Abstract
Aim: Bullous pemphigoid (BP) is an organ-specific autoimmune bullous disease characterized by autoantibodies that target the cellular adhesion molecules BP180 and BP230. Both immunoglobulin (Ig)G and IgE are involved in the induction of subepidermal blisters. Specifically, IgE autoantibodies are presumed to be responsible for the pruritic and erythematous features of BP. Histologically, eosinophil infiltration is a prominent feature in BP. Eosinophils and IgE are mostly associated with the Th2 immune response. Th2 cytokines, particularly interleukin (IL)-4 and IL-13, are presumed to contribute to the pathology of BP. The aim of this review is to discuss the role of IL-4/13 in the pathogenesis of BP and the potential of using IL-4/13 antagonists for treatment.Methods: After searching in PubMed and Web of Science databases using ‘bullous pemphigoid’, ‘interleukin-4/13’, and ‘dupilumab’ as keywords, studies related was compiled and examined.Results: Overall, IgE, eosinophils, IL-4, and IL-13 may interact with each other in the pathogenesis of BP; these potential interactions provide clues concerning targets for molecular treatment.Conclusion: Anti-IL-4/13 treatment has been experimentally used in patients with BP, with satisfactory outcomes and few side effects. However, before this novel therapy can be approved for regular usage, further studies are needed concerning the long-term safety and systemic usage of IL-4/13 monoclonal antibody treatment in BP.
BP is an autoimmune skin disease with Th2-mediated autoimmune response involvement.
As typical Th2 cytokines, IL-4 and IL-13 may contribute to the pathogenesis of BP in multiple ways, such as promoting Th2 cell polarization, driving the immunoglobulin class switching, recruiting eosinophils and basophils, and inducing pruritus.
As a promising therapeutic approach for BP, IL-4/13 antagonists have shown satisfactory outcomes in preliminary clinical studies.
KEY MESSAGES
Introduction
Bullous pemphigoid (BP) is an autoimmune bullous skin disease mediated by pathogenic autoantibodies that target the hemidesmosome proteins BP antigen 180 (BP180) and BP antigen 230 (BP230) [Citation1–3]. Clinically, BP is characterized by large, tense blisters and erythema, and it mostly affects senior adults [Citation4]. Histologically, BP presents with subepidermal blisters with neutrophil and eosinophil infiltration. BP180 is a transmembrane glycoprotein with a globular cytoplasmic N-terminal domain, whereas BP230 is an intracellular constituent of hemidesmosome plaques [Citation1,Citation2]. Most patients with BP have circulating immunoglobulin (Ig) G autoantibodies that target BP180, particularly in non-collagenous domain 16A (NC16A), which is the immunodominant region recognized by autoreactive T and B cells [Citation5]. In BP, T cell responds with both T helper 1 (Th1) and T helper 2 (Th2) cells. Thus, in patient serum, both Th2-mediated IgG4 and Th1-mediated IgG1 autoantibodies are present [Citation6]. However, the presence of IgG autoantibodies does not explain all of the clinical features involved in BP. Factors other than IgG autoantibodies may also contribute to the pathogenesis of cutaneous inflammation in BP, such as T-helper autoreactive lymphocytes, cytokines, IgE, and eosinophils [Citation7,Citation8]. Whereas interleukin-4 (IL-4) and interleukin-13 (IL-13) are two key cytokines in Th2 autoimmune response, IL-4 and IL-13 are presumed to contribute to the pathogenesis of BP.
Current treatment options for BP are primarily corticosteroids, with or without immunosuppressive drugs, such as methotrexate, azathioprine, and mycophenolate mofetil. The combination of such treatments can have life-threatening effects, including severe infections, osteoporosis, and metabolic disorders [Citation9]. Therefore, new therapies with fewer side effects are needed. Atopic dermatitis (AD) is a common pruritic dermatosis with a Th2-dominant immune response [Citation10]. AD is characterized by the overexpression of Th2 cytokines, such as IL-4 and IL-13. IL-4/13 antagonists, such as dupilumab have yielded satisfactory outcomes in the treatment of AD, with few side effects [Citation11]. Because of the important role of Th2 immune response in BP and the possible contribution of IL-4/13 in maintaining Th2 immune response, IL-4/13 antagonists might yield similar outcomes in the treatment of BP. There have already been abundant case reports and case series studying the use of dupilumab in BP with promising results [Citation12–33] (). However, the mechanisms underlying the therapeutic effects have not been fully revealed.
Table 1. Case reports and studies of BP patients treated with dupilumab.
Our review briefly summarizes the autoimmune mechanisms involved in BP, the roles of IL-4 and IL-13 in immune disorders, and the roles of IL-4 and IL-13 in the pathophysiology of BP. Additionally, we describe current anti-IL-4/13 strategies and their potential side effects in the treatment of BP.
Bullous pemphigoid and immune response
BP is regarded as an IgG-mediated disease. In patients with BP, IgG1, and IgG4 are the predominant circulating subtypes of IgG [Citation34]. IL-4 reportedly can interfere with IgG isotype switching by promoting the production of IgG4 [Citation35]. When IgG1 autoantibodies bind to their targets, complement activation is induced. Complement fragments (e.g. C3a and C5a) then recruit neutrophils and eosinophils to BP lesions [Citation6]. Subsequently, neutrophils and eosinophils release proteolytic enzymes, such as matrix metallopeptidase-9 and neutrophil elastase, which destroy the linkage between dermis and epidermis and result in subepidermal blistering [Citation6]. In contrast to IgG1, IgG4 autoantibodies have a more complex function in BP; they may promote blister formation in a complement-independent manner [Citation36]. Patients with dual IgG1 and IgG4 responses to BP180 often exhibit severe skin evolvement [Citation37]. Mihai et al. suggested that, although IgG4 does not fix complement, it contributes to the formation of blisters by activating leukocytes and inducing dermal-epidermal separation [Citation38]. However, another study showed that IgG4 autoantibodies may play an immunoregulatory role in BP by inhibiting the binding of IgG1 and IgG3 to the NC16A region, thereby attenuating complement activation and blister formation [Citation39].
In addition to IgG autoantibodies, IgE autoantibodies are associated with BP [Citation40]. Whereas the pathogenic role of IgE in allergic disorders is well-known, the potential role of IgE in BP is also under research. Similar to IgG, IgE BP180 NC16A-specific autoantibodies have been identified [Citation41,Citation42]. Moreover, the levels of IgE autoantibodies targeting NC16A correspond to BP severity and activity [Citation41,Citation43].
IgE has two receptors, FcεRI and FcεRII (CD23). FcεRI is a high-affinity receptor, primarily distributed on mast cells and basophils; CD23 is a low-affinity receptor, found mainly on mature B cells and eosinophils. Both receptors are involved in stimulating the Th2 response [Citation44]. The binding of IgE autoantibodies to mast cells and basophils induces degranulation, which is responsible for eosinophil infiltration in the BP lesions [Citation45]. Mast cells express high levels of FcεRI for eosinophils recruitment and can produce major regulating cytokines for eosinophils like IL-5 [Citation46]. Consistent with the distribution of receptors, Freire et al. reported that IgE autoantibodies could not only be detected in the serum of patients with BP but also in the basement membrane zone and on the surface of mast cells and eosinophils, which indicates the interaction between IgE and cells with high-affinity receptors [Citation45]. Furthermore, there is evidence that both FcεR-dependent and -independent immune reactions promote blister development in BP, and that IgE is involved in the onset of BP [Citation40,Citation47–50]. For in vivo studies, Lin et al. found that the severity of the disease depends on IgE dose and is related to the degree of eosinophil infiltration in lesions [Citation51]. In addition to inducing blister formation, the administration of IgE autoantibodies can also recapitulate similar symptoms, such as pruritus, erythema, and eosinophilia, which are absent in sole IgG-based mouse models [Citation40,Citation47]. This finding may explain why patients with BP often exhibit pruritic erythema and eosinophilic infiltration.
Eosinophilia is a typical pathological feature of BP. The numbers of eosinophils and secretory granules (e.g. eosinophil cationic protein) in serum are reportedly correlated with the severity of BP [Citation52,Citation53]. Eosinophils can promote the pathogenesis of blistering in BP by releasing proteolytic enzymes and producing extracellular traps [Citation35,Citation54]. Pruritus in BP could last for months or remain the only symptom, which is difficult to control [Citation55]. The mechanism underlying the onset of pruritus in BP may include multiple mediators, such as cytokines, chemokines, proteases, and associated receptors [Citation56]. The study by Hashimoto et al. indicated that eosinophil is related to pruritus severity of BP [Citation56], presumably because of chemokines activated by eosinophils. A meta-analysis revealed that numerous chemokines were elevated in BP. The levels of CCL11 (eotaxin 1), CCL17, and tumor necrosis factor-α were elevated in blister fluid, whereas CCL26 (eotaxin 3) was elevated in serum [Citation57]. CCL11 and CCL26 are important chemokines that mediate eosinophil infiltration and degranulation. Additionally, IL-31, a Th2 cytokine primarily expressed by eosinophils in BP [Citation58], may also contribute to pruritus in BP.
Because IgE and eosinophils are mostly associated with the Th2 immune response, further investigations are needed to determine whether BP fits in the category of Th2-dominant disease. The cytokine patterns of Th1-dominant and Th2-dominant immune diseases are distinct. Most autoimmune diseases regarded as Th1-associated conditions exhibit high levels of IL-2, interferon-γ, and IL-12. Th2-associated diseases, such as allergic and atopic dermatitis, are characterized by high levels of IL-4, IL-5, and IL-13. By detecting the cytokine levels in BP, researchers identified greater numbers of IL-4/13-producing cells at the lesion site or perilesional site of BP [Citation59,Citation60], indicating an unneglectable involvement of Th2-mediated autoimmunity in BP pathogenesis.
Roles of IL-4 and IL-13 in BP
Sources of IL-4 and IL-13
Th2 cells are presumed to have key roles in the pathogenesis of allergic disorders, such as AD, asthma, and chronic rhinosinusitis. As described above, BP is regarded as a Th2-related immune response disease that involves overexpression of IL-4, IL-5, and IL-13 in the past decades, however, the discoveries of cells other than Th2 cells that produce IL-4 and IL-13 add to its complexity. It was revealed that group 2 innate lymphoid cells (ILC2s) are important sources of IL-13, while follicular helper T cells (Tfh) are sources of IL-4 [Citation61,Citation62].
ILC2 cells are innate lymphoid cells that can produce large amounts of type 2 cytokines. Considerable research has been conducted regarding the role of ILC2s in asthma. In mouse studies regarding the pathogenesis of asthma, it was revealed that ILC2s are major sources of IL-5 and IL-13 [Citation63]. Interestingly, the authors found that when patients with asthma were treated with corticosteroids, cytokines produced by Th2 cells decreased but cytokines of ILC2s were not suppressed. These findings may explain the corticosteroid resistance in asthma patients [Citation63]. The study from Bartemes et al. revealed that ILC2s were abundant in the peripheral blood of patients with allergic asthma, and ILC2s could be recruited to mucosal tissues through peripheral blood circulation. Furthermore, ILC2s also have the ability to induce eosinophil acumination in tissue [Citation64]. However, allergic rhinitis, another Th2-dominant immune response disease, did not show an increase in ILC2s [Citation64]. In skin diseases, ILC2s have been proven to be involved in AD [Citation65], but there has been no research concerning ILC2s in BP. Although ILC2s may not able to be markers for Th2 immune response, further studies are needed to fully elucidate their role.
Tfh cells are a subset of CD4+ helper T cells, with distinct but overlapping molecular mechanisms as Th2 cells to regulate IL-4 [Citation66]. King et al. identified Tfh responses in the context of Th2 immunity, suggesting that Tfh cells might be the main source of IL-4 in vivo [Citation67]. It was also reported that IgE and IgG1 antibody responses in allergic disease were mainly controlled by IL-4-secreting Tfh cells, rather than Th2 cells [Citation61]. In the study of autoimmune bullous disease, it was revealed that Tfh-like CD4+ tissue-resident memory cells were present in pemphigus lesions [Citation68]. In BP, Li et al. found that Tfh cell counts were significantly higher in the peripheral blood of patients with BP and they observed an obvious decrease in Tfh levels after effective therapy. This indicates that Tfh cells are involved in the pathogenesis of BP [Citation69]. However, to our knowledge, no research has been conducted regarding the presence and involvement of Tfh-like cells in the skin of patients with BP.
Signaling of IL-4 and IL-13
IL-4 can bind to two receptors, the type I receptor and type II receptor. Type I receptors are mainly distributed on lymphocytes and myeloid cells, whereas type II receptors are present on myeloid cells and all non-hematopoietic cells () [Citation70]. Type I receptors are composed of IL-4Rα and γc, whereas type II receptors are composed of IL-4Rα and IL-13Rα1 [Citation71]. IL-13Rα1 not only serves as a subunit of type II receptors for binding IL-4 but also a receptor for IL-13 (). Once IL-4 binds with type I receptors or type II receptors, downstream signaling molecules, such as Janus kinase (JAK) 3, JAK1, or JAK2/tyrosine kinase 2 (TYK2) are activated [Citation71]. These signaling molecules then phosphorylate with each other to induce changes in the cytoplasmic tails of receptors, which serve as docking sites for downstream signaling molecules like signal transducer and activator of transcription 6 (STAT) and insulin receptor substrate (IRS) [Citation71]. STAT6 and IRS are the two main pathways in IL-4/13 signaling. STAT6 can bind with DNA sequences to initiate gene transcription, while the IRS-2 pathway does not translocate to the nucleus but activate signaling molecules like PI3-K [Citation72,Citation73] to initiate gene transcription. The STAT6 pathway has been well-studied in asthma, where it is responsible for Th2 differentiation and eosinophil migration. However, the IRS pathway is presumed to be critical for cancer proliferation and metastasis [Citation71].
Figure 1. IL-4 and IL-13 receptor structure IL-4 can bind to both type I and type II receptors. (A) Type I receptors consist of IL-4Rα and γc, whereas type II receptors consist of IL-4Rα and IL-13Rα1. When IL-4 binds to a type I receptor, Janus kinase (JAK) 1 and JAK3 are activated; both can induce tyrosine phosphorylation of the type I receptor intracellular domain, forming docking sites for downstream signaling molecules, such as signal transducer and activator of transcription (STAT) 6 and insulin receptor substrate-2 (IRS-2). Homodimers of STAT6 then translocate to the nucleus to facilitate transcription of IL-4- and IL-13-dependent genes. IRS-2 can activate signaling molecules, such as PI3K to induce gene transcription. (B) IL-13 binds to IL-13Rα2 with greater affinity than IL-13Rα1; the IL-13Rα2 receptor is considered a decoy receptor because it lacks a cytoplasmic signaling tail. However, the cytoplasmic domain of IL-13Rα2 may attenuate IL-4 signaling by inhibiting dimerization with γc or IL-13Rα1. (C) When IL-4 or IL-13 binds to a type II receptor, JAK1 and JAK2/TYK2 are activated.
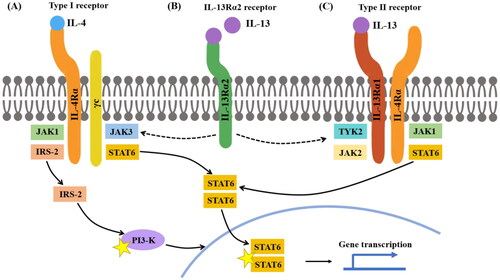
Similar to IL-4, IL-13 can activate STAT6 by combining the type II receptor [Citation71]. When IL-13 combines with the type II receptors, the following signaling pathway is similar to IL-4 signaling. JAK1 or JAK2/TYK2 will be activated and gene transcription will be conducted by the STAT6 pathway. In addition to the IL-13Rα1 in type II receptors, IL-13Rα2 is an alternative receptor chain for IL-13 that exhibits higher binding affinity [Citation71]. When combined with IL-13, IL-13Rα2 does not initiate the typical STAT6/IRS signaling to promote IL-4-driven inflammation. Therefore, IL-13Rα2 used to be considered merely a decoy receptor of IL-13 for the lacking of cytoplasmic tail signaling motifs. However, there has been increasing evidence that IL-13Rα2 has additional functions [Citation74–76]. Andrews et al. reported that IL-13Rα2 can attenuate IL-4 signaling by interacting with the cytoplasmic tail of IL-4α [Citation75].
Relationship between IL-4/13 and BP
IL-4 plays an important role in the differentiation of naive CD4+ T cells into Th2, forming a positive feedback loop between Th2 cells and IL-4 [Citation77,Citation78]. By suppressing pro-inflammatory cytokines like IL-1, IL-6, IL-8, interferon-γ, and tumor necrosis factor-α, IL-4 can also hinder the differentiation of naive CD4+ cells into Th1 cells [Citation79]. Therefore, IL-4 can enhance the Th2 response and inhibit the Th1 response to induce Th2 polarization. As a B cell helper, the enhanced Th2 immune response promotes the production of autoantibodies, such as autoimmune IgG and IgE, leading to tissue damage in BP. In addition to promoting IgE production by regulating Th2 differentiation, IL-4 is an essential factor for class switching to IgG1 and IgE in B cells. IL-13 is also able to induce class switching, however with less ability compared to IL-4 [Citation80]. As noted above, IgG1 and IgE autoantibodies are key factors in the development of BP.
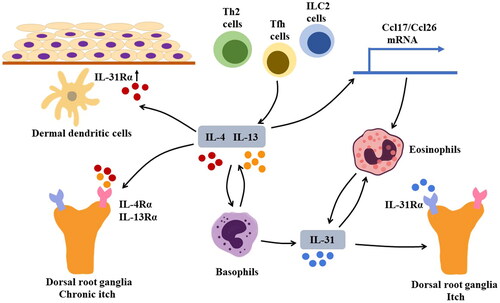
The underlying pathogenesis of pruritis in BP is complicated, where both IL-4 and IL-13 play important roles (). IL-4 and IL-13 are known to activate mast cells, basophils, and macrophages [Citation80]. Among these cells, basophils can produce IL-31, a cytokine that stimulates IL-31Rα on sensory neurons and induces cutaneous nerve growth and branching, leading to severe pruritus [Citation81–84]. High levels of IL-31 have been detected in the serum and blister fluid of patients with BP [Citation85]. There are studies suggesting that basophils secreted IL-31 could recruit eosinophils to the site of the lesions [Citation86]. As the recruited eosinophils produce IL-4 and IL-13, more basophils are activated to produce IL-31, thus forming a positive feedback loop to promote pruritus in BP. Furthermore, basophils can produce IL-4 and IL-13 [Citation65,Citation87]. In addition to its effects on the levels of IL-31, IL-4 can interfere with the expression of IL-31Rα. In one study, IL-4 was found to stimulate the expression of IL-31Rα in mouse bone marrow-derived dermal dendritic cells in a dose-dependent manner [Citation82]. Increased interactions between IL-31 and IL-31Rα led to greater production of chemokines that promote Th2 cell response, resulting in the exacerbation of pruritis symptoms [Citation82]. Apart from IL-31, IL-4/13 can directly induce pruritis. Oetjen et al. observed that IL-4Rα and IL-13Rα were expressed on mouse dorsal root ganglia (neurons that mediate skin sensation), revealing that IL-4 and IL-13 can act directly on sensory neurons and might promote chronic itch through the JAK/STAT signaling pathway discussed above [Citation88]. However, Hashimoto et al. indicated that IL-13, rather than IL-4, was correlated with pruritus severity in BP [Citation56]. They speculated that in addition to interacting with IL-13R, IL-13 may contribute to pruritis symptoms by recruiting eosinophils to sites of inflammation in BP lesions.
Figure 2. Mechanism of pruritus in BP Sources of IL-4 and IL-13 may include T helper 2 (Th2) cells, group 2 innate lymphoid cells (ILC2s), and follicular helper T (Tfh) cells. IL-4 and IL-13 can activate basophils that produce IL-31, a cytokine that induces pruritus by stimulating IL-31Rα on sensory neurons. Additionally, basophils can produce IL-4 and IL-13; IL-31 can recruit eosinophils to lesion sites, contributing to the formation of a positive feedback loop that exacerbates pruritis. In addition to IL-31, IL-4, and IL-13 can directly cause chronic pruritus by interacting with IL-4Rα and IL-13Rα on sensory neurons. Furthermore, IL-4 can induce upregulation of IL-31Rα on dermal dendritic cells. The augmented IL-31/IL-31Rα interaction can lead to increased production of CCL17 and CCL22, thus promoting Th2-related immune response.
The distribution pattern and disease correlation of IL-4/13 with BP were also studied. The study by Teraki et al. showed that BP was a unique organ-specific autoimmune disease with increasing numbers of skin-homing IL-4- and IL-13-producing cells [Citation59]. In their study, treatment with corticosteroids led to a significant reduction in the number of IL-13-producing cells, along with an improvement in clinical symptoms. The distribution of these IL-4/13 producing cells may be different since it was also reported that IL-4 was mostly localized within the superficial dermis while IL-13 was localized both in the upper and deep dermis [Citation60]. The mechanisms underlying these cytokine distribution patterns require further exploration; the patterns highlight the complexity of local immunological pathogenesis in BP. It was also reported that IL-4 concentration was remarkably higher in blisters than in serum in patients, which indicates that IL-4 may be a major contributor to local skin lesion formation than IL-13 [Citation89].
Considering the roles of IL-4 and IL-13 in amplifying Th2 polarization, autoantibody class switching, pruritus, and skin-homing T cells-cell accumulation, it is reasonable to target both cytokines in the treatment of BP.
Anti-IL-4/13 therapy as a potential treatment for BP
Th2-related diseases, including BP, often present with similar characteristics: elevated levels of IgE autoantibodies, higher numbers of circulating and infiltrating eosinophils, and increased production of downstream chemokines [Citation90,Citation91]. When considering the reduction of the overall Th2 response, it is reasonable to target the Th2 axis. Drugs targeting cytokines in the Th2 axis have already been used for AD treatment with satisfactory efficacy [Citation11]. Due to the similarity of cytokine patterns between BP and AD, the anti-IL-4/13 treatment is promising in managing BP.
Dual targeting of IL-4/13: dupilumab
AD is immunologically regarded as a Th2-dominant immune response disease. IL-13 acts as a disease-inducing agent, whereas IL-4 acts as a Th2 response amplifier [Citation10]. Dupilumab targets both IL-4 and IL-13 and therefore have a potential impact on the Th2 axis, such as interfering with positive feedback loops in the Th2 response, downregulating the concentration of IgE levels, and infiltration of eosinophils. Dupilumab has been reported to significantly downregulate serum levels of CCL17, a key regulator of the Th2 immune response [Citation10]. In multiple clinical trials, dupilumab has demonstrated impressive efficacy in managing AD and showed satisfying tolerability in AD patients under 18 years old (Table S1). Considering the impressive efficacy of dupilumab, ongoing clinical trials are studying the use of dupilumab in other dermatoses, such as allergic contact dermatitis (NCT03935971), cholinergic urticaria (NCT03749148), and chronic hand eczema (NCT04512339).
Similar to AD, in patients with BP, dupilumab may play a major part in relieving itch by interrupting the neuronal stimulation caused by IL-4 and IL-13. In one multicenter case series, the use of dupilumab was investigated in 13 patients with BP [Citation13] (). Some patients received concomitant medications, including systemic or topical corticosteroids and methotrexate. The study used the same dosing approved for AD: initially, 600 mg subcutaneously, followed by 300 mg subcutaneously every 2 weeks [Citation13]. In that case series, 53.8% (7 of 13) patients achieved disease clearance, defined as the clearance of both bullae and pruritus. 76.9% (10 of 13) patients showed improvement of bullae with residual pruritus. Furthermore, no obvious adverse events were reported in patient records with dupilumab administration. In a recent systematic review, similar results were reported (). The complete remission rate in patients was 66.7% (24 of 36) within 4.5 months of dupilumab treatment; the recurrence rate was 5.6% (2 of 36) [Citation28]. The usage of dupilumab has also been studied in a case-control paradigm, along with methylprednisolone and azathioprine, for the treatment of moderate-to-severe BP (). In that study, the group with additional dupilumab treatment demonstrated better performance, compared with the group receiving methylprednisolone and azathioprine. The dupilumab group exhibit a shorter time to blistering cessation and steroid tapering, whereas the difference in length of hospital stay was not statistically significant. Nevertheless, patients in the dupilumab group achieved more rapid pruritus relief within the first 2 weeks. Surprisingly, there was no difference in the time to the reduction of eosinophil count between the two groups. A possible explanation for this lack of difference is that methylprednisolone may be sufficiently effective to reduce eosinophil count within a few hours [Citation14]. There is also an ongoing clinical trial (NCT04206553) that aims to assess the safety and tolerability of dupilumab administered to patients with BP, and to evaluate its effect on pruritus. This may provide additional evidence for the use of dupilumab in BP, but further clinical trials are needed.
Antibodies targeting IL-13
Tralokinumab is a humanized monoclonal IgG4 antibody that inhibits the binding of IL-13 to IL-13Rα1 and IL-13Rα2. The binding of tralokinumab to IL-13Rα1 interferes with the heterodimerization of IL-13Rα1 with IL-4Rα, whereas the binding of tralokinumab to IL-13Rα2 inhibits endogenous regulation. In a series of clinical trials (ECZTRA 1–6), tralokinumab demonstrated satisfactory efficacy and safety in the treatment of AD (Table S1). In 52-week phase 3 trials (NCT03131648 and NCT03160885), participants treated with tralokinumab demonstrated improvements in primary endpoints and all key secondary endpoints, compared with participants given placebo. The clinical trials also showed that most tralokinumab responders at week 16 continued to exhibit good tolerance at week 52 without rescue treatment [Citation92]. In the safety assessment, most adverse events were non-serious; upper respiratory tract infection and conjunctivitis were the most common side effects.
Lebrikizumab is also an autoantibody drug that targets IL-13. A phase 2b study (NCT03443024) found that lebrikizumab provided rapid, dose-dependent efficacy with a favorable safety profile in adult AD patients [Citation93]. In that study, the lebrikizumab group showed significant improvement in the primary endpoint at week 16, compared with the placebo group. In contrast to tralokinumab, lebrikizumab only inhibits the binding of IL-13 to IL-13Rα1; it cannot inhibit the binding of IL-13 to IL-13Rα2 [Citation94]. Ideally, by analyzing the efficacy of tralokinumab and lebrikizumab, it may provide evidence of the different biological effects of IL-13Rα1 and IL-13Rα2 signaling. To our knowledge, no clinical trials have explored these effects thus far. However, Miyano et al. developed a mathematical model to study the efficacies of biological drugs based on a meta-analysis of the most recent clinical trials in AD [Citation95]. According to their model, lebrikizumab was more effective than tralokinumab in the treatment of AD. Furthermore, the effectiveness of tralokinumab was 44% of the lebrikizumab effectiveness in terms of inhibiting IL-13 signaling, which may be explained by the finding that IL-13Rα2 mainly acts as a decoy receptor for IL-13 and may inhibit the IL-4 signaling cascade. They also reported that dupilumab and lebrikizumab showed comparable efficacy, suggesting that IL-13 is the main contributor to the efficacy of dupilumab in AD because lebrikizumab does not target IL-4. Therefore, we expect that IL-13 antibodies will find applications in the treatment of BP.
Possible side effects of anti-IL-4/13 therapies
Although anti-IL-4/13 therapy sounds promising, it may have various side effects. The target receptors are not exclusive to hematopoietic cells. They are also distributed in myeloid cells and all non-hematopoietic cells [Citation70]. The side effects of anti-IL-4/13 therapy may be complex because of the wide receptor distribution. Because of similarities in the pathological mechanisms of BP and AD, the potential side effects of BP can be explored by reviewing relevant studies of AD.
Notably, IL-4/13 signaling has been shown to participate in metabolism, tissue regeneration, remodeling, cancer, as well as cognitive function [Citation96]. IL-4 deficient mice and IL-13 deficient mice both showed severe cognitive impairment, as measured by the Morris water maze test [Citation97,Citation98]. The authors suggested that by stimulating astrocytes in the meninges and hippocampus, Th2 cytokines may improve cognitive functions [Citation97]. There is a consensus that BP is closely associated with neurodegenerative diseases, and that patients with BP are more likely to develop cognitive impairment [Citation99–101]. To our knowledge, there is no consensus regarding the levels of IL-4 and IL-13 in the cerebrospinal fluid of patients with BP, or whether IL-4 and IL-13 contribute to cognitive impairment in BP. In healthy conditions, the blood-brain barrier restricts the access of macromolecules (e.g. anti-IL-4/13 drugs) to the central nervous system. However, in patients with neurodegenerative diseases, disruption of the blood-brain barrier is common [Citation102]. It is unclear whether anti-IL-4/13 drugs can cross the blood-brain barrier in certain pathological conditions. Because there is no consensus regarding the mechanisms that underlie the concurrent onset of BP and neurodegenerative diseases, further studies are needed to determine whether anti-IL-4/13 therapy will increase susceptibility to cognitive damage or dementia among patients with BP.
A systematic review was performed concerning the side effects of anti-IL-4/13 therapy in preclinical and clinical studies from 2006 to 2016. It found no significant increases in major side effects, such as severe infections, malignancies, or cardiovascular events. Furthermore, compared with IL-4/13 dual targeting therapies, biologics targeting IL-13 alone did not exhibit differences in terms of safety [Citation103]. Overall, anti-IL-4/13 therapy is presumed to be safe for most patients.
Conclusion
As typical Th2 cytokines, IL-4 and IL-13 may contribute to the pathogenesis of BP in multiple ways. The possible mechanisms include: promoting Th2 cell polarization, driving immunoglobulin class switching to IgG1 and IgE, interfering with IgG isotype switching by promoting the production of IgG4, recruiting eosinophils and basophils, and mediating pruritus by increasing the production of IL-31. The management of BP is challenging due to the side effects of traditional therapies including corticosteroids and immunosuppressants, whereas IL-4/13 antagonists, such as dupilumab had demonstrated satisfactory outcomes in preliminary BP clinical studies. Thus, IL-4/13 monoclonal antibodies in BP deserve further study and might be regularly used for BP therapy in the future.
Author contributions
Fangyuan Chen and Li Li contributed to the conceptualization of the review. Fangyuan Chen and Yiman Wang wrote the manuscript. Xinyi Chen constructed the figures. Fangyuan Chen and Nan Yang conducted the literature search and constructed the tables.
Supplemental Material
Download MS Word (17.5 KB)Acknowledgements
We thank Ryan Chastain-Gross, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109(5):679–683.
- Di Zenzo G, Thoma-Uszynski S, Fontao L, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. 2008;128(3):415–426.
- Thoma-Uszynski S, Uter W, Schwietzke S, et al. Autoreactive T and B cells from bullous pemphigoid (BP) patients recognize epitopes clustered in distinct regions of BP180 and BP230. J Immunol. 2006;176(3):2015–2023.
- Amber KT, Murrell DF, Schmidt E, et al. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol. 2018;54(1):26–51.
- Giudice GJ, Wilske KC, Anhalt GJ, et al. Development of an ELISA to detect anti-BP180 autoantibodies in bullous pemphigoid and herpes gestationis. J Invest Dermatol. 1994;102(6):878–881.
- Bağcı IS, Horváth ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16(5):445–455.
- Liu Z, Giudice GJ, Swartz SJ, et al. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95(4):1539–1544.
- Sams WMJr., Gammon WR. Mechanism of lesion production in pemphigus and pemphigoid. J Am Acad Dermatol. 1982;6(4 Pt 1):431–452.
- Patton T, Korman NJ. Bullous pemphigoid treatment review. Expert Opin Pharmacother. 2006;7(17):2403–2411.
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 Suppl 1):S28–S36.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139.
- Russo R, Capurro N, Cozzani E, et al. Use of dupilumab in bullous pemphigoid: where are We now? J Clin Med. 2022;11(12):3367.
- Abdat R, Waldman RA, de Bedout V, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52.
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate-to-severe bullous pemphigoid. Front Immunol. 2021;12:738907.
- Seyed Jafari SM, Feldmeyer L, Bossart S, et al. Case report: combination of omalizumab and dupilumab for recalcitrant bullous pemphigoid. Front Immunol. 2021;11:611549.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154(10):1225–1226.
- Wang M, Wang J, Shi B. Case report: dupilumab for the treatment of bullous pemphigoid. Dermatol Ther. 2022;35(7):e15541.
- Li W, Cai S, Man X. The treatment of refractory atypical bullous pemphigoid with generalized eczema and intense pruritus with dupilumab. Dermatol Ther. 2022;35(2):e15243.
- Yang J, Gao H, Zhang Z, et al. Dupilumab combined with low-dose systemic steroid therapy improves efficacy and safety for bullous pemphigoid. Dermatol Ther. 2022;35(8):e15648.
- Shan Y, Zuo YG. A successful case of vesicular pemphigoid concurrent with pulmonary tuberculosis with dupilumab. Dermatol Ther. 2022;35(4):e15330.
- Pop SR, Strock D, Smith RJ. Dupilumab for the treatment of pembrolizumab-induced bullous pemphigoid: a case report. Dermatol Ther. 2022;35(8):e15623.
- Jendoubi F, Bost C, Tournier E, et al. Severe pemphigoid nodularis successfully treated with dupilumab. Dermatol Ther. 2022;35(9):e15727.
- Bruni M, Moar A, Schena D, et al. A case of nivolumab-induced bullous pemphigoid successfully treated with dupilumab. Dermatol Online J. 2022;28(2):6.
- Zhang Y, Zhang J, Chen J, et al. Dupilumab successfully treated refractory bullous pemphigoid with early clinical manifestations imitating atopic dermatitis: a case letter. Australas J Dermatol. 2021;62(4):525–527.
- Saleh M, Reedy M, Torok H, et al. Successful treatment of bullous pemphigoid with dupilumab: a case and brief review of the literature. Dermatol Online J. 2021;27(4):7.
- Klepper EM, Robinson HN. Dupilumab for the treatment of nivolumab-induced bullous pemphigoid: a case report and review of the literature. Dermatol Online J. 2021;27(9):6.
- Seidman JS, Eichenfield DZ, Orme CM. Dupilumab for bullous pemphigoid with intractable pruritus. Dermatol Online J. 2019;25(11):12.
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621.
- Velin M, Dugourd PM, Sanchez A, et al. Efficacy and safety of methotrexate, omalizumab and dupilumab for bullous pemphigoid in patients resistant or contraindicated to oral steroids. A monocentric real-life study. J Eur Acad Dermatol Venereol. 2022;36(7):e539–e542.
- Takamura S, Teraki Y. Treatment of bullous pemphigoid with dupilumab: dupilumab exerts its effect by primarily suppressing T-helper 2 cytokines. J Dermatol. 2022;49(9):845–850.
- Bal A, Sorensen A, Ondreyco SM. Nonbullous erythrodermic pemphigoid with florid lymphadenopathy, response to dupilumab. JAAD Case Rep. 2021;17:58–60.
- Liu X, Ma J, Qiu X, et al. Dupilumab, an emerging therapeutic choice for recalcitrant subepidermal autoimmune bullous diseases: a case series of three patients. Eur J Dermatol. 2021;31(6):846–847.
- Valenti M, De Giacomo P, Lavecchia A, et al. A severe case of IgA bullous pemphigoid successfully treated with dupilumab. Dermatol Ther. 2022;35(11):e15890.
- Sitaru C, Mihai S, Zillikens D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch Dermatol Res. 2007;299(1):1–8.
- Giomi B, Caproni M, Calzolari A, et al. Th1, Th2 and Th3 cytokines in the pathogenesis of bullous pemphigoid. J Dermatol Sci. 2002;30(2):116–128.
- Dainichi T, Nishie W, Yamagami Y, et al. Bullous pemphigoid suggestive of complement-independent blister formation with anti-BP180 IgG4 autoantibodies. Br J Dermatol. 2016;175(1):187–190.
- Hofmann S, Thoma-Uszynski S, Hunziker T, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol. 2002;119(5):1065–1073.
- Mihai S, Chiriac MT, Herrero-Gonzalez JE, et al. IgG4 autoantibodies induce dermal-epidermal separation. J Cell Mol Med. 2007;11(5):1117–1128.
- Zuo Y, Evangelista F, Culton D, et al. IgG4 autoantibodies are inhibitory in the autoimmune disease bullous pemphigoid. J Autoimmun. 2016;73:111–119.
- Fairley JA, Burnett CT, Fu CL, et al. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. 2007;127(11):2605–2611.
- Döpp R, Schmidt E, Chimanovitch I, et al. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42(4):577–583.
- Dresow SK, Sitaru C, Recke A, et al. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol. 2009;160(2):429–432.
- Kalowska M, Ciepiela O, Kowalewski C, et al. Enzyme-linked immunoassay index for anti-NC16a IgG and IgE auto-antibodies correlates with severity and activity of bullous pemphigoid. Acta Derm Venereol. 2016;96(2):191–196.
- Messingham KN, Holahan HM, Frydman AS, et al. Human eosinophils express the high affinity IgE receptor, FcepsilonRI, in bullous pemphigoid. PLOS One. 2014;9(9):e107725.
- Freire PC, Muñoz CH, Stingl G. IgE autoreactivity in bullous pemphigoid: eosinophils and mast cells as major targets of pathogenic immune reactants. Br J Dermatol. 2017;177(6):1644–1653.
- Messingham KN, Crowe TP, Fairley JA. The intersection of IgE autoantibodies and eosinophilia in the pathogenesis of bullous pemphigoid. Front Immunol. 2019;10:2331.
- Zone JJ, Taylor T, Hull C, et al. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol. 2007;127(5):1167–1174.
- Messingham KN, Srikantha R, DeGueme AM, et al. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187(1):553–560.
- Amber KT, Valdebran M, Kridin K, et al. The role of eosinophils in bullous pemphigoid: a developing model of eosinophil pathogenicity in mucocutaneous disease. Front Med. 2018;5:201.
- Yu X, Holdorf K, Kasper B, et al. FcγRIIA and FcγRIIIB are required for autoantibody-induced tissue damage in experimental human models of bullous pemphigoid. J Invest Dermatol. 2010;130(12):2841–2844.
- Lin L, Hwang BJ, Culton DA, et al. Eosinophils mediate tissue injury in the autoimmune skin disease bullous pemphigoid. J Invest Dermatol. 2018;138(5):1032–1043.
- Bushkell LL, Jordon RE. Bullous pemphigoid: a cause of peripheral blood eosinophilia. J Am Acad Dermatol. 1983;8(5):648–651.
- Giusti D, Gatouillat G, Le Jan S, et al. Eosinophil cationic protein (ECP), a predictive marker of bullous pemphigoid severity and outcome. Sci Rep. 2017;7(1):4833.
- Simon D, Hoesli S, Roth N, et al. Eosinophil extracellular DNA traps in skin diseases. J Allergy Clin Immunol. 2011;127(1):194–199.
- Bakker CV, Terra JB, Pas HH, et al. Bullous pemphigoid as pruritus in the elderly: a common presentation. JAMA Dermatol. 2013;149(8):950–953.
- Hashimoto T, Kursewicz CD, Fayne RA, et al. Pathophysiologic mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol. 2020;83(1):53–62.
- Kowalski EH, Kneibner D, Kridin K, et al. Serum and blister fluid levels of cytokines and chemokines in pemphigus and bullous pemphigoid. Autoimmun Rev. 2019;18(5):526–534.
- Rudrich U, Gehring M, Papakonstantinou E, et al. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Derm Venereol. 2018;98(8):766–771.
- Teraki Y, Hotta T, Shiohara T. Skin-homing interleukin-4 and -13-producing cells contribute to bullous pemphigoid: remission of disease is associated with increased frequency of interleukin-10-producing cells. J Invest Dermatol. 2001;117(5):1097–1102.
- Rico MJ, Benning C, Weingart ES, et al. Characterization of skin cytokines in bullous pemphigoid and pemphigus vulgaris. Br J Dermatol. 1999;140(6):1079–1086.
- Kubo M. T follicular helper and T(H)2 cells in allergic responses. Allergol Int. 2017;66(3):377–381.
- Kabata H, Moro K, Koyasu S, et al. Group 2 innate lymphoid cells and asthma. Allergol Int. 2015;64(3):227–234.
- Kabata H, Moro K, Fukunaga K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675.
- Bartemes KR, Kephart GM, Fox SJ, et al. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671–678.e4.
- Ebbo M, Crinier A, Vely F, et al. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017;17(11):665–678.
- Vijayanand P, Seumois G, Simpson LJ, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36(2):175–187.
- King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206(5):1001–1007.
- Zou Y, Yuan H, Zhou S, et al. The pathogenic role of CD4(+) tissue-resident memory T cells bearing T follicular helper-like phenotype in pemphigus lesions. J Invest Dermatol. 2021;141(9):2141–2150.
- Li Q, Liu Z, Dang E, et al. Follicular helper T cells (Tfh) and IL-21 involvement in the pathogenesis of bullous pemphigoid. PLOS One. 2013;8(7):e68145.
- Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. 2018;9:888.
- McCormick SM, Heller NM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine. 2015;75(1):38–50.
- Sun XJ, Wang LM, Zhang Y, et al. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377(6545):173–177.
- Heller NM, Qi X, Gesbert F, et al. The extracellular and transmembrane domains of the gammaC and interleukin (IL)-13 receptor alpha1 chains, not their cytoplasmic domains, dictate the nature of signaling responses to IL-4 and IL-13. J Biol Chem. 2012;287(38):31948–31961.
- Chandriani S, DePianto DJ, N’Diaye EN, et al. Endogenously expressed IL-13Ralpha2 attenuates IL-13-mediated responses but does not activate signaling in human lung fibroblasts. J Immunol. 2014;193(1):111–119.
- Andrews AL, Nordgren IK, Campbell-Harding G, et al. The association of the cytoplasmic domains of interleukin 4 receptor alpha and interleukin 13 receptor alpha 2 regulates interleukin 4 signaling. Mol Biosyst. 2013;9(12):3009–3014.
- Zheng T, Liu W, Oh SY, et al. IL-13 receptor alpha2 selectively inhibits IL-13-induced responses in the murine lung. J Immunol. 2008;180(1):522–529.
- Hsieh CS, Heimberger AB, Gold JS, et al. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89(13):6065–6069.
- Seder RA. Acquisition of lymphokine-producing phenotype by CD4+ T cells. J Allergy Clin Immunol. 1994;94(6 Pt 2):1195–1202.
- Tavakolpour S, Tavakolpour V. Interleukin 4 inhibition as a potential therapeutic in pemphigus. Cytokine. 2016;77:189–195.
- Matsunaga K, Katoh N, Fujieda S, et al. Dupilumab: basic aspects and applications to allergic diseases. Allergol Int. 2020;69(2):187–196.
- Raap U, Gehring M, Kleiner S, et al. Human basophils are a source of and are differentially activated by IL-31. Clin Exp Allergy. 2017;47(4):499–508.
- Miake S, Tsuji G, Takemura M, et al. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced ccl 17 and ccl 22 production in dendritic cells: implications for atopic dermatitis. Int J Mol Sci. 2019;20(16):4053.
- Datsi A, Steinhoff M, Ahmad F, et al. Interleukin-31: the “itchy” cytokine in inflammation and therapy. Allergy. 2021;76(10):2982–2997.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138(2):500–508.e24.
- Salz M, Haeberle S, Hoffmann J, et al. Elevated IL-31 serum levels in bullous pemphigoid patients correlate with eosinophil numbers and are associated with BP180-IgE. J Dermatol Sci. 2017;87(3):309–311.
- Hashimoto T, Rosen JD, Sanders KM, et al. Possible roles of basophils in chronic itch. Exp Dermatol. 2019;28(12):1373–1379.
- Izuhara K, Arima K, Kanaji S, et al. IL-13: a promising therapeutic target for bronchial asthma. Curr Med Chem. 2006;13(19):2291–2298.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228 e13.
- Ameglio F, D’Auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138(4):611–614.
- Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–1485.
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4s):S65–S76.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449.
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156(4):411–420.
- Ultsch M, Bevers J, Nakamura G, et al. Structural basis of signaling blockade by anti-IL-13 antibody lebrikizumab. J Mol Biol. 2013;425(8):1330–1339.
- Miyano T, Irvine AD, Tanaka RJ. A mathematical model to identify optimal combinations of drug targets for dupilumab poor responders in atopic dermatitis. Allergy. 2022;77(2):582–594.
- Karo-Atar D, Bitton A, Benhar I, et al. Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: in allergy and beyond. BioDrugs. 2018;32(3):201–220.
- Brombacher TM, Nono JK, De Gouveia KS, et al. IL-13-Mediated regulation of learning and memory. J Immunol. 2017;198(7):2681–2688.
- Derecki NC, Cardani AN, Yang CH, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–1080.
- Zhao W, Wang Y, Mao X, et al. Detection of underlying dementia in bullous pemphigoid patients using cognitive evaluation tests: a multicenter case-control study. Ann Transl Med. 2020;8(21):1397.
- Papakonstantinou E, Limberg MM, Gehring M, et al. Neurological disorders are associated with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2019;33(5):925–929.
- Kokkonen N, Herukka SK, Huilaja L, et al. Increased levels of the bullous pemphigoid BP180 autoantibody are associated with more severe dementia in Alzheimer’s disease. J Invest Dermatol. 2017;137(1):71–76.
- Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276.
- Braddock M, Hanania NA, Sharafkhaneh A, et al. Potential risks related to modulating interleukin-13 and interleukin-4 signalling: a systematic review. Drug Saf. 2018;41(5):489–509.
