Abstract
Objective
This retrospective study aimed to observe the efficacy of transcatheter arterial chemoembolization (TACE) combined with sirolimus in the treatment of haemangioma combined with the Kasabach–Merritt phenomenon (KMP).
Methods
A total of 11 infants with KMP who were treated at our hospital from January 2016 to September 2021 were selected and treated with arteriosclerosis embolotherapy using a microsphere emulsion formed by bleomycin + ultra-fluid lipiodol + dexamethasone + contrast agent or bleomycin mixed microspheres as the embolising agent. The patients were administered sirolimus orally after TACE. The clinical efficacy and examination indicators before and after treatment were observed and compared.
Results
The 11 infants underwent TACE treatment by arteriosclerosis embolotherapy a total of 21 times; of these cases, 10 were cured, and 1 showed a moderate response. There were no cases of non-response or death. The platelet count rose from 10.0 (7.0, 18.0) x 109/L before TACE to 236.0 (188.0, 275.0) x 109/L six months after the first TACE, and the tumour size decreased from 49.0 (43.0, 111.7) cm3 before TACE to 7.0 (3.5, 17.0) cm3 six months after the first TACE. The differences were statistically significant (the Z values were −2.943 and −2.934, respectively, p < 0.05).
Conclusion
The combination of TACE and sirolimus has significant efficacy on critical children with KMP.
Background
Kasabach–Merritt syndrome (KMS), which refers to giant haemangioma combined with thrombocytopenia syndrome, was first reported by Kasabach and Merritt in 1940 [Citation1]. In the 1990s, it was found that this disease was caused by Kaposiform hemangioendothelioma (KHE) and tufted haemangioma (TA) rather than infantile haemangioma. In the same year, Professor Sarker suggested that severe thrombocytopenia, microangiopathic haemolytic anaemia, secondary fibrinogenopenia, and consumptive coagulation disorder caused by KHE and TA should be known as the Kasabach–Merritt phenomenon (KMP). Infants and young children are prone to KMP. Although the condition is rare, the fatality rate is as high as 10%–37% [Citation2], and there is no agreed treatment regimen. Traditional treatment methods include compression, support, drug therapy, radiotherapy, endovascular embolization, and surgery. Drug therapy includes treatment with prednisone, interferon, propranolol, and sirolimus, added in recent years. However, compression and drug therapies are slow to work. For large lesions, it is usually difficult to surgically resect them completely, and the risk is high. The action time of endovascular embolization alone on platelet elevation is usually limited.
Therefore, in this paper, we retrospectively reviewed 11 cases of KMP treated by transcatheter arterial chemoembolization (TACE) combined with sirolimus at the Haemangioma and Vascular Malformation Centre of Anhui Provincial Children’s Hospital.
Subjects and methods
Subjects
A total of 19 children with KMP were hospitalized for treatment in our centre from January 2016 to September 2021. A case with a Localized tumour undergo surgical excision alone and seven cases were treated using more than two approaches of combination therapies, including sirolimus/corticoid, compression therapy and surgical excision. A clinical, imaging, and surgical data of 11 cases with KMP treated with TACE and sirolimus were analyzed retrospectively. The inclusion criteria were as follows: 1) infants and young children; 2) patients confirmed the diagnosis of KMP; 3) patients treated with TACE and sirolimus. Four children reported previously were also included [Citation3]. Six patients have received pre-treatment before TACE, such as sirolimus and compression therapy, but the lesions could not be controlled.
Treatment
For children who have been pre-treatment, cryoprecipitate (5-10 ml/kg) will be applied to correct hypofibrinogenemia and coagulopathy. Platelet infusion (10–15 mL/kg) was conducted within 24 h before TACE. The interventional operation was performed under general anaesthesia. A Cobra-2 catheter was inserted into the haemangioma feeding artery via the femoral artery approach and a 4-F puncture sheath, and angiography was performed with a high-pressure syringe at 3 mL/s 8 mL at 300 psi. The haemangioma feeding artery was identified according to the angiographic findings. A 2.7-Fr or 2.2-Fr microcatheter was used to select the feeding artery one by one. After the injection of bleomycin emulsion, polyvinyl alcohol (PVA) particles or a spring ring were used for embolization until a satisfactory result was obtained.
Sirolimus was administered orally from the second day after TACE at an initial dose of 0.8 mg/m2 daily, 12-h/episode. The drug concentration was determined 1 week after medication, and the dose was appropriately adjusted to maintain the minimum blood concentration of 10–15 ng/mL. The medication lasted for 3–6 months. The routine blood parameters, coagulation function, liver and kidney functions, serum lipid, and sirolimus levels were monitored regularly. The changes in the patients’ platelets and tumour sizes were evaluated 4 weeks after each TACE, which constituted one course of treatment. If patients failed to reach the criteria for a cure, a second embolotherapy was performed up to a maximum of three. All patients were followed up for between 8 months and 6 years.
Efficacy evaluation criteria
Six months after the first TACE, the patients’ tumour volume was measured by ultrasound or computed tomography (CT). The criteria for a cure were as follows: The tumour had completely or mainly disappeared (the volume was less than or equal to 20% of the original volume), the platelet count was maintained stably within the normal range, and the patient did not relapse for more than 6 months. According to a previous study, the criteria for a basic cure are as follows [Citation4]. The tumour size was reduced significantly (tumour size reduction of 20%–50%), the platelet count was maintained stably within the normal range, and the patient did not relapse for more than 6 months. The criteria for an improvement were as follows [Citation4]: The tumour size had decreased (tumour size reduction of 20%–50%), the platelet count was maintained stably within the normal range, and the patient did not relapse for more than 6 months. The criteria for no response were as follows: Six months later, the haemangioma volume increased, the platelet count showed no response, or the disease relapsed within 6 months.
Statistical method
The statistical analysis was conducted using SPSS 20.0 (IBM Corporation, Chicago, Illinois) software. The normally distributed enumeration data were expressed as means and standard deviations, while the non-normally distributed enumeration data were expressed as medians (interquartile range [IQR]). A non-parametric test of paired samples was used for comparison before and after TACE, and a value of p < 0.05 indicated that the difference was statistically significant.
Results
Infants’ general information
The infants’ general information is presented in . There were 7 males and 4 females, aged 6 days to 7 months; the IQR of the median was 30.0 (8.0, 120.0) days. Among the patients, the lesions were located on the body surface in 9 cases, in the liver in 1 case, and in the throat in 1 case, where it pressed against the airway. Six infants were treated with 1 ∼ 3mg/(kg.d) prednisone orally, support, and compression for 1 to 2 weeks before TACE, but the lesions could not be controlled. The range of lesions further increased, the platelet count further decreased, and the clinical symptoms continued to deteriorate. Among the 6 infants who were treated previously, 2 patients were administered sirolimus orally before TACE. For those infants with lesions located on the body surface, compression bandaging treatment was implemented after the first TACE; all infants did not receive surgery (suture and excision) and radiotherapy.
Table 1. Clinical characteristics.
Clinical efficacy
Eleven infants underwent a total of 21 interventional embolizations, ranging from 1 to 3 times per patient, with a mean of (1.9 ± 0.8) times, as shown in . The femoral artery approach was used in all cases. In one case, due to the failure of the right femoral artery puncture and the subsequent formation of a subcutaneous haematoma, the left femoral artery approach was used. One week later, the subcutaneous haematoma was absorbed, and it disappeared. In one case, the lesion was located in the right inguinal region, so the left femoral artery approach was used. The sirolimus medication lasted for a median (IQR) of 5.0 (3.0, 6.0) months, without side effects.
A total of 10 infants met the criteria for a cure, and their tumour volume was reduced significantly. No cases met the criteria for a basic cure. The case with the lesion located in the inguinal region improved, and there were no cases without a response. The median (IQR) of platelet count rose from 10.0 (7.0, 18.0) x 109/L before TACE to 236.0 (188.0, 275.0) x 109/L six months after the first TACE, and the median (IQR) of tumour size decreased from 49.0 (43.0, 111.7) cm3 before TACE to 7.0 (3.5, 17.0) cm3 six months after the first TACE. Local skin necrosis occurred after the third TACE on the lesion on the right shoulder. Later, it scabbed over and fell off, leaving behind a small scar. Two patients had low-grade fever after TACE, which abated after symptomatic treatment. All patients were followed up for between 9 months and 6 years, and there were no cases of relapse or death.
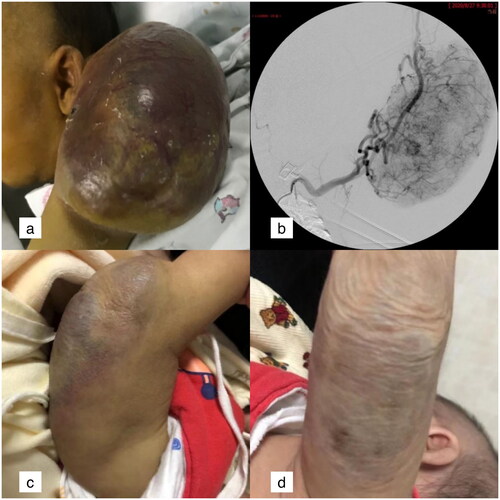
The mass on the infant’s left upper limb shown in was found immediately after birth. The CT examination indicated KHE. On day 14, the tumour suddenly increased, and its surface was swollen and congested. After prednisone and blood transfusion treatment for 10 days, the patient’s coagulation function deteriorated. The prothrombin time was 20.5 s, the activated partial thromboplastin time was 50.6 s, and the D dimer was 80.0. Meanwhile, the infant was accompanied by liver function impairment. After undergoing TACE and sirolimus treatment three times, the lesion was completely absorbed 6 months later.
Figure 1. The mass on the infant’s left upper limb was found immediately after birth.
a: Lesions located on the left upper limb in a 24-day-old boy, with the surface swollen and purple. b: The angiography of the left brachial artery showed that the feeding arteries of the lesion came from multiple brachial artery branches. c: The swelling was alleviated, tumour size was reduced and skin colour got recovered 1 month after TACE. d: The tumour was completely absorbed and the surface skin was flabby 6 months after TACE.
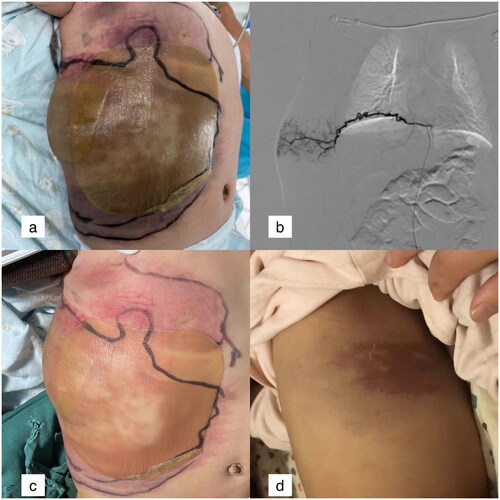
In , the lesion on the right abdominal wall of a 4-month-old boy increased suddenly, and the diameter of the tumour increased by 6 cm within 1 day after hospitalization. Digital subtraction angiography revealed the blood for the lesion was supplied via multiple intercostal arteries, internal thoracic arteries, and lateral thoracic arteries. After three treatments with TACE and the oral administration of sirolimus for 3 months, the lesion was completely absorbed in month 6.
Figure 2. The lesion on the right abdominal wall of a 4-month-old boy.
a: Lesions in the right abdominal wall. The inner ring showed the range of lesions in the first day of hospitalization, and the outer ring showed the range of lesions in the 2nd day of hospitalization. b: One of feeding vessels of the lesion, tortuous intercostal artery. c: The swelling was alleviated 1 week after TACE. d: The tumour was completely absorbed and a little pigment deposition was remained 6 months after TACE.
Discussion
The incidence of KMP is clinically rare. Its incidence is low, but its fatality rate is high. The leading causes of death include haemorrhage, pulmonary infection, hepatic failure, airway compression, respiratory failure, and cardiopulmonary. It should be diagnosed and treated in the early stages.
Kasabach–Merritt phenomenon treatment by sirolimus
Rapamycin (mTOR) inhibitor sirolimus, a substrate for CYP3A4, targeting the PI3K-Akt-mTOR pathway is emerging as a novel treatment option in KMP [Citation5,Citation6]. Sirolimus seems to exert both antiproliferative and antiangiogenic/−lymphangiogenic effects by inhibiting mTOR, a serine-threonine kinase regulated by PI3K [Citation4]. Blatter et al. [Citation7] first reported a new effect of sirolimus (0.1 mg/kg/day): a mammalian target of rapamycin (mTOR) inhibitor on KMP. Later, multiple individual reports on KMP treatment by sirolimus appeared around the world. Sirolimus achieved very good efficacy in KMP cases who relapsed after surgical resection and showed no response to other drug therapies [Citation6, Citation8–12], proving the safety and efficacy of sirolimus in the treatment of KMP. In 2017, Yi et al. [Citation13] reported that among 37 KMP children, there was no significant difference in tumour reduction between treatment with sirolimus alone and sirolimus combined with prednisone. In 2018, Wang et al. [Citation14] reported that sirolimus had been used as the initial medication to treat 8 patients with KHE and TA, among whom 6 cases were combined with KMP. Since then, sirolimus has gradually become the first-line treatment regimen for KMP as a replacement for combined prednisone/vincristine treatment and vincristine alone [Citation14–17]. However, considering the slow onset of sirolimus and the high mortality of KMP, we believe that the necessary invasive treatment is necessary for critically ill children with KMP (large tumour, small age, low platelet, etc.).
Among the infants in the present study, 3 cases were administered prednisone orally for 1 to 2 weeks before TACE, without any response. All infants were administered sirolimus alone after TACE. Sirolimus is an immunosuppressant, which can lower patients’ immunity, increasing the risk of infection. Furthermore, some patients die of pulmonary infection after taking sirolimus [Citation8]. Considering the pulmonary toxicity of bleomycin used in treating TACE, the author recommended combined anti-infective therapy. Luckily, only a single case had a postoperative low-grade fever, which improved spontaneously after 24 h and may have been related to the thermogenic effect of bleomycin. The remaining cases had no severe complications. Once infection symptoms occur during sirolimus treatment, it is necessary to actively treat them to avoid severe consequences. To reduce the side effects of sirolimus, some researchers have attempted to reduce its dosage [Citation10,Citation18,Citation19]; the therapeutic dose was reduced to half the standard dose and even lower, and very good results were obtained. Among the cases in the present study, the standard dose (0.8 mg/L, bid) was used for all infants. Sirolimus concentration was monitored 1 week after administration and appropriately adjusted to maintain the valley plasma concentration at 10–15 ng/mL. In the present study, sirolimus was used for 5.0 (3.0, 6.0) months, less than 6 months. Relative to sirolimus combined with other drugs [Citation10], the course of treatment was shorter.
Interventional therapy for the Kasabach–Merritt phenomenon
In 1982, Argenta treated KMS using gel sponge mbolization [Citation20]. In 2007, Yesudian used PVA to embolize a child with KMP in the lower limb [Citation21]. In 2009, O’Regan used the embolization technique for 2 out of 3 patients with KHE [Citation22]. In 2010, Ryan used trans-arterial embolization in 10 out of 15 cases, among whom 1 case was embolized 7 times (mean: 2.8 times) [Citation23]. In 2012, Ryan used microspheres to embolize 2 children with KMP [Citation24]. In 2013, Yuan et al. [Citation25] found evidence that tumours in patients with KMS could capture blood components, while interventional embolization could embolize the feeding artery of the tumour, make the tumour ischaemic and smaller, and reduce the capture of blood components by the tumour. Their findings provided a theoretical basis for interventional embolization. Later, multiple centres reported cases receiving interventional embolization of KMP [Citation16,Citation26] using embolization materials including PVA, microspheres, and spring rings.
However, embolization alone seems to be associated with a high relapse rate of KHE [Citation16]. From 2013, the interventional therapy of KMP has no longer been limited solely to embolotherapy. The Guangzhou Women and Children’s Medical Centre began to use an emulsion comprising bleomycin, lipiodol, and dexamethasone combined with other embolizing agents to treat KMP [Citation27–29]. This is consistent with the TACE materials used in the cases of this study. Bleomycin inhibits abnormal vascular growth in tumours by inhibiting the DNA synthesis of malformed vascular endothelial cells. Furthermore, it can lead to local inflammatory responses and cause local fibrosis. Lipiodol is a super-liquefied embolizing agent that can be embolized to the capillary level. After mixing, it can prolong the destructive effect of bleomycin on vascular endothelial cells. The addition of dexamethasone to the emulsion not only inhibits the release of endogenous pyrogen caused by bleomycin but is also anti-inflammatory and anti-allergy, and it alleviates tissue edoema. In the present study, all cases were first embolized with bleomycin emulsion combined with PVA or microspheres. Subsequently, PVA/microspheres may be used for embolotherapy according to the blood supply of the lesions. In the present study, the cases were embolized an average of 1.9 times, and the time of action was longer lasting, relative to embolization alone. Embolization times can be reduced for critical cases.
Complications caused by TACE mainly include ectopic embolization and lesion necrosis [Citation22,Citation23]. The present study mainly used PVA and microsphere embolization, and a spring ring was used for the embolization of liver lesions. In addition to local skin necrosis on the shoulder lesion (recovery after 2 months), no ectopic embolization or skin necrosis occurred.
Considering that most feeding arteries in KMP are numerous and slender, and there exist arteriovenous fistulas in the lesions in some cases [Citation29], the interventional therapy of KMP should be appropriate, and to prevent complications, perfection should not be pursued. In the meantime, interventional therapy is not applicable to all children with KMP [Citation9]. In this study, the improvement in cases may be related to the fact that the lesions were located in the right inguinal region; their blood was supplied by the branch of the bilateral internal iliac artery, and it was difficult to access the left internal iliac artery, so it was not treated. Most children with KMP have a complicated blood supply and slender feeding arteries, even if they have rich blood supply lesions. Thus, it tests operators’ patience.
Choices for treating the Kasabach–Merritt phenomenon
Drug therapy has been the first-line treatment method for KMP. However, the response time for drug therapy is long, so it is not usually considered for critical KMP cases. Surgical excision under the circumstance of no full improvement of the coagulation function may cause more troublesome diffuse haemorrhage and greatly increase the risk of death. Radiotherapy was once a treatment method for critical KMP cases [Citation30]. However, given its considerable side effects, it is no longer widely applied. Haemorrhage is the leading cause of death in KMP. For cases without a response to drug therapy or those with a high risk of surgical excision due to a wide range of lesions, the interventional embolization combined with sirolimus approach is a choice, with good safety, efficacy, and high sensitivity as well as high repeatability. It may be used as the first choice for children with critical KMP. Since the age of KMP onset is young, the difficulty in performing intervention operations may be one of the reasons affecting its widespread application.
There are several limitations to this study. First, this was a single-centre retrospective study, and the number of cases was small. Secondly, lack of control group which treated with only sirolimus in this study. The follow-up time was limited, and it was difficult to evaluate the long-term efficacy of the combined therapy. In addition, because the leading cause of death in KMP is haemorrhage, all infants did not undergo pathological biopsy and were diagnosed by clinical and laboratory examinations as well as imaging results.
Conclusion
For KMP with no response to drug therapy, high risk of surgical excision and life-threatening consumptive coagulopathy, the interventional embolization combined with sirolimus approach was an alternative treatment for KMP, which can provide a rapid improvement in haematopoietic parameters and remission of the primary tumour.
Ethics approval and consent to participate
This study was conducted with approval from the Ethics Committee of Anhui Provincial Children’s Hospital (EYLL-2017-002). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all patient guardians.
Consent for publication
Not applicable.
Authors’ contributions
Conception and design of the research: Chuan-gao Yin.
Acquisition of data: Wei-Wei Qi, Song Wang, Deng Pan, Xiao-Li Chen
Analysis and interpretation of the data: Song Wang
Statistical analysis: Deng Pan, Shi-Yu Li
Obtaining financing: Chuan-gao Yin
Writing of the manuscript: Chuan-gao Yin, Wei-Wei Qi
Critical revision of the manuscript for intellectual content: Chuan-gao Yin
All authors read and approved the final draft.
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kasabach HH, Merritt KK. Capillary hemangioma with extensive purpura: report of a case. Arch Pediatr Adolesc Med. 1940;59:1–8.
- Kwok-Williams M, Perez Z, Squire R, et al. Radiotherapy for life-threatening mediastinal haemangioma with kasabach-merritt syndrome. Pediatr Blood Cancer. 2007;49(5):739–744.
- Wang Y, Wang S, Wang L, et al. Transarterial embolization in neonatal Kasabach-Merritt syndrome. Front Pediatr. 2021;9:788120.
- Brill R, Uller W, Huf V, et al. Additive value of transarterial embolization to systemic sirolimus treatment in aposiform hemangioendothelioma. Int J Cancer. 2021;148(9):2345–2351.
- Freixo C, Ferreira V, Martins J, et al. Efficacy and safety of sirolimus in the treatment of vasculKar anomalies: a systematic review. J Vasc Surg. 2020;71(1):318–327.
- Sakata N, Suenobu SI, Okano M, et al. Impact of sirolimus treatment for refractory Kaposiform hemangioendothelioma with exacerbation of the disease 10 years after initial diagnosis. Rare Tumors. 2018;10: 2036361318776185.
- Blatt J, Stavas J, Moats-Staats B, et al. Treatment of childhood Kaposiform hemangioendothelioma with sirolimus. Pediatr Blood Cancer. 2010;55(7):1396–1398.
- Wang Y, Kong L, Sun B, et al. Sirolimus for Kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon in two infants. J Craniofac Surg. 2020;31(4):1074–1077.
- MacFarland SP, Sullivan LM, States LJ, et al. Management of refractory pediatric Kaposiform hemangioendothelioma with sirolimus and aspirin. J Pediatr Hematol Oncol. 2018;40(4):e239–e242.
- Mariani LG, Schmitt IR, Garcia CD, et al. Low dose sirolimus treatment for refractory tufted angioma and congenital Kaposiform hemangioendothelioma, both with Kasabach-Merritt phenomenon. Pediatr Blood Cancer. 2019;66(8):e27810.
- Cashell J, Smink GM, Helm K, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon in an infant: successful treatment with prednisolone, vincristine, and addition of sirolimus. Pediatr Blood Cancer. 2018;65(12):e27305.
- Jahnel J, Lackner H, Reiterer F, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon: from vincristine to sirolimus. Klin Padiatr. 2012;224(6):395–397.
- Ji Y, Chen S, Xiang B, et al. Sirolimus for the treatment of progressive Kaposiform hemangioendothelioma: a multicenter retrospective study. Int J Cancer. 2017;141(4):848–855.
- Wang H, Guo X, Duan Y, et al. Sirolimus as initial therapy for Kaposiform hemangioendothelioma and tufted angioma. Pediatr Dermatol. 2018;35(5):635–638.
- Peng S, Yang K, Xu Z, et al. Vincristine and sirolimus in the treatment of Kaposiform haemangioendothelioma. J Paediatr Child Health. 2019;55(9):1119–1124.
- Liu XH, Li JY, Qu XH, et al. Treatment of kaposiform hemangioendothelioma and tufted angioma. Int J Cancer. 2016;139(7):1658–1666.
- Ying H, Qiao C, Yang X, et al. A case report of 2 sirolimus-related deaths among infants with kaposiform hemangioendotheliomas. Pediatrics. 2018;141(Suppl 5):S425–S429.
- Labonnelie A, Soupre V, Maruani A, et al. Management of sirolimus treatment for tumours associated with Kasabach-Merritt phenomenon. Acad Dermatol Venereol. 2022;36(7):e586–e588.
- Cabrera TB, Speer AL, Greives MR, et al. Sirolimus for Kaposiform hemangioendothelioma and Kasabach-Merritt phenomenon in a neonate. AJP Rep. 2020;10(4):e390–e394.
- Argenta LC, Bishop E, Cho KJ, et al. Complete resolution of life-threatening hemangioma by embolization and corticosteroids. Plast Reconstr Surg. 1982;70(6):739–744.
- Yesudian PD, Klafkowski J, Parslew R, et al. Tufted angioma-associated Kasabach-Merritt syndrome treated with embolization and vincristine. Plast Reconstr Surg. 2007;119(4):1392–1393.
- O’Regan GM, Irvine AD, Yao N, et al. Mediastinal and neck Kaposiform hemangioendothelioma: report of three cases. Pediatr Dermatol. 2009;26(3):331–337.
- Ryan C, Price V, John P, et al. Kasabach-Merritt phenomenon: a single centre experience. Eur J Haematol. 2010;84(2):97–104.
- Garcia-Monaco R, Giachetti A, Peralta O, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon: successful treatment with embolization and vincristine in two newborns. J Vasc Interv Radiol. 2012;23(3):417–422.
- Yuan SM, Hong ZJ, Chen HN, et al. Kaposiform hemangioendothelioma complicated by Kasabach-Merritt phenomenon: ultrastructural observation and immunohistochemistry staining reveal the trapping of blood components. Ultrastruct Pathol. 2013;37(6):452–455.
- Khant ZA, Hirai T, Ikeda O, et al. Successful transarterial embolization with cellulose porous beads for occipital haemangioma in an infant with Kasabach-Merritt syndrome. BJR Case Reports. 2017;3(3):20170004.
- Zhou SY, Li HB, Mao YM, et al. Successful treatment of Kasabach-Merritt syndrome with transarterial embolization and corticosteroids. J Pediatr Surg. 2013;48(3):673–676.
- Wang P, Zhou W, Tao L, et al. Clinical analysis of Kasabach-Merritt syndrome in 17 neonates. BMC Pediatr. 2014;14:146.
- Tan X, Chen M, Zhang J, et al. Treatment of corticosteroid-resistant vascular tumors associated with the Kasabach-Merritt phenomenon in infants: an approach with transcatheter arterial embolization plus vincristine therapy. J Vasc Interv Radiol. 2016;27(4):569–575.
- Leong E, Bydder S. Use of radiotherapy to treat life-threatening Kasabach-Merritt syndrome. J Med Imaging Radiat Oncol. 2009;53(1):87–91.
