Abstract
Background
Vascular calcification (VC) is one of the complications of chronic kidney disease (CKD) patients. Previous studies have confirmed that oxidative stress (OS) plays an important role in developing VC and that antioxidants have anti-VC effects.
Objectives
Our study aimed to determine the relationship between the intake of antioxidants from dietary sources and the prevalence of VC, especially in the CKD population.
Methods
This cross-sectional study analyzed population-based data from the National Health and Nutrition Examination Survey (NHANES; 2013–2014). Participants were noninstitutionalized adults >40 years of age. Diet-derived antioxidants were obtained from the first 24-h dietary recall interviews. The abdominal aortic calcification (AAC) score was measured by a DXA scan. We divided the AAC scores into three groups: no calcification (AAC =0), mild to moderate calcification (0< AAC ≤6), and severe calcification (AAC >6).
Results
A total of 2897 participants were included in the main analysis. Our results showed that vitamin B6, α-tocopherol, and lycopene were associated with severe AAC in unadjusted models (odds ratio (OR): 0.81, 95% confidence interval (CI): 0.72–0.91, p = 0.001; OR: 0.97, 95% CI: 0.95–0.99, p = 0.008; OR: 0.98, 95% CI: 0.96–0.99, p = 0.01, respectively). However, only dietary lycopene was associated with severe AAC after adjusting covariates based on clinical and statistical significance. Per 1 mg higher intake of diet-derived lycopene per day, the odds of having severe AAC were 2% lower in the fully adjusted model (OR: 0.98, 95% CI: 0.95–0.999, p = 0.04). Moreover, in subgroup analysis, diet-derived antioxidant was not associated with AAC in patients with CKD.
Conclusion:
Our findings indicate that a higher intake of diet-derived lycopene was independently associated with lower odds of having severe AAC in humans. Therefore, a high intake of diet-derived lycopene may help prevent severe AAC.
KEY MESSAGES
Dietary lycopene was inversely associated with vascular calcification (VC) in adults.
Patients with chronic kidney disease (CKD) have a higher chance of having severe VC.
Dietary antioxidants were not significantly associated with VC in patients with CKD.
1. Introduction
The prevalence of cardiovascular disease in patients with chronic kidney disease (CKD) is higher than that of the general population, and it is also the leading cause of death in patients with CKD [Citation1,Citation2]. This is partly due to a high incidence of vascular calcification (VC) [Citation3], which causes increased vessel stiffness, pulse pressure, and left ventricular hypertrophy, leading to cardiovascular disease, myocardial infarction, and cardiac arrest [Citation4,Citation5]. In vitro and in vivo experiments, oxidative stress (OS) is thought to play an important role in the development and progression of VC [Citation6], which may regulate the PI3K/Akt pathway [Citation7], p38 MAPK [Citation8], and Runx2 [Citation9], thus inducing the transformation of vascular smooth muscle cells (VSMCs) into the phenotypic transformation of collagen-secreting osteoblasts. Furthermore, the uremic state is characterized by increased p-cresyl sulfate (pCS) and indoxyl sulfate (I.S.) in the blood circulation, which promotes OS by increasing the production of reactive oxygen species (ROS) [Citation10,Citation11].
Recent studies have shown that certain natural antioxidants are also dietary components, such as apocynin [Citation12], diosgenin [Citation13,Citation14], quercetin [Citation15,Citation16], resveratrol [Citation17], and vitamin E [Citation18], which have shown anti-VC properties. Many studies have found that dietary intake of nutrients has a protective effect on diseases, such as antioxidant intake reduces CKD mortality [Citation19] and zinc intake reduces calcification [Citation20]. Foods rich in these natural antioxidants may also benefit people with VC. Consequently, it raises an open question of whether the intake of dietary antioxidants is related to the occurrence of VC. On the other hand, if the development and progression of VC can be inhibited by dietary intake, then the intake of drugs can be reduced. after all, the potential side effects of drugs may be multifaceted. Thus, we hypothesized that a higher intake of antioxidants from dietary sources is associated with the prevalence of VC, especially for the CKD population.
To study the effects of the association of dietary antioxidants with VC, we first conducted an observational study using data from National Health and Nutrition Examination Survey (NHANES) cohort 2013–2014, which monitors nutritional status among the US civilian population.
2. Methods
2.1. Study population
The NHANES is a cross-sectional, multistage, stratified, clustered probability sample of the civilian, noninstitutionalized population of the United States conducted by the National Center of Health Statistics. The protocol was approved by the National Center for Health Statistics Ethics Review Board, and written informed consent was obtained from all participants. All NHANES data used in our analysis are publicly available at https://www.cdc.gov/nchs/nhanes [Citation21]. We examined descriptive data from NHANES 2013–2014. Participants aged ≥40 years were included, and those lacking dual-energy X-ray absorptiometry (DXA) scan or having an invalid scan, lacking dietary data, being pregnant, and weighing >450 g were excluded. After excluding 6467 participants aged <40, 481 with no DXA scan, 190 with invalid DXA scan, 243 with no dietary data, three pregnant women, and 1 participant weighing >450 g, 2897 participants were finally included for the main analysis () [Citation22]. The missing data of the included respondents is shown in supplementary Table 1.
2.2. Dietary antioxidants
To study the association between dietary antioxidants and AAC, six diet-derived antioxidants were selected, including retinol, β-carotene, vitamin B6, vitamin C, α-tocopherol, and lycopene [Citation23–27]. Information on the intake of these antioxidants was obtained from the total nutrient intake file on the NHANES website. NHANES derived the total nutrient intake file by performing a 24-hour dietary recall of participants, which diet included foods and dietary supplements, and calculating it based on the amount of each nutrient in the food.
2.3. Abdominal aortic calcification
Severe AAC is associated with a higher risk of cardiovascular heart disease and stroke even after adjusting for the traditional risk factors [Citation28]. In 2013–2014, lateral DXA scans of the thoraco-lumbar spine were administered in the NHANES mobile examination center (MEC). The Instant Vertebral Assessment (IVA) lateral spine scans provide vertebral fracture information for 13 individual vertebrae from T4-L4 and AAC measurement for vertebrae L1–L4. AAC-24 scoring semi-quantitative (SQ) techniques were used for the AAC evaluation [Citation28,Citation29]. In the scoring method for AAC-24, the anterior and posterior aortic walls are divided into four segments, corresponding to the areas in front of the lumbar vertebrae L1-L4. The scores were obtained separately for each segment according to the degree of calcification, resulting in a range from “0” to “6” for each vertebral level and “0” to “24” for the total score. A higher AAC score corresponded to a much more serious calcification condition of the abdominal aorta. According to AAC scores, we divided the calcification into three groups: no calcification (AAC = 0), mild to moderate calcification (0< AAC ≤6), and severe calcification (AAC >6) [Citation30].
2.4. Covariates
In the analysis, we included covariates such as age (40–49, 50–59, 60–69, or ≥70 years old), sex (male or female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other race), an education level (below high school, high school graduate or general educational development (GED) test, or some college and above), marital status (married or without married), currently smoking (yes or no), and family income (<1.30 or ≥1.30 of the Poverty-Income Ratio (PIR) [Citation31]. Family income was categorized using PIR information, an index of income in relation to the federally established poverty thresholds, which accounts for economic inflation and family size, and was applied to measure family income. Diabetes was defined as having a diagnosis of diabetes, taking hypoglycemic medications, glycated hemoglobin ≥6.5%, or fasting plasma glucose ≥126 mg/dL [Citation32]. We defined hypertension as having a diagnosis of hypertension, taking antihypertensive medications, or having three consecutive systolic blood pressure (SBP) readings ≥140 mmHg or diastolic blood pressure (DBP) readings ≥90 mmHg [Citation33]. Mean SBP and DBP were calculated from all available measurements for each respondent using the NHANES recommended method. Haemoglobin A1c, fasting plasma glucose, total cholesterol (TC), high-density lipoprotein (HDL), and other biochemical examinations were measured with the standard experimental methods in the laboratory. Albuminuria was defined as a urine albumin-to-creatinine ratio (ACR) of ≥30 mg/g. The CKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 with albuminuria. CKD groups were categorized as CKD Stages 1–2 (presence of albuminuria and eGFR ≥60 mL/min/1.73 m2) and Stages 3–5 (eGFR <60 mL/min/1.73 m2). The eGFR was calculated by using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation [Citation34]. Serum creatinine was measured using a kinetic rate Jaffe method and re-calibrated to standardized serum creatinine measurements obtained at the Cleveland Clinic Research Laboratory [Citation35].
2.5. Statistical analysis
Participant characteristics were reported as frequency (percentages) for categorical variables and compared with chi-square, Fisher’s exact tests, or Cochran–Mantel–Haenszel test (CMH). For continuous variables, data were shown as mean ± standard deviation if they followed a normal distribution and compared with Student’s t-test between or one-way analysis of variance (ANOVA), otherwise as median (interquartile range) and compared with Wilcoxon rank-sum test. Due to the large number of risk factors investigated in this study, only factors with p < 0.05 in the univariable analysis and traditional factors associated with AAC and dietary antioxidants were included in the multivariable logistic regression analysis [Citation36]. Model 1 was a multivariate logistic regression model adjusted by age, sex, race/ethnicity, and other diet-derived antioxidants. Model 2 included age, sex, marital status, race/ethnicity, body mass index (BMI), diabetes, hypertension, smoking history, eGFR, hemoglobin A1c, TC/HDL, mean systolic pressure, mean diastolic pressure, and other diet-derived antioxidants as adjusting factors. Senility, diabetes, hypertension, and CKD are risk factors for arterial calcification and may mask the association between dietary zinc and AAC. Subgroup analysis stratified by age, hypertension, diabetes, and CKD was also performed using stratified multivariate regression analysis. All tests were two-sided, and p < 0.05 was considered statistically significant. Statistical analysis was carried out using SAS 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Participants’ characteristics
A characteristics summary of 2897 participants was shown in . Among all participants, 29.9% had AAC (20.8% mild-moderate AAC and 9.1% severe AAC), 22.2% had diabetes, 57.7% had hypertension, and 21.1% had CKD (9.7% stage 1–2 and 11.5% stage 3–5). Relative to those without AAC, participants with severe AAC were older (71.54 vs 56.00 years old) and more likely to be Non-Hispanic White (64.3% vs 40.9%), unmarried (52.9% vs 38.9%), and have diabetes (39.2% vs 19.4%), hypertension (86.7% vs 51.2%), and CKD (49.0% vs 16.5%). They have lower BMI (26.70 vs 28.10 kg/m2), lower eGFR (66.81 vs 89.25 mL/min/1.73 m2), lower mean DBP (64.00 vs 72.00 mmHg), higher glycated hemoglobin (5.90 vs 5.60%), and higher mean SBP (133.00 vs 123.00 mmHg).
3.2. Associations between diet-derived antioxidants and AAC
Intakes of diet-derived vitamin A, β-carotene, and vitamin C did not differ significantly among AAC groups. Intake of diet-derived vitamin B6 was significantly higher in participants with AAC than in participants without AAC (group without AAC: 1.77 mg/day; group with mild-moderate AAC: 1.67 mg/day, group with severe AAC: 1.56 mg/day, p = 0.002). Intake of α-tocopherol and lycopene in participants without AAC (α-tocopherol: 7.17 mg/day; lycopene: 1.28 mg/day) was similar to those with mild-moderate AAC (α-tocopherol: 7.16 mg/day; lycopene: 1.56 mg/day), whereas those with severe AAC had a lower intake of dietary α-tocopherol and lycopene (α-tocopherol: 6.42 mg/day, p = 0.03; lycopene: 0.76 mg/day, p = 0.04).
The association between diet-derived antioxidants and having AAC was presented in . Intake of vitamin A, carotene, and vitamin C was not associated with mild-moderate AAC or severe AAC (p > 0.05). In unadjusted models, a higher intake of vitamin B6 and α-tocopherol were not associated with the lower odds of having mild-to-moderate calcification (odds ratios (OR): 0.96, 95% confidence interval (CI): 0.89–1.02, p =0.19; OR:1.00, 0.99–1.02, p = 0.66), but were associated with the lower odds of having severe calcification (0.81, 0.72–0.91, p = 0.001; 0.97, 0.95–0.99, p = 0.008, respectively). They were not associated with mild-moderate AAC or severe AAC after adjusting models with other covariates (p > 0.05). Per 1 mg higher intake of diet-derived lycopene per day, the odds of having severe AAC were 2% lower in the unadjusted model (p = 0.01) and were 2% lower in the fully adjusted model (model 1: 0.98, 0.96–0.999, p = 0.04; model 2: 0.98, 0.95–0.999, p = 0.04).
3.3. Associations between other covariates and AAC
Age, race/ethnicity, BMI, smoking history, hypertension, and blood pressure remained significantly associated with the odds of having mild-moderate and severe AAC in the fully adjusted model (). With one year older, the odds of having mild-to-moderate AAC were 3% higher, and having severe AAC was 12% higher after fully adjusted (p < 0.001). Compared with non-Hispanic whites, non-Hispanic blacks and Hispanics were associated with lower odds of mild-moderate and severe AAC (mild-moderate AAC: 0.66, 0.49–0.88, p = 0.005, 0.76, 0.58–0.99, p = 0.04; severe AAC: 0.31, 0.19–0.52, p < 0.001; 0.47, 0.29–0.77, p = 0.003, respectively). Per 1 kg/m2 higher BMI, the odds of having mild-moderate AAC were 4% lower, and severe AAC was 7% lower (p < 0.001). Smoker and hypertension patients had a higher risk of having mild-moderate and severe AAC in the reference group (p < 0.05). For blood pressure, the risk of having AAC increased as SBP increased and DBP decreased (mild-moderate AAC: SBP (per 1 mm Hg), 1.01, 1.00–1.02, DBP (per 1 mm Hg), 0.99, 0.98–0.995; severe AAC: SBP (per 1 mm Hg), 1.01, 1.00–1.02, DBP (per 1 mm Hg), 0.98, 0.96–0.99, respectively). Participants with diabetes had an almost double risk of having severe AAC compared to the reference group (1.97, 1.22–3.03, p = 0.005). Severe AAC was more common in participants with stage 3–5 CKD than in participants without CKD (1.75, 1.15-2.67, p = 0.01) and associated with CKD severity (p for trend = 0.03). TC/HDL was significantly associated with the risk of mild-moderate AAC (p = 0.006) but not with the risk of severe AAC (p = .49).
3.4. Subgroup analysis
To further assess the relationship between diet-derived antioxidants and AAC, subgroup analyses were performed according to age, diabetes, hypertension, and CKD ( and supplementary Figure 2). It was noted that intake of vitamin B6 negatively correlated with mild-moderate AAC in the population of age <60 (0.78, 0.62–0.97, p = 0.03). In contrast, in the population of age ≥60, this association was not statistically significant, indicating that this association could be more significant in younger individuals. Intake of lycopene was inversely associated with a higher risk of severe AAC in people without diabetes (0.97, 0.93–0.999, p = 0.04), whereas this association was not statistically significant among people with diabetes. However, diet-derived antioxidant was not associated with AAC in the patients with CKD (p > 0.05). And in other subgroup analyses, no statistically significant relationship was found.
4. Discussion
Here, our initial hypothesis was partially confirmed, suggesting that an increased intake of lycopene from dietary sources was independently associated with lower odds of developing severe AAC. After adjusting for the covariates of demographics, comorbidities, renal function, and other dietary sources of antioxidants, we found that taking 1 mg more of dietary sources of lycopene was associated with a 2% reduction in the odds of developing severe AAC. However, in subgroup analysis, diet-derived antioxidant was not associated with AAC in the patients with CKD.
Most studies have found that lycopene is beneficial for individuals with antioxidant deficiency or humans exposed to higher levels of OS, like metabolic syndrome [Citation37], patients on hemodialysis [Citation37], and patients with acute myocardial infarction [Citation38]. OS may age VSMC and cause loss of smooth muscle cell phenotype by causing telomere shortening, mitochondrial DNA damage, and mutagenic effects on gene fragments involved in contractile function, along with activation of osteogenic transcription factors, which are central to the underlying pathophysiology of VC [Citation39]. A large number of experimental evidence in vitro and vivo have revealed the association between antioxidants and VC. Ji et al. found that rosmarinic acid (RA), a common phenolic compound that possesses antioxidation, anti-inflammatory, and antimicrobial effects, improves VC by regulating the Nrf2 pathway and significantly reduced the levels of ALP, MDA, Ca, and P but increases SOD levels [Citation40]. Curcumin (CUR) was a natural polyphenolic antioxidant compound that increased miR-92b-3p expression and decreased KLF4 expression in the aorta thereby attenuating vitamin D3-induced VC [Citation41]. Furthermore, Huang et al. also found that antioxidant resveratrol ameliorated AAC in rats with chronic renal failure and VSMCs cultured in a high-phosphate environment [Citation17]. Using cultured rat VSMCs and a rat model of CKD with VC, resveratrol attenuated hypophosphite-induced β-catenin activation and inhibits Runx2 expression during VSMC osteogenic transdifferentiation [Citation17]. Runx2 is a key transcription factor that drives VC through a combination of osteogenic transition and apoptosis [Citation9,Citation42]. Therefore, lycopene can prevent the occurrence of severe calcification most likely by clearing ROS such as singlet molecular oxygen [Citation43]. There may be other concrete mechanisms that we don’t yet know, which is needed further exploration through experimental validation.
However, the Cox regression analysis recognized that the other diet-derived antioxidants intake was not associated with severe AAC. These differences may be due to the following reasons. First, the bioavailability of each diet-derived antioxidant is completely inconsistent. And the absorption, distribution, and metabolism of various diet-derived antioxidants are also not completely consistent. Through a meta-regression of in vitro studies, Yao et al. found that the bioavailability of β-carotene was positively correlated with the concentration of dietary fat [Citation44]. As was expected and already known, fat-soluble vitamins were found to have the best oral absorption and the best bioavailability [Citation45]. In addition, interactions between antioxidant substances can have different effects [Citation46]. Second, lycopene is the most potent antioxidant. Di Mascio et al. found that lycopene’s singlet oxygen quenching ability is higher than γ-carotene, tocopherols, and β-carotene [Citation43].
Our research found that diet-derived antioxidant was not associated with AAC in patients with CKD. The reason why the results we obtained differ from other studies [Citation17,Citation47] may be due to the small sample size (n = 589). We need to expand the sample size further to study its effect on calcification in patients with CKD. Consistent with the conclusions of published literature [Citation48], we found that although the incidence and prevalence of AAC in patients with CKD have increased significantly compared to the general population, patients with advanced CKD (stage CKD 3–5) have shown a significant higher chance of severe AAC.
Our study found that getting enough lycopene from a diet was associated with a lower chance of severe AAC, suggesting that lycopene may reduce the chance of severe calcification and demonstrating that the function of lycopene was not affected by dietary intake. Furthermore, because of the conjugated structure of lycopene, (all-E)-lycopene is quite an unstable molecule when isolated. However, lycopene was shown to be rather stable in its natural matrix, i.e. tomato [Citation49]. Adults may be able to prevent severe AAC by increasing their intake of foods rich in lycopene.
There are several strengths to our study. First, this is the first study in humans to study the relationship between dietary antioxidants and VC in the CKD state or the non-CKD state, and it can provide a dosage reference for dietary intake. Second, NHANES not only has a large sample size but also adopts standardized methods and has high data quality. Third, we adjusted for confounding covariates, chosen covariates based on our analysis and previous studies assessing the association of AAC with other exposure variables, to ensure that our results were robust. Finally, subgroup analyses of the main factors affecting ACC were performed to determine the study’s reliability further.
Our study has several limitations. Firstly, due to the cross-sectional nature of the study, we were unable to obtain a causal relationship between diet-derived lycopene and AAC. However, together with the findings that high-takes or high-serum concentrations of lycopene were associated with significant reductions in the risk of stroke [Citation50] and risk of mortality in the CKD population [Citation19], our findings create a foundation for further human studies to reveal the relationship between dietary lycopene intake and arterial calcification. Secondly, participants in our analysis were enrolled from a single country, and their ethnicity may not apply to many countries worldwide. Thirdly, due to the study design, the 24-hour dietary recall could not ensure that participants’ diet habits had not changed and whether there was a bias in the participants’ memory. Lastly, since the NHANES does not collect the content of individual substances in the serum since the serum levels of each antioxidant are not on the NHANES test list, it is impossible to exclude the certain influence of reasons such as absorption and excretion on statistical results.
Figure 1. Screening flow of participants included in the research. A total of 10,175 participants from the 2013–2014 NHANES were recruited for this study. The AAC score was measured by a dual-energy X-ray absorptiometry (DXA) scan. A total of 3140 participants completed DXA scans and obtained available data. Diet-derived antioxidants were obtained from 24-h dietary recall interviews. After excluding 243 participants who did not undergo 24-h dietary recall interviews, 2897 participants were included in the analysis. NHANES: National Health and Nutritional Examination Survey; AAC: abdominal aortic calcification.
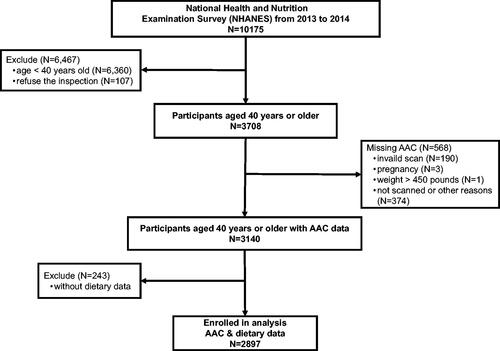
Figure 2. Subgroup analysis for the association between diet-derived lycopene and AAC. Subgroup analysis stratified by age, hypertension, diabetes, and CKD was also performed using stratified multivariate logistic regression analysis. *Logistic regression model adjusted for variables in age, sex, marital status, race/ethnicity, body mass index, diabetes, hypertension, smoking history, eGFR, Haemoglobin A1c, TC/HDL, mean SBP, mean DBP, and diet-derived antioxidants. AAC: abdominal aortic calcification; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; TC: total cholesterol; HDL: high-density lipoprotein; SBP: systolic blood pressure; DBP: diastolic blood pressure; OR: Odds ratio; CI: confidence interval.
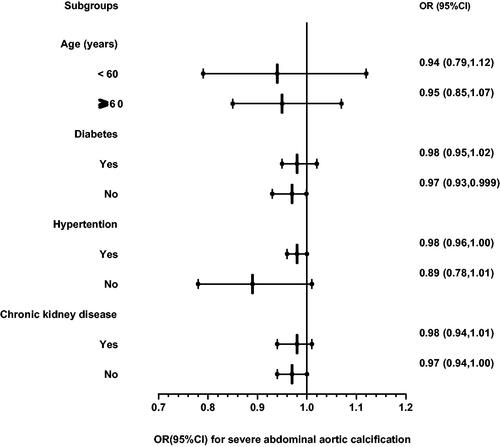
Table 1. Characteristics and dietary antioxidant assessment based on no, mild-moderate, and severe calcification in the ACC of participants.
Table 2. Multivariate analysis to evaluate associations between AAC group and diet-derived antioxidants in included participants*.
Table 3. Multivariate analysis to evaluate associations between the AAC group and other relevant covariates in included participants*.
In conclusion, we found that a higher intake of lycopene from dietary sources was independently associated with lower odds of having severe AAC. Combined with the findings in other cardiovascular disease studies, our findings suggest that dietary lycopene potentially benefits AAC.
Author contributions
ML and LMH conceived and designed the study. LMH and QJL analyzed the data and drafted the manuscript. ML reviewed and edited the manuscript. YYO, DDL, YDW, HYL, and ZGZ contributed to data collection. All the authors have read and agreed to the published version of the manuscript.
Ethical statement
The protocol was approved by the National Center for Health Statistics Ethics Review Board, and written informed consent was obtained from all participants.
Supplemental Material
Download MS Word (21.5 KB)Data availability statement
The data used in this study are freely available from the following sources: https://www.cdc.gov/nchs/nhanes/index.htm.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Foley R, Parfrey P, Sarnak M. Clinical epidemiology of the cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):1–10. .
- Matsushita K, Ballew SH, Wang AY-M, et al. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18(11):696–707.
- Górriz JL, Molina P, Cerverón MJ, et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10(4):654–666.
- Toussaint ND, Kerr PG. Vascular calcification and arterial stiffness in chronic kidney disease: implications and management. Nephrology. 2007;12(5):500–509.
- Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117(22):2938–2948.
- Al-Aly Z. Phosphate, oxidative stress, and nuclear factor-κB activation in vascular calcification. Kidney Int. 2011;79(10):1044–1047.
- Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283(22):15319–15327.
- Tanikawa T, Okada Y, Tanikawa R, et al. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 2009;46(6):572–580.
- Boström KI. DNA damage response, Runx2 and vascular calcification. Arterioscler Thromb Vasc Biol. 2021;41(4):1358–1359.
- Watanabe H, Miyamoto Y, Enoki Y, et al. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol Res Perspect. 2015;3(1):e00092.
- Yu M, Kim YJ, Kang D-H. Indoxyl sulfate–induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011;6(1):30–39.
- Feng W, Zhang K, Liu Y, et al. Apocynin attenuates angiotensin II-induced vascular smooth muscle cells osteogenic switching via suppressing extracellular signal-regulated kinase 1/2. Oncotarget. 2016;7(50):83588–83600.
- Manivannan J, Barathkumar TR, Sivasubramanian J, et al. Diosgenin attenuates vascular calcification in chronic renal failure rats. Mol Cell Biochem. 2013;378(1-2):9–18.
- Manivannan J, Shanthakumar J, Arunagiri P, et al. Diosgenin interferes coronary vasoconstriction and inhibits osteochondrogenic transdifferentiation of aortic VSMC in CRF rats. Biochimie. 2014;102:183–187.
- Chang X, Cui L, Wang X, et al. Quercetin attenuates vascular calcification through suppressed oxidative stress in adenine-induced chronic renal failure rats. Biomed Res Int. 2017;2017:5716204.
- Cui L, Li Z, Chang X, et al. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vascul Pharmacol. 2017;88:21–29.
- Hammad SK, Eissa RG, Shaheen MA, et al. Resveratrol ameliorates aortic calcification in ovariectomized rats via SIRT1 signaling. Curr Issues Mol Biol. 2021;43(2):1057–1071.
- Peralta-Ramírez A, Montes de Oca A, Raya AI, et al. Vitamin E protection of obesity-enhanced vascular calcification in uremic rats. Am J Physiol Renal Physiol. 2014;306(4):F422–9.
- Hu Y, Cai X, Zhang N, et al. Relation between dietary carotenoid intake, serum concentration, and mortality risk of CKD patients among US adults: national health and nutrition examination survey 2001–2014. Front Med (Lausanne). 2022;9:871767.
- Chen W, Eisenberg R, Mowrey WB, et al. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant. 2020;35(7):1171–1178.
- Ahluwalia N, Dwyer J, Terry A, et al. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7(1):121–134.
- Schousboe JT, Lewis JR, Kiel DP. Abdominal aortic calcification on dual-energy X-ray absorptiometry: methods of assessment and clinical significance. Bone. 2017;104:91–100.
- Berger RG, Lunkenbein S, Ströhle A, et al. Antioxidants in food: mere myth or magic medicine? Crit Rev Food Sci Nutr. 2012;52(2):162–171.
- Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;652:18–26.
- Lindschinger M, Tatzber F, Schimetta W, et al. A randomized pilot trial to evaluate the bioavailability of natural versus synthetic vitamin B complexes in healthy humans and their effects on homocysteine, oxidative stress, and antioxidant levels. Oxid Med Cell Longev. 2019;2019:1–14.
- Khan UM, Sevindik M, Zarrabi A, et al. Lycopene: food sources, biological activities, and human health benefits. Oxid Med Cell Longev. 2021;2021:1–10.
- M, Nunez-Cordoba, Martinez-Gonzalez M. Antioxidant vitamins and cardiovascular disease. CTMC. 2011;11(14):1861–1869.
- Szulc P. Abdominal aortic calcification: a reappraisal of epidemiological and pathophysiological data. Bone. 2016;84:25–37.
- Schousboe JT, Vo TN, Langsetmo L, et al. Abdominal aortic calcification (AAC) and ankle-brachial index (ABI) predict health care costs and utilization in older men, independent of prevalent clinical cardiovascular disease and each other. Atherosclerosis. 2020;295:31–37.
- Kauppila LI, Polak JF, Cupples LA, et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–250.
- Ogden CL, Lamb MM, Carroll MD, et al. Obesity and socioeconomic status in adults: United States, 2005–2008. NCHS Data Brief. 2010;50:1–8.
- Fang M, Wang D, Coresh J, et al. Undiagnosed diabetes in US adults: prevalence and trends. Diabetes Care. 2022;45(9):1994–2002.
- Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190–1200.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the national health and nutrition examination surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918–926.
- Bartstra JW, Mali WPTM, Spiering W, et al. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prev Cardiol. 2021;28(12):1386–1391.
- Albrahim T, Robert AA. Lycopene effects on metabolic syndrome and kidney injury in rats fed a High-Fat diet: an experimental study. ACS Omega. 2022;7(35):30930–30938.
- Tong C, Peng C, Wang L, et al. Intravenous administration of lycopene, a tomato extract, protects against myocardial ischemia-reperfusion injury. Nutrients. 2016;8(3):138.
- Sulistyowati E, Hsu J-H, Lee S-J, et al. Potential actions of baicalein for preventing vascular calcification of smooth muscle cells in vitro and in vivo. IJMS. 2022;23(10):5673. .
- Ji R, Sun H, Peng J, et al. Rosmarinic acid exerts an antagonistic effect on vascular calcification by regulating the Nrf2 signalling pathway. Free Radic Res. 2019;53(2):187–197.
- Chen C, Li Y, Lu H, et al. Curcumin attenuates vascular calcification via the exosomal miR-92b-3p/KLF4 axis. Exp Biol Med . 2022;247(16):1420–1432.
- Heath J, Mao X, Huang L, et al. Abstract 603: o -GlcNAcylation of Runx2 is essential for vascular calcification. ATVB. 2014;34(suppl_1):A603–A603.
- Mascio PD, Devasagayam TPA, Kaiser S, et al. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem Soc Trans. 1990;18(6):1054–1056.
- Yao Y, Tan P, Kim JE. Effects of dietary fats on the bioaccessibility and bioavailability of carotenoids: a systematic review and meta-analysis of in vitro studies and randomized controlled trials. Nutr Rev. 2022;80(4):741–761.
- Nicolov M, Cocora M, Buda V, et al. Hydrosoluble and liposoluble vitamins: new perspectives through ADMET analysis. Medicina. 2021;57(11):1204.
- Shardell MD, Alley DE, Hicks GE, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the third national health and nutrition examination survey. Nutr Res. 2011;31(3):178–189.
- Yonova DH, Vazelov ES, Trendafilov II, et al. First impressions of cardiovascular calcification treatment in hemodialysis patients with a new dialysis fluid containing sodium thiosulphate (STS). Int J Artif Organs. 2014;37(4):308–314.
- Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172.
- Müller L, Caris-Veyrat C, Lowe G, et al. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—a critical review. Crit Rev Food Sci Nutr. 2016;56(11):1868–1879.
- Cheng HM, Koutsidis G, Lodge JK, et al. Lycopene and tomato and risk of cardiovascular diseases: a systematic review and meta-analysis of epidemiological evidence. Crit Rev Food Sci Nutr. 2019;59(1):141–158.
