Abstract
Background
Adjuvant durvalumab after chemoradiation has become the standard of care for patients with stage III NSCLC, according to the PACIFIC trial. Whether biomarkers before durvalumab for patients with stage III NSCLC showed predictive and prognostic effects remains unknown.
Methods
This is a retrospective study in the Fujieda Municipal General Hospital between October 2018 and March 2022. We assessed the predictive value of the Prognostic Nutritional Index (PNI) in stage III non-small cell lung cancer (NSCLC) patients treated with durvalumab after chemoradiation.
Results
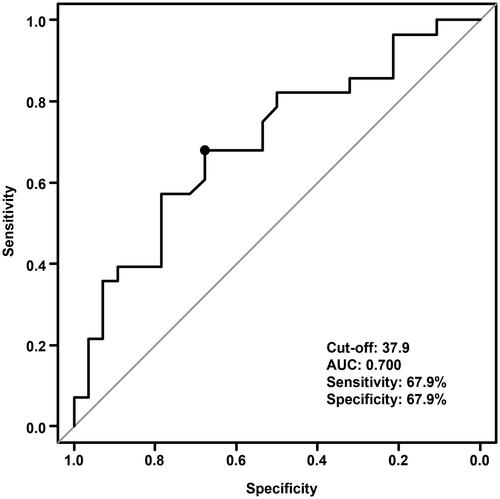
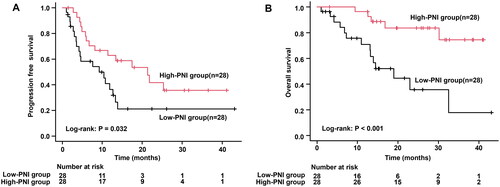
After applying the inclusion and exclusion criteria, the study included 56 patients for further analysis. The median follow-up period was 17.6 months (range, 3.0–45.4 months). According to receiver operating characteristic curve results, the PNI cutoff value to predict overall survival (OS) was 37.9, with sensitivity and specificity at 67.9% and 67.9%. Accordingly, the patients were divided into low- and high-PNI groups. Patients with the low-PNI group had a significantly shorter progression-free survival compared to the high-PNI group (median, 9.1 vs. 21.3 months, p = 0.032). OS was also shorter in the low-PNI group (median, 19.0 months vs. not reached, p < 0.001). In the multivariate Cox hazards regression analyses, the high-PNI was an independent prognostic factor for OS (hazard ratio, 0.187; 95% confidence interval, 0.046–0.760; p = 0.019).
Conclusions
It seems that PNI could be used as a predictor for OS in patients with stage III NSCLC treated with durvalumab after chemoradiation.
Inadequate immunocompetence and nutritional status after chemoradiation therapy may result in poor antitumor efficacy of ICIs.
Pretreatment immune and nutritional assessment using PNI could be considered an independent predictor for the survival of stage III NSCLC patients treated with durvalumab after chemoradiation therapy.
KEY MESSAGES
Introduction
Patients with stage III non-small cell lung cancer (NSCLC) are treated with platinum-based doublet chemotherapy and radiotherapy; however, chemoradiation alone has a poor prognosis [Citation1]. Adjuvant durvalumab after chemoradiation has become the standard of care for patients with stage III NSCLC, according to the PACIFIC trial [Citation2]. Predicting treatment efficacy with durvalumab for patients with stage III NSCLC is critical for clinical administration and maintenance decisions.
The progression of lung cancer is closely linked to cancer-related inflammation and nutrition. PNI, which is calculated by the count of peripheral lymphocyte and albumin concentration [Citation3], is widely accepted as an index for nutritional and inflammatory conditions and it correlates with therapeutic efficacy and long-term outcome in various advanced malignancies [Citation4–7]. It is unclear, whether PNI can be used to predict the therapeutic efficacy of immune check inhibitors (ICI) after chemoradiation therapy in stage III NSCLC. We hypothesized that PNI value after chemoradiation therapy and before durvalumab treatment could predict progression-free survival (PFS) and overall survival (OS) in stage III NSCLC patients.
Patients and methods
Study population
This retrospective study included 56 patients with stage III NSCLC who received durvalumab after chemoradiation therapy. The patients were selected from the Fujieda Municipal General Hospital between October 2018 and March 2022, and the ethical committee of the same hospital approved the study conduction (R04-22). The patients were included in the study only if they were (a) ≥18 years old, (b) clinically diagnosed as stage IIIA, IIIB, or IIIC according to the eighth edition of the International Union Against Cancer Tumor, Node, Metastasis Classification was used to stage lung cancer [Citation8], (c) available clinicopathological and pretreatment laboratory data, (d) Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 − 1, and (e) no prior history of lung cancer or no intervention for lung cancer including surgery, chemotherapy, and radiotherapy. The exclusion criterion was the presence of hematologic disorder and advanced cancer.
Clinical data collection
Using an electronic medical record system, we extracted relevant demographic data, ECOG-PS baseline values, pathological diagnosis, stage, and PD-L1 expression, as well as therapeutic data.
Nutritional assessment using PNI
PNI was calculated based on a peripheral blood sample and using the formula:
10 × serum albumin (g/dl) + 0.005 × lymphocyte count (cells/mm3) (3). The peripheral blood obtained within 3 days immediately preceding durvalumab treatment.
Statistical analysis
EZR (Saitama Medical Center, Jichi Medical University) was used to conduct all statistical tests in this paper [Citation9], which is a user interface for R (ver. 4.0.3). Variables were presented using median (range). Fisher’s exact test was selected for categorical data, while Mann–Whitney U-test or Kruskal–Wallis one-way analysis was for continuous one. The receiver operating characteristic (ROC) curve determined the cutoff PNI value for predicting OS. PFS was considered the time from the first durvalumab dose to the disease progression or death date and censored on the day of the last disease assessment. Conversely, OS was considered the time from treatment to death date from any cause, with censoring on the last known alive date. OS and PFS parameters were calculated using Kaplan–Meier survival analysis, and the survival differences were calculated using a log-rank test. For identification of prognostic factors for survival, after testing the proportional hazard assumption, univariate and multivariate Cox proportional hazards regression analyses were performed to estimate hazard ratios (HRs) and 95% confidence intervals (CI).
Furthermore, univariate logistic regression analysis was selected to determine which factors affect PFS and OS. The chosen factors for analysis were as follows: age, sex, ECOG-PS, smoking history, pathology, stage, chemotherapy, PD-L1 expression, and PNI. Finally, based on the results of the univariate analysis, significant variables (p-value < 0.05) were included in the multivariate logistic regression analysis. All statistical tests were two-sided tests.
Results
Patient characteristics
displays clinical characteristics. The median age of the 56 patients was 70 years (41–84), with 47 being men, 23 (41.1%) having adenocarcinoma, and 28 (50.0%) had squamous cell carcinoma. In terms of ECOG-PS, 44 (78.6%) and 12(21.4%) patients received a score of 0 and 1, respectively. There were 22 (39.3%), 30 (53.6%), and 4 (7.1%) patients with stage IIIA, IIIB, and IIIC, respectively.
Table 1. The clinical characteristics of patients with Stage III nonsmall cell lung cancer.
Oncological outcomes in the entire cohort
The median follow-up period was 17.6 months (range, 3.0–45.4). Overall, 34 patients showed evidence of progression, and 19 died during the follow-up period. The median PFS and OS of all patients were 12.6 months (95% CI, 6.7–21.3) and 32.5 months (95% CI, 22.9 to not reached), respectively.
Independent factors associated with oncological outcomes
According to univariate analysis, age, gender, ECOG-PS, smoking, pathology, stage, chemotherapy, PD-L1 expression, and PNI significantly affected OS and PFS (). ECOG-PS, pathology, PD-L1 expression, and PNI were identified as significant OS predictors. shows the results of the multivariate Cox hazards regression analyses of oncological outcomes after adjusting for ECOG-PS, pathology, PD-L1 expression, and PNI. PNI value can significantly predict the OS of the patients (HR, 0.187; 95% CI, 0.046–0.760; p = 0.019).
Table 2. Univariate analysis of several parameters as predictors of PFS and OS in patients with stage III NSCLC.
Table 3. Multivariate analysis for parameters as predictors of PFS and OS in patients with stage III NSCLC.
Identification of PNI cutoff values
PNI was measured on median 9.5 days (range, 3.0–55.0) after chemoradiation. According to the ROC curve and Youden index, the pretreatment PNI cutoff value to predict OS was 37.9, and the area under the curve was 0.70 (95% CI: 0.56–0.84) (). However, the specificity and sensitivity of PNI cutoff values were 67.9% and 67.9%, respectively. According to the PNI cutoff value, patients were divided into low-PNI (PNI ≤ 37.9) and high-PNI (PNI > 37.9) groups. is a comparison of patient characteristics according to PNI. The low PNI group was significantly correlated with elderly and poor ECOG-PS.
Oncological outcomes in low-PNI and high-PNI groups
We investigated how PNI could influence the OS and PFS in the included patients. Low-PNI group had a significantly shorter PFS compared to the high-PNI group (median, 9.1 vs. 21.3 months, p = 0.032; ). OS was also shorter in the low-PNI group (median, 19.0 months vs. not reached, p < 0.001; ). As a result, patients with low PNI had significantly worse PFS and OS than those with high PNI.
Figure 2. Based on PNI, a Kaplan–Meier curve analysis of PFS and OS for 56 NSCLC patients treated with durvalumab after chemoradiation therapy was performed. (A) PFS based on PNI. (B) OS based on PNI. PFS: progressive free survival; OS: overall survival; PNI: prognostic nutritional index; NSCLC: non-small cell lung cancer.
Discussion
This study looked at the predictive power of PNI as a measure of immunity and nutrition in stage III NSCLC patients. According to our results, PNI could be considered an independent predictor for the survival of stage III NSCLC patients treated with durvalumab after chemoradiation therapy. Consequently, pretreatment immune and nutritional assessment using PNI are important to evaluate the prognosis of those patients.
The immunological alterations due to chemoradiation therapy should be considered when predicting durvalumab outcomes. Blood components, including lymphocytes, can be directly affected by chemotherapy-induced myelosuppression and radiation to the spinal cord. Albumin synthesis may decrease due to anorexia caused by chemotherapy and inflammation by radiotherapy, which is consequently associated with short survival [Citation10]. PNI is calculated as a prognostic factor for cancer using lymphocyte count and serum albumin [Citation3]. We had previously shown that low PNI before first-line therapy was an important indicator of poor OS in patients with advanced NSCLC [Citation11]. In the present study, lower PNI before durvalumab was also associated with shorter survival. Inadequate immunocompetence and nutritional status after chemoradiation therapy may result in poor antitumor efficacy of ICIs.
The level of PD-L1 expression in tumor specimens before treatment for advanced lung cancer is considered a predictive marker of the antitumor efficacy of ICI [Citation12–13]. It remains uncertain whether PD-L1 expression in tumor specimens before chemoradiation therapy is a predictive marker for antitumor efficacy of ICI after chemoradiation. The antitumor efficacy is highly dependent on the patient’s immune competence. A previous study showed that PNI level before treatment was a prognostic factor for NSCLC patients on ICI therapy [Citation14]. After chemoradiation therapy, our results show that the low PNI before durvalumab treatment is an independent predictor of OS in patients with stage III NSCLC. PNI provides a measure of both immunological and nutritional status in patients with malignancies and warrants further studies as a predictive marker of ICI.
The first limitation of this study is the retrospective design, and the modes or small sample size. It is thought that this is because it is a single institution, and we carefully selected patients with stage III NSCLC who had undergone chemoradiation therapy without severe side effects or progression and who could be administered durvalumab. Therefore, a study with a larger sample size, standardized treatment, and a longer follow-up period should be conducted to validate the significance of PNI. Second, we did not perform PD-L1 testing in all cases. PD-L1 expression has been shown to be effective for ICI in advanced NSCLC [Citation12,Citation13], but the effectiveness of ICI in stage III NSCLC is uncertain and is not an essential test. Third, we only investigated the PNI for predicting the efficacy of durvalumab and did not examine other immunological and nutritional indexes. PNI, calculated from lymphocyte counts and serum albumin, appears convenient and reasonable for predicting ICI response.
To summarize, this is the first study to look at the prognostic impact of PNI on survival in patients with stage III NSCLC receiving durvalumab after chemoradiation therapy.
Author contributions
SM performed and designed this study. SS, RY, KM, YI, TH, EM, KT, NA, MT, NK, and TK provided clinico-pathological data and analyses. SM wrote the manuscript. All the authors reviewed the manuscript.
Acknowledgment
The authors would like to thank all of the patients as well as the medical staff who cared for the patients in practice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on a reasonable request.
Additional information
Funding
References
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013.143(5):1–5.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929.
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984; 85(9):1001.
- Geng Y, Qi Q, Sun M, et al. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41(11):1508–1514.
- Bruixola G, Caballero J, Papaccio F, et al. Prognostic Nutritional Index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO Open. 2018;3(6):e000425.
- Salati M, Filippi R, Vivaldi C, et al. The prognostic nutritional index predicts survival and response to first-line chemotherapy in advanced biliary cancer. Liver Int. 2019;20(3):704–711.
- Wang Y, Battseren B, Yin W, et al. Predictive and prognostic value of prognostic nutritional index for locally advanced breast cancer. Gland Surg. 2019;8(6):618–626.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classifi cation for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
- Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458.
- Wang JY, Chen KY, Wang JT, et al. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002;54(3):735–741.
- Matsuura S, Morikawa K, Ito Y, et al. The geriatric nutritional risk index and prognostic nutritional index predict the overall survival of advanced non-small cell lung cancer patients. Nutr Cancer. 2022;74(5):1603–1613.
- Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830.
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339.
- Shoji F, Takeoka H, Kozuma Y, et al. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2019;136:45–51.