] reported SII’s prognostic significance to OS, while 14 studies [Citation15,Citation16,Citation18,Citation20–30] presented the SII–PFS correlation among the ICI-treated carcinoma patients. For the enrolled studies, the NOS score scope was 7–9, with a median of 8, revealing high quality of the entire studies. lists the particular NOS scores.
Table 1. Basic characteristics of included studies in the current meta-analysis.
Table 2. The details of NOS scale for included studies in this meta-analysis.
Prognostic role of SII for OS
SII was shown prognostically significant for carcinoma patients undergoing ICIs in 13 studies involving 1758 patients in total [Citation11,Citation15–21,Citation24–28]. Significant heterogeneity (I2 = 88.5%, p < .001) was detected, and we employed a random-effects model. The pooled HR was 2.62, whereas 95% CIs ranged 1.76–3.90 (p < .001) (, ), implying prominent correlation of high SII with inferior OS. According to the subgroup assessments stratified by multiple factors, SII’s prognostic significance in OS was unaffected by study design, sample size, study centre, TNM stage, cut-off value, geographical region, cancer type, and survival analysis type in cancer patients treated with ICIs (all p < .05) ().
Figure 2. Forest plot of HR for the relationship between SII and overall survival in cancer patients receiving ICIs.
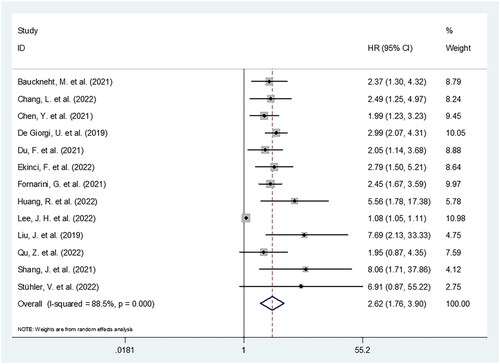
Figure 3. Forest plot of HR for the association between SII and progression-free survival in cancer patients receiving ICIs.
Table 3. The prognostic value of SII for OS in cancer patients treated with ICIs.
Prognostic impact of SII on PFS
SII produced a prognostic influence on PFS in 14 studies comprising 1464 patients [Citation15,Citation16,Citation18,Citation20–30]. Given the significant inter-study heterogeneity (I2 = 80.2%, p < .001), we adopted a random-effects model. As displayed in and , the pooled HR was 2.09, while 95% CIs ranged from 1.48 to 2.95 (p < .001). As revealed by subgroup assessments, SII’s prognostic impact was kept stable across various subgroups of sample size, research design, centre, geographical location, threshold, as well as type of survival analysis (all p < .05) ().
Figure 4. Results of the analysis of publication bias. (A) OS, Begg’s test, p = .583; (B) PFS, Begg’s test, p = .956.
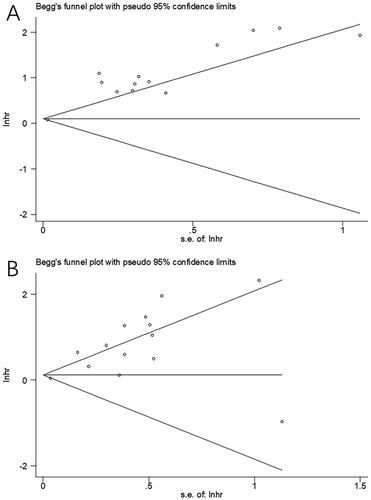
Table 4. The prognostic value of SII for PFS in cancer patients treated with ICIs.
Association between SII and clinicopathological features
Four studies including 636 patients [Citation17,Citation19,Citation26,Citation28] reported the associations of SII with the clinicopathological traits for carcinoma patients receiving ICIs. As shown in , SII was linked insignificantly to the age (OR = 1.08, 95% CI = 0.39–2.98, p = .881), the gender (OR = 1.01, 95% CI = 0.59–1.73, p = .959), the lymph node (LN) metastasis (OR = 1.41, 95% CI = 0.92–2.17, p = .117), or the metastatic site quantity (OR = 1.49, 95% CI = 0.90–2.46, p = .119).
Table 5. The correlation between SII and clinicopathological variables in cancer patients undergoing ICIs treatment.
Publication bias
Possible publication bias was assessed by employing Begg’s test combined with funnel plot test. As shown in , the funnel plots for OS and PFS were not evidently asymmetric in shape. With the Begg’s test, the p values for OS and PFS were .583 and .956, respectively. Thus, insignificant bias was present in our meta-analysis.
Discussion
The previous works have shown that SII was prognostically significant among carcinoma patients receiving ICIs, whereas the results were inconsistent. In the present meta-analysis, data were extracted from 17 studies involving 1990 patients for quantitatively analysing the prognostic role of SII for OS and PFS. Our results demonstrated that elevated SII was a significant biomarker of outcome for both OS and PFS among the ICI-receiving carcinoma patients. Besides, SII exhibited a reliable prognostic efficiency, which was unaffected in diverse subgroups. However, SII was found associated significantly with age, gender, number of metastasis sites, or LN metastasis in our meta-analysis. Assessment of publication bias revealed the reliability of the results. In summary, our meta-analysis implied the significance and validity of SII as a biomarker of outcome for the ICI-treated carcinoma patients. Those cancer patients treated with ICIs who have high pretreatment SII may experience high risk of disease progression and should be cautiously managed.
The SII was derived as the formula: SII = (platelet quantity * neutrophil quantity)/lymphocyte quantity. Accordingly, a high SII would be indicative of high platelet and neutrophil quantities, and/or low quantity of lymphocytes. For the ICI-treated carcinoma patients, it is possible to explain the mechanisms of high SII with inferior survival prognosis in the following aspects: first, neutrophils can promote cancer development through directly interacting with tumour cells or indirectly remodelling the tumour microenvironment [Citation33]. Growth and metastasis of tumours can also be promoted by neutrophils through the proinflammatory cytokine and chemokine discharge in tumour microenvironment [Citation34]. Second, platelet quantity is an extra indicator for a systemic inflammatory reaction and possible microvascular thrombosis. Platelets also enables the angiogenesis facilitation via the cytokine VEGF, thereby promoting the growth of tumours [Citation35]. Upon activation, the platelets also probably regulate the tumour site migration of immunocytes and haematopoietic cells, and thus facilitates the cancer-related inflammation [Citation36]. Thirdly, as a major cellular immunity component in humans, lymphocytes are implicated in anti-cancer immunoresponses [Citation37]. T lymphocytes, in particular, function pivotally in the tumour cell identification and killing, thereby suppressing the multiplication and motility of these cells [Citation38]. Therefore, the reduction of lymphocytes can result in impairment of anti-tumour activity of the host.
The subgroup analysis was performed to examine the impact of treatment, cancer type, cut-off value, and study design on the results of this meta-analysis in and Citation4. The subgroup analysis indicated that sample size, study centre, country, study centre did not significantly affect the prognostic role of SII for both OS and PFS for cancer patients receiving ICIs. Additionally, SII showed obvious prognostic effect on OS and PFS for patients treated with Anti-PD-1, Anti-PD-1/PD-L1 + Anti-CTLA-4, and Anti-PD-L1 ( and ). Inherent heterogeneity may exist in included studies; however, subgroup analysis and publication bias test confirmed the reliability of our meta-analysis.
ICIs are applied in multiple cancer types and become standard treatment strategy for some cancers. The use of PD-1 inhibitors as adjuvant therapy in high-risk resected stage III or IV melanoma has become standard practice [Citation39]. In the treatment of advanced gastric cancers, ICIs including nivolumab and pembrolizumab have emerged as a promising treatment option [Citation40]. Different ICIs have been successfully introduced into clinical medicine for lung cancer treatment since 2014, including pembrolizumab, atezolizumab, and durvalumab [Citation41]. Immune checkpoint inhibitors (ICIs) have been approved as first-line therapy for patients ineligible for cisplatin or as second-line therapy for patients with metastatic bladder cancer [Citation42].
Recently, a series of studies also investigated SII’s prognostic value in diverse solid tumours through meta-analysis [Citation14,Citation43–45]. According to a meta-analysis involving 3515 patients by Zhang and colleagues, an elevated pre-therapy SII was linked significantly to worse RFS/PFS and OS among BTC patients [Citation14]. Li et al. found significant associations of high SII with inferior PFS, cancer-specific survival (CSS) and OS among oesophageal squamous cell carcinoma (ESCC) patients receiving operation in a meta-analysis including nine retrospective studies [Citation44]. In a meta-analysis on 10 studies enrolling 7087 patients by J. Li et al. SII displayed association with poor OS and RFS among the bladder cancer patients [Citation45]. M. Li et al. demonstrated the connection of elevated pre-therapy SII to inferior CSS/disease-free survival (DFS)/PFS and poor OS for pancreatic cancer in their meta-analysis comprising 2132 patients [Citation46]. In addition, a meta-analysis containing 12 studies identified the prognostic role of high SII for worse OS and PFS in patients with colorectal cancer [Citation47]. Another recent meta-analysis with 2365 subjects reported the association between SII and short-term and long-term survival outcomes in patients with pancreatic cancer [Citation48]. Our current meta-analysis demonstrated the significant prognostic value of SII in cancer patients treated with ICIs, which were in accordance with observations in other cancer.
The present meta-analysis has a few shortcomings. First of all, only those articles published in English were considered. Since the enrolled patients were predominantly Asians and partially Caucasians, subjects other than Caucasian and Asian ethnicities were assessed inadequately. Second shortcoming was the rather small sample size. Despite the inclusion of 17 studies, only 1990 patients were enrolled. The small sample size may also lead to the negative results regarding SII’s correlations with the clinicopathological parameters. Thirdly, the uniformed SII thresholds across the enrolled studies is a probable contributor to the meta-analytical heterogeneity. Hence, verifying our results by large-scale trials is necessary, where a unified SII threshold should be utilized.
In summary, elevated SII was linked significantly to the inferior survival prognosis (both short- and long-terms) among the ICI-receiving carcinoma patients. For these patients, SII may serve as a reliable and cheap prognostic biomarker in the clinical settings.
Author contributions
YW convinced the study. YW and QN performed literature search, data collection, and study quality evaluation. YW performed statistical analyses and interpreted the results. QN drafted the manuscript. All authors revised the manuscript and approved the submission.
Disclosure statement
No conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135.
- Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39.
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727–742.
- Twomey JD, Zhang BL. Cancer immunotherapy update: FDA-Approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23:39.
- Ren DX, Hua YZ, Yu BY, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19(1):19.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer [Review]. Cell. 2010;140(6):883–899.
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7.
- Qi Y, Liao D, Fu X, et al. Elevated platelet-to-lymphocyte corresponds with poor outcome in patients with advanced cancer receiving anti-PD-1 therapy. Int Immunopharmacol. 2019;74:105707.
- Brown JT, Liu Y, Shabto JM, et al. Baseline modified Glasgow prognostic score associated with survival in metastatic urothelial carcinoma treated with immune checkpoint inhibitors. Oncologist. 2021;26(5):397–405.
- Bauckneht M, Genova C, Rossi G, et al. The role of the immune metabolic prognostic index in patients with non-small cell lung cancer (NSCLC) in radiological progression during treatment with nivolumab. Cancers. 2021;13(13):3117.
- Wang S, Yang X, Yu Z, et al. The values of Systemic Immune-Inflammation Index and neutrophil-lymphocyte ratio in predicting testicular germ cell tumors: a retrospective clinical study. Front Oncol. 2022;12:893877.
- Cao W, Shao Y, Zou S, et al. Prognostic significance of Systemic Immune-Inflammation Index in patients with bladder cancer: a systematic review and meta-analysis. Medicine. 2022;101(36):e30380.
- Zhang B, Yao W. Prognostic role of the Systemic Immune-Inflammation Index in biliary tract cancers: a meta-analysis of 3,515 patients. World J Surg Oncol. 2022;20(1):320.
- Chang L, Cheng Q, Ma Y, et al. Prognostic effect of the controlling nutritional status score in patients with esophageal cancer treated with immune checkpoint inhibitor. J Immunother. 2022:45(9):415–422.
- Chen Y, Zhang C, Peng Z, et al. Association of lymphocyte-to-monocyte ratio with survival in advanced gastric cancer patients treated with immune checkpoint inhibitor. Front Oncol. 2021;11:589022.
- De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25(13):3839–3846.
- Du F, Qiu Z, Ai W, et al. Blood tests predict the therapeutic prognosis of anti-PD-1 in advanced biliary tract cancer. J Leukoc Biol. 2021;110(2):327–334.
- Ekinci F, Balcik OY, Demir B, et al. Systemic immune inflammation index as a key marker of survival and immune-related adverse events in immune checkpoint inhibitor therapy. J Coll Phys Surg Pak. 2022;32(8):996–1003.
- Fornarini G, Rebuzzi SE, Banna GL, et al. Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: an analysis of the Italian SAUL cohort. ESMO Open. 2021;6(3):100118.
- Huang R, Zheng Y, Zou W, et al. Blood biomarkers predict survival outcomes in patients with hepatitis B Virus-Induced hepatocellular carcinoma treated with PD-1 inhibitors. J Immunol Res. 2022;2022:3781109.
- Iinuma K, Enomoto T, Kawada K, et al. Utility of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index as prognostic, predictive biomarkers in patients with metastatic renal cell carcinoma treated with nivolumab and ipilimumab. JCM. 2021;10(22):5325.
- Kauffmann-Guerrero D, Kahnert K, Kiefl R, et al. Systemic inflammation and pro-inflammatory cytokine profile predict response to checkpoint inhibitor treatment in NSCLC: a prospective study. Sci Rep. 2021;11(1):10919.
- Lee JH, Hyung S, Lee J, et al. Visceral adiposity and systemic inflammation in the obesity paradox in patients with unresectable or metastatic melanoma undergoing immune checkpoint inhibitor therapy: a retrospective cohort study. J Immunother Cancer. 2022;10(8):e005226.
- Liu J, Li S, Zhang S, et al. Systemic Immune-Inflammation Index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964.
- Qu Z, Wang Q, Wang H, et al. The effect of inflammatory markers on the survival of advanced gastric cancer patients who underwent anti-programmed death 1 therapy. Front Oncol. 2022;12:783197.
- Shang J, Han X, Zha H, et al. Systemic Immune-Inflammation Index and changes of neutrophil-lymphocyte ratio as prognostic biomarkers for patients with pancreatic cancer treated with immune checkpoint blockade. Front Oncol. 2021;11:585271.
- Stühler V, Herrmann L, Rausch S, et al. Role of the Systemic Immune-Inflammation Index in patients with metastatic renal cell carcinoma treated with first-line ipilimumab plus nivolumab. Cancers. 2022;14(12):2972.
- Wu X, Han R, Zhong Y, et al. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Cancer Cell Int. 2021;21(1):356.
- Xiong Q, Huang Z, Xin L, et al. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol Immunother. 2021;70(3):713–720.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601–620.
- Jaillon S, Ponzetta A, Di Mitri D, et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20(9):485–503.
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249.
- Palacios-Acedo AL, Mège D, Crescence L, et al. Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front Immunol. 2019;10:1805.
- Li Y, Zhang Z, Hu Y, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) may predict the outcomes of advanced non-small-cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs). Front Oncol. 2020;10:654.
- Yang J, Liao D, Chen C, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/sox-2 signaling pathway. Stem Cells. 2013;31(2):248–258.
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398(10304):1002–1014.
- Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23(4):565–578.
- Onoi K, Chihara Y, Uchino J, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9:1362.
- Lopez-Beltran A, Cimadamore A, Blanca A, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers. 2021;13:131.
- Zhou Y, Dai M, Zhang Z. Prognostic significance of the Systemic Immune-Inflammation Index (SII) in patients with small cell lung cancer: a meta-analysis. Front Oncol. 2022;12:814727.
- Li X, Zhang S, Lu J, et al. The prognostic value of Systemic Immune-Inflammation Index in surgical esophageal cancer patients: an updated meta-analysis. Front Surg. 2022;9:922595.
- Li J, Cao D, Huang Y, et al. The prognostic and clinicopathological significance of Systemic Immune-Inflammation Index in bladder cancer. Front Immunol. 2022;13:865643.
- Li M, Li Z, Wang Z, et al. Prognostic value of Systemic Immune-Inflammation Index in patients with pancreatic cancer: a meta-analysis. Clin Exp Med. 2022:22:637–646.
- Dong M, Shi Y, Yang J, et al. Prognostic and clinicopathological significance of Systemic Immune-Inflammation Index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920937425.
- Shui Y, Li M, Su J, et al. Prognostic and clinicopathological significance of Systemic Immune-Inflammation Index in pancreatic cancer: a meta-analysis of 2,365 patients. Aging. 2021;13(16):20585–20597.