Abstract
Malnutrition is very common in patients with chronic kidney disease, especially in those on maintenance dialysis. Malnutrition is one of the major factors affecting survival and death of dialysis patients, and reducing their activity tolerance and immunity. There are numerous and interacting risk factors for malnutrition, such as reduced nutritional intake, increased energy expenditure, hormonal disorders, and inflammation. Selenium, in the form of selenoproteins, is involved in many physiological processes in the body and plays an important role in maintaining redox homeostasis. Oxidative stress and infection are very common in dialysis patients, and selenium levels in dialysis patients are significantly lower than those in the healthy population. It has been shown that there is a correlation between selenium levels in hemodialysis patients and their nutrition-related indicators, and that selenium supplementation may improve malnutrition in patients. However, further studies are needed to support this conclusion and there is a lack of basic research to further characterize the potential mechanisms by which selenium may improve malnutrition in dialysis patients. The purpose of this review is to provide a comprehensive overview of factors associated with malnutrition in dialysis patients and to describe the progress of research on nutritional status and selenium levels in dialysis patients.
KEY MESSAGES
Malnutrition is very common and one of the main factors affecting the survival and mortality of dialysis patients
Risk factors for malnutrition in dialysis patients are numerous and interact with each other; controlling and reducing these risk factors is important to improve the nutritional status of patients.
The trace element selenium acts to improve the nutritional status of patients by reducing oxidative stress and inflammation in their bodies.
1. Introduction
The prevention and treatment of chronic kidney disease (CKD) has become an important public health concern globally, with about 10% adults worldwide having CKD and about 1.2 million people dying from CKD each year. CKD is expected to be the fifth leading cause of death worldwide by 2040 [Citation1]. With the progress of CKD, the renal and glomerular filtration function of patients is gradually reduced, and toxins in the body are continuously accumulated. Renal failure and multiple system damage such as nerve, muscle, and respiratory and metabolic disorders occur towards the end stage of the disease. Renal replacement therapy should be done on time. It has been shown that 77.5% patients with end-stage renal disease (ESRD) have received renal replacement therapy (RRT), of which 43.1% were treated with dialysis [Citation2].
Long-term dialysis can remove metabolic waste and excess water from the body of CKD patients; however, a series of complications such as malnutrition, disorders of calcium and phosphorus metabolism, and electrolyte imbalance can occur. The global prevalence rates of malnutrition in patients with stage 3–5 CKD who did not receive dialysis and those who needed to maintain dialysis were 11–54% and 28–54%, respectively [Citation3]. It is evident that malnutrition is more common in maintenance dialysis patients. Malnutrition can lead to reduced immune function and physical activity in patients, is closely associated with various infectious and non-infectious complications, severely reduces patients’ quality of life, and is one of the main factors affecting the survival and mortality of dialysis patients [Citation4–6].
Selenium (Se) is an essential trace element that inhibits oxidation and suppresses inflammation [Citation7]. In recent years, scholars across the globe have conducted an increasing number of studies on the relationship between Se and chronic kidney disease and found a correlation between Se and malnutrition in dialysis patients, which has great potential research value. The purpose of this review is to provide a comprehensive compilation of factors associated with malnutrition in dialysis patients and assess the progress of research regarding the effects of Se on the nutritional status of dialysis patients.
2. Definition of malnutrition
Along with clinicians’ attention to malnutrition in dialysis patients and numerous scholarly studies on the etiology of malnutrition, a variety of terms, such as cachexia, malnutrition, protein-energy wasting(PEW, and malnutrition-inflammatory-atherosclerosis syndrome (MIA) have evolved to describe the malnutrition status of dialysis patients [Citation8–11]. Although the general meanings of these terms are similar, there are subtle differences [Citation12].
The International Society of Renal Nutrition and Metabolism (ISRNM) (2008) states that the term cachexia is not used to describe the malnutrition status of all dialysis patients; it is more often used to describe the final stage of malnutrition in dialysis patients. In contrast, the definition of malnutrition is intuitive and clear. It mainly emphasizes on muscle wasting, hypoalbuminemia, and other low nutritional markers in dialysis patients due to inadequate nutritional intake or excessive protein loss [Citation13], and does not comprehensively describe other aspects of malnutrition in dialysis patients. With a comprehensive understanding of malnutrition, the relationship between malnutrition and inflammation and its comorbidities has come into focus, resulting in a new definition of malnutrition due to inflammation and comorbidities – ‘malnutrition-inflammation-atherosclerosis syndrome’ – that focuses more on the study of cardiovascular complications in patients with ESRD. With progress in research, scholars have found that malnutrition in dialysis patients not only stems from inadequate nutrient intake, but metabolism-related factors also play a large role in it. Therefore, in 2008, the ISRNM introduced the term ‘protein-energy wasting’ to highlight the complexity of malnutrition in dialysis patients and defined the term as a state of decreased protein and energy reserves in the body [Citation14]. This definition is currently the most widely used. However, it has a shortcoming. The definition only considers albumin and cholesterol; therefore, it may cause some bias in assessment of the nutritional status of hyperlipidemic patients. Although body mass index (BMI) is included in the diagnostic criteria of this definition, obesity sarcopenia may still be overlooked [Citation12].
These ‘definitions’ suggest that the etiology of malnutrition in dialysis patients is complex and that there is no uniformity in the diagnosis of malnutrition due to dialysis.
3. Related index of malnutrition in dialysis patients
At present, there are many indexes for evaluating malnutrition in patients. Subjective Global Assessment (SGA), Malnutrition-Inflammation Score (MIS), Geriatric Nutritional Risk Index (GNRI), Mini Nutritional Assessment (MNA), and Nutritional Risk Screening 2002 (NRS-2002) are widely used for dialysis patients.
In 1987, Detsky et al. [Citation15] proposed SGA in order to evaluate the nutritional status of patients undergoing gastrointestinal surgery. SGA mainly evaluates the nutritional status of patients from six aspects (weight change, dietary intake, gastrointestinal symptoms, functional ability, complications, and physical examination). As SGA is simple, convenient, cost effective, and has been validated in patients with ESRD, Kidney Disease Outcomes Quality Initiative (K DOQI) recommends SGA as a routine tool for assessing the nutritional status of dialysis patients [Citation16]. SGA can not only assess the nutritional status of patients, but has also been found to be associated with a high risk of nutrition-related death in dialysis patients [Citation17–20]. In addition, in a meta-analysis, Khor et al. demonstrated that SGA is an effective tool for assessing the nutritional status of patients with acute kidney injury (AKI) [Citation20]. However, SGA also has some limitations: it is highly subjective and its scoring results are highly variable among each rater, which means that SGA may not be able to accurately determine the degree of malnutrition in patients, and is more suitable for distinguishing patients with severe malnutrition and good nutrition. Therefore, in order to evaluate the accuracy of SGA, it is better to conduct a formal training for raters [Citation21–24].
With an increasing emphasis on the role of inflammation in malnutrition in dialysis patients, MIS is derived from SGA and includes three new parameters: BMI, serum albumin level, and total iron binding capacity, which makes it a more comprehensive evaluation system than SGA [Citation25–27]. Similar to SGA, MIS is significantly associated with mortality in dialysis patients [Citation28–30]. However, MIS has the following advantages over SGA: it is associated with a higher risk of hospitalization in dialysis patients [Citation28], inflammation and quality of life [Citation31], and can predict the occurrence of cardiovascular events in dialysis patients [Citation32]. It is an important tool for identification of early malnutrition in patients with renal failure [Citation6]. Dialysis-malnutrition score (DMS) [Citation33,Citation34], another SGA-based nutritional assessment tool, can also assess the nutritional status and cardiovascular disease risk in dialysis patients.
Both SGA and MIS are subjective. In order to seek a simpler and more objective nutritional assessment method, Bouillanne et al. [Citation35] invented GNRI in 2005, which includes only three objective parameters: weight, height, and serum albumin level. The calculation formula is as follows: GNRI = [14.89 * albumin g/dL] + [41.7 + (weight/ideal weight)], < 82, major nutrition-related risk; between 82 and < 92, moderate nutrition-related risk; between 92 and ≤ 98, low nutrition-related risk; > 98, no risk. GNRI can assess the nutritional status of dialysis patients [Citation34,Citation36], and the changes of its parameters are significantly correlated with all-cause mortality [Citation37–39] and cardiovascular events [Citation37,Citation40]. In addition, low GNRI score is associated with an increased risk of CKD progression to ESRD [Citation41]. Two recent studies have shown that simultaneous assessment of the patient ‘s GNRI and modified creatinine index (mCI) can stratify the risk and improve the predictability of mortality in dialysis patients [Citation42,Citation43]. GNRI can also evaluate the muscle strength of dialysis patients and is a useful tool for screening sarcopenia in dialysis patients [Citation44,Citation45]. Therefore, GNRI is a simple and effective nutritional assessment method for long-term dialysis patients.
MNA was originally used to screen the nutritional status of individuals over 65 years of age, and later proved to be equally applicable in the hemodialytic population [Citation46]. Another study by Tsai et al. showed that MNA was more effective than SGA in predicting the risk of malnutrition in peritoneal dialysis patients [Citation47]. NRS-2002 is the preferred nutritional risk screening tool for inpatients recommended by ESPEN [Citation48]. A retrospective study by Li et al. [Citation49] showed that NRS-2002 score was strongly associated with AKI risk; the risk of death in patients with low NRS-2002 score was significantly lower than that in patients with high NRS-2002 score in terms of short- and long-term survival. The original Nutritional Risk Index (NRI) [Citation50] was created by Buzby et al. to assess the nutritional status of postoperative patients. In 2019, Japanese scholars described a new NRI (the Nutritional Risk Index-Japanese Hemodialysis (NRI-JH)) based on the characteristics of dialysis patients in Japan. The new index included only four objective indicators, namely BMI, serum albumin, and total cholesterol and creatinine levels [Citation51]. Subsequent studies have confirmed [Citation24,Citation52] that NRI-JH is a useful tool for assessing the nutritional status of dialysis patients and that its score is significantly associated with sarcopenia in dialysis patients.
Bioelectrical impedance analysis (BIA) is also a nutritional assessment method that analyzes body composition [Citation53]. It is a non-invasive tool based on the conduction of human AC current to estimate the hydration state of human body [Citation54]. As a nutritional assessment method, BIA has been validated in patients with CKD and dialysis [Citation55]. It is also an effective tool for early diagnosis of malnutrition in dialysis patients [Citation18].
Other nutritional assessment methods that can be used for dialysis patients include renal inpatient screening tools (renal iNUTs) [Citation56,Citation57]. The application area of this method is limited; hence, it has not been widely used.
Many studies suggest that appropriate nutrition assessment methods should be selected for identification and management of malnutrition in dialysis patients at the earliest as it is important to improve the survival rate and quality of life of dialysis patients.
4. Risk factors of malnutrition in dialysis patients
Risk factors of malnutrition in dialysis patients are numerous and interact with each other (). Factors such as reduced nutrient intake, microinflammatory state, metabolic acidosis, increased energy expenditure, and endocrine and gastrointestinal disorders will be addressed one by one in the following text.
Figure 1. Schematic diagram of malnutrition risk factors and consequences. Abbreviations: DM: diabetes mellitus; CVD: cardiovascular disease; UPS: ubiquitin-proteasome system.
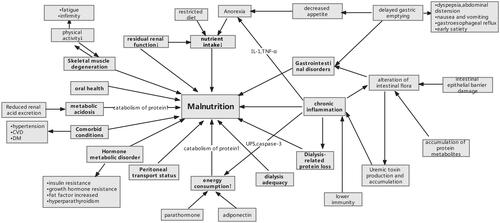
4.1. Reduced nutrient intake
A historical cohort study including 2221 maintenance hemodialysis patients [Citation58] showed a gradual increase in their BMI >20 kg/m2 with increase in protein catabolic rate (nPCR), demonstrating that reduced nutrient intake contributes largely to malnutrition in dialysis patients.
Anorexia nervosa and dietary restrictions are the main reasons for experience of reduced nutrient intake among dialysis patients [Citation58,Citation59]. Anorexia nervosa is common in dialysis patients, with a prevalence of 29.5–50% [Citation60–62]. According to many previous studies, it has been found that the causes of anorexia may be related to central loss of appetite caused by toxins of uremic substances, chronic inflammation, changes in hormones affecting appetite, accumulation of metabolic wastes in the body, and abnormalities in taste buds or taste sensation due to long-term application of oral medications [Citation10,Citation59,Citation63], which leads to a reduced intake of active nutrients in dialysis patients with ESRD. Gołębiewska et al. showed [Citation64] that after 3 months of treatment with megestrol (a synthetic progestin that has been shown to increase appetite in patients [Citation65]), the weight and BMI of dialysis patients increased significantly compared to the previous period and the changes were statistically significant (p < 0.05); a decreasing trend towards total cholesterol concentration in the first 2 months of treatment (p = 0.052) was also seen. This suggests that as the appetite of dialysis patients improves, so does their nutritional status.
In addition to this, in order to prevent and correct some metabolic complications and delay progression of the disease, dialysis patients are usually recommended to restrict the intake of certain nutrients, such as protein, potassium, sodium, and phosphorus [Citation1,Citation66,Citation67]. Although restriction of protein intake is relatively reduced after initiation of regular dialysis, the intake of potassium, sodium, and phosphorus is still quite restricted, which means that patients can consume a reduced variety of foods, such as some sodium-rich condiments, fruits, vegetables, animal offal, and other crops that are rich in potassium and phosphorus. Although strict sodium restriction can effectively reduce the patient’s water and sodium retention and slow down edema, sodium restriction also makes the dialysis patient’s diet lighter, which undoubtedly aggravates the patient’s loss of appetite and further reduces the intake of nutrients.
In addition to anorexia and dietary restrictions, advanced age, loss of residual renal function, gastrointestinal dysfunction, depression, poor socioeconomic status, and early satiety in peritoneal dialysis may cause reduced nutrient intake in dialysis patients [Citation68–70].
4.2. Increased energy consumption
In addition to reduced nutrient intake, increased energy expenditure also plays an important role in malnutrition of dialysis patients. A study by Ikizler et al. [Citation71] reported that resting energy expenditure on non-dialysis days was significantly higher in dialysis patients than in healthy controls and that resting energy expenditure increased further on dialysis days. Resting energy expenditure during dialysis is increased by approximately 15–20% [Citation71]. Increased resting energy expenditure accelerates the consumption of fat and muscle tissue and promotes the catabolism of fat and protein.
The key to increased resting energy expenditure is transformation of white fat to brown fat, a phenomenon known as adipose browning [Citation72]. Mitochondrial uncoupling protein 1 (UCP1) uncouples mitochondrial respiration to generate more heat and brown adipose tissue, leading to lipid mobilization and energy consumption [Citation73]. An animal experiment by Cheung et al. also showed [Citation74] that the activity of UCP1 is increased in mice after nephrectomy, which increases the metabolic rate. It was also found that the mice after nephrectomy had decreased appetite and body weight, and even after force-feeding, the mice did not show a significant increase in their body weight and fat mass. This suggests that the kidneys play a role in regulating metabolism.
Hyperparathyroidism is common in end-stage dialysis patients. It has been found that parathyroid hormone plays a key role in the browning of adipose tissue and increased resting energy expenditure in dialysis patients. Cuppari et al. [Citation75] measured resting energy expenditure in dialysis patients with hyperparathyroidism and found that parathyroid hormone was an independent determinant of resting energy expenditure. And the researcher also found that 6 months after surgery in patients with severe hyperparathyroidism, parathyroid hormone levels and the patients’ resting energy expenditure were significantly reduced. Regarding the mechanism by which parathyroid hormone increases resting energy expenditure and adipose tissue browning, Kir et al. [Citation76] found that parathyroid hormone and parathyroid hormone-related protein (PTHrP) in dialysis patients can increase the expression of thermogenic genes, accelerate adipose tissue browning, and increase resting energy expenditure in dialysis patients. In 2022, a retrospective study by Disthabanchong et al. [Citation77] showed that patients with severe hyperparathyroidism had a poorer nutritional status than dialysis patients with normal or moderate hyperparathyroidism.
Additionally, dialysis patients are generally in a chronic inflammatory state and these inflammatory factors can act on the central nervous system to reduce appetite and increase resting energy expenditure of patients [Citation78].
4.3. Metabolic acidosis
Metabolic acidosis is also prevalent in dialysis patients due to decreased ability of the kidneys to excrete acid in patients with ESRD. A higher pH may be more conducive to protein synthesis and may improve the patient’s malnutrition [Citation79,Citation80]. In 2009, in a cell culture study by Chiu et al. [Citation81], it was found that the rate of intracellular protein synthesis in cell cultures increased progressively with increasing pH of the culture medium. In the same year, Mehrotra et al. [Citation82] also found that a significant increase in net positive nitrogen balance was observed when the arterial pH was increased from 7.37 to 7.44 in patients with peritoneal dialysis. In a randomized controlled trial including 134 patients with stage 4 CKD [Citation83], it was found that an increase in serum bicarbonate levels to 24 mmol/L compared to maintaining these levels at 20 mmol/L showed a significant improvement in mid-arm muscle circumference and serum albumin of patients and also delayed the progression of CKD.
Why does acidosis accelerate protein catabolism and exacerbate malnutrition in CKD patients? The mechanism of this was shown in a study by Bailey et al. [Citation84] in 1996, who suggested that metabolic acidosis exacerbates malnutrition in CKD patients by activating the ubiquitin-proteasome system (UPS) to increase protein catabolism. A recent study [Citation85] shed new light on the mechanism and stated that cysteine aspartate protease-3 (Caspase-3) also plays a role. The researchers found that caspase-3 cleaves myosin and myogenic fibers, providing a suitable substrate for UPS-mediated proteolysis. In addition, caspase-3 can also activate 26S proteinosome-mediated protein decomposition by cleaving subunits of 19S proteinosome particles (Rpt2 and 6). Therefore, these tests reveal that the presence or absence of acidosis is closely related to good or bad nutritional status of the patient, and that correction of acidosis in the patient is beneficial for improvement of his nutritional status.
4.4. Chronic inflammatory state
CKD is a chronic wasting disease; patients on long-term dialysis therapy are commonly immunocompromised and prone to infection. Therefore, chronic inflammatory states are prevalent in this population. In addition to the common inflammatory cytokines such as interleukin (IL)-6, IL-1β, IL-18, tumor necrosis factor (TNFα), IL-8, and C-reactive protein (CRP), hypoalbuminemia and elevated ferritin levels are also considered as inflammatory markers in patients with ESRD [Citation86–89]. Inflammation-induced malnutrition is achieved through increased proteolytic metabolism and/or decreased protein intake [Citation90]. Inflammatory cytokines can promote protein catabolism by inhibiting phosphatidylinositol 3-kinase (PI3K) activity, which in turn activates two proteolytic pathways, the ubiquitin-proteasome proteolytic system (UPP) and caspase-3, causing a negative nitrogen balance in patients, which in turn leads to malnutrition in dialysis patients [Citation91]. As for reduced protein intake, the inflammatory factor IL-1 can cause anorexia by directly affecting the satiety center, suppressing the patient’s appetite, resulting in reduced protein intake, and malnutrition [Citation92].
4.5. Disorders of hormone metabolism
Kidneys have a very important endocrine function and play an important role in the synthesis, metabolism, and regulation of many hormones. In patients with CKD, the ability of the kidneys to regulate various hormones is gradually reduced due to the deteriorating kidney function. Therefore, disorders of hormone metabolism are very common in patients with CKD, especially in those with ESRD.
In 1981, DeFronzo et al. [Citation93] found that insulin resistance occurs early in the course of CKD and becomes more pronounced as renal function continues to deteriorate. There is a strong link between insulin resistance and increased protein catabolism and muscle atrophy [Citation94,Citation95]. Insulin resistance activates the UPP and increases caspase-3 activity, which promotes protein catabolism and causes muscle atrophy [Citation96,Citation97].
In addition to insulin resistance, growth hormone resistance also occurs in patients with ESRD [Citation98,Citation99]. In a study on pediatric patients with CKD [Citation100], the use of recombinant growth hormone (rhGH) was reported to promote growth among these patients. This finding may also demonstrate that growth hormone resistance plays a role in the development of malnutrition in patients with ESRD.
In addition to disturbances in insulin and growth hormone metabolism, elevated adipokines such as leptin, adiponectin, and endolipin can be found in patients with ESRD. Adipocytokines such as adiponectin and leptin are important nutrients for dialysis patients. Coimbra et al. showed that [Citation101] increased adiponectin in chronic dialysis patients induces a more protective high-density lipoprotein profile for the human body, and adiponectin may also have the effect of improving common oxidative stress in hemodialysis patients [Citation102]. Studies [Citation101,Citation103,Citation104] have also shown that higher leptin levels are associated with higher BMI and adipose tissue mass. Unlike healthy individuals, dialysis patients with obesity or higher BMI have better nutritional status and clinical outcomes [Citation105]. However, leptin is a hormone that can regulate appetite [Citation106]. High levels of leptin suppress appetite in patients with ESRD and can lead to increased energy expenditure [Citation107–109]. Although adiponectin has anti-inflammatory, anti-atherosclerosis, and insulin sensitization effects, adiponectin can also increase the energy consumption of patients [Citation110,Citation111], thereby accelerating body’s catabolism and causing malnutrition. There is a significant positive correlation between serum lipocalin levels and malnutrition (p < 0.0001) [Citation112]. Elevated levels of endolipin in dialysis patients suppress appetite and reduce serum amino acid levels [Citation113].
In addition, disorders of parathyroid hormone metabolism are also prevalent in dialysis patients, and the mechanisms by which parathyroid hormones cause malnutrition in dialysis patients have been described previously and will not be repeated here. Therefore, it is important to pay attention to and make efforts to correct hormone metabolism disorders in patients with ESRD for the prevention and treatment of malnutrition.
4.6. Gastrointestinal disorders
Two major features of gastrointestinal dysfunction in patients with CKD are dysbiosis of the intestinal flora [Citation114–116] and delayed gastric emptying [Citation117]. Dysbiosis of the intestinal flora is caused by exacerbation of the chronic inflammatory state of dialysis patients, which in turn leads to malnutrition [Citation63]. According to the preferential metabolic pathways, the human intestinal flora can be divided into two categories, namely, preferentially fermented carbohydrates and preferentially fermented proteins. Among them, the intestinal bacteria that preferentially ferment proteins produce some potentially toxic substances while decomposing proteins. In healthy people, these harmful substances can be discharged in vitro through the kidney; however, in case of renal failure, these harmful substances accumulate in the body, thereby further aggravating the intestinal flora imbalance. In addition to this, re-hydrolysis of urea accumulated in the patient’s intestine by microorganisms produces large amounts of ammonia; this can be further converted into ammonium hydroxide, which damages the integrity of the intestinal epithelial barrier, increases permeability of the intestine, and promotes transfer of toxic substances from the intestine to the circulatory system [Citation118]. Therefore, dysbiosis of the intestinal flora in patients with ESRD promotes the production and accumulation of uremic toxins, while disruption of the intestinal epithelial barrier promotes the absorption of uremic toxins; these absorbed uremic toxins induce or even aggravate the inflammatory state of dialysis patients and makes them malnourished [Citation119]. A recent randomized controlled study [Citation120] has confirmed that supplementation of dialysis patients with enteric probiotics for 2 months significantly increases the patients’ albumin levels, upper arm circumference, and triceps skinfold thickness, improves the patients’ malnutrition status, and decreases the patients’ inflammatory factor levels.
Delayed gastric emptying (gastroparesis) is also very common in patients with ESRD [Citation117,Citation121]. Due to long-term chronic delayed gastric emptying, patients experience gastrointestinal symptoms such as dyspepsia, bloating, nausea and vomiting, gastroesophageal reflux, and early satiety; these gastrointestinal symptoms reduce the patient’s appetite, resulting in decreased nutrient intake and progressive malnutrition [Citation122–124]. When patients improve delayed gastric emptying with the application of pro-gastric motility drugs, their nutritional status improves along with it [Citation125]. In patients on peritoneal dialysis, high retention of peritoneal fluid (which can increase intra-abdominal pressure) and reabsorption of glucose from the peritoneal fluid can cause delayed gastric emptying [Citation126,Citation127]. Other factors affecting delayed gastric emptying include diabetes mellitus and abnormal gastric electromyographic activity [Citation128]. In conclusion, aggressive treatment of gastrointestinal dysfunction not only leads to improvement of malnutrition in patients, but also delays the progression of renal disease.
4.7. Skeletal muscle degeneration
Patients on long-term dialysis treatment often experience fatigue due to various physical and psychosocial factors, which directly leads to less physical activity [Citation129,Citation130]. Prolonged reduction in physical activity decreases the muscle mass and strength of patients [Citation131], which in turn leads to degeneration of skeletal muscles, while degeneration of skeletal muscles further reduces physical activity and aggravates malnutrition in patients. It is important to provide appropriate physical exercise to ESRD patients for prevention of skeletal muscle degeneration [Citation132,Citation133]. In a study, Johansen showed that appropriate resistance exercise not only improves physical function in daily life among patients with ESRD, but also has a direct effect on reducing muscle wastage in patients [Citation134]. A recent study [Citation135] revealed that dialysis patients show a significant decrease in the levels of inflammatory markers after 6 months of resistance exercise training. The study also showed significant improvements in serum albumin levels, muscle mass, and physical performance in patients after exercise training. Therefore, increasing resistance exercise training in dialysis patients may reduce muscle atrophy, prevent skeletal muscle degeneration, improve the nutritional status of patients, and delay progression of the disease.
4.8. Dialysis-related protein loss
Dialysis can discharge various harmful substances and excess metabolic wastes from the body of patients with ESRD, correct water and electrolyte acid-base balance disorders, and facilitate blood purification. Although the current dialysis technology is quite sophisticated, many complications directly related to dialysis still prevail, one of which is protein and nutrient loss from the body [Citation136–138].
For patients treated with peritoneal dialysis, the most commonly used peritoneal dialysis solution in clinical practice is glucose as the permeate. A single 1.5–4.25% instillation of peritoneal dialysis fluid can have a caloric load of 50–300 kcal, which is equivalent to 0.30% of the total daily energy intake of patients on peritoneal dialysis [Citation21]. Although absorption of glucose in the peritoneal dialysis solution does not reduce the patient’s caloric and protein intake, it is still believed that these ‘calories’ in the peritoneal dialysis solution have an impact on the patient’s protein and other nutrient intake. In addition to caloric absorption of peritoneal dialysis fluid, there is also a significant loss of protein during the process of peritoneal dialysis. Patients treated with peritoneal dialysis lose 6–8 g of albumin-based protein per day with the effluence of peritoneal dialysis fluid, and protein loss in this manner is further increased during peritonitis [Citation139,Citation140].
As with peritoneal dialysis, hemodialysis can also lead to loss of protein and other nutrients. Related studies [Citation137,Citation141,Citation142] have shown that approximately 6–12 g of amino acids and 7–8 g of protein are lost during each dialysis session in patients and these lost proteins are likely to lead to hypoalbuminemia and aggravation of malnutrition in patients.
In addition to the direct loss of protein caused by dialysis itself, Ikizler [Citation143] et al. clearly demonstrated that hemodialysis also increases protein catabolism, by measuring protein synthesis and catabolism in hemodialysis patients.
In addition to the risk factors mentioned above, loss of residual renal function, peritoneal transit status, dialysis adequacy, oral health of the elderly, and comorbidities also have an impact on the nutritional status of dialysis patients, and further studies are needed on the topic [Citation12,Citation19,Citation21,Citation144,Citation145].
5. Advances in research on trace elements and malnutrition
Numerous studies have shown that [Citation146–148] the imbalance of trace elements, such as Zinc (Zn), Se, Copper (Cu), etc., in dialysis patients is very common. These trace elements are not only involved in oxidative stress and anti-inflammatory processes in patients, but their imbalance can also lead to malnutrition in dialysis patients.
Zn is an essential trace element in the human body, which has anti-inflammatory and anti-oxidant effects [Citation149,Citation150]. Zn deficiency is common in dialysis patients [Citation148,Citation151]. In 2009, Sahin et al. [Citation152] proposed that low Zn levels in dialysis patients may cause malnutrition, and pointed out that dialysis patients need Zn supplementation to prevent progressive malnutrition. Subsequent studies have also confirmed that [Citation153–158] lower Zn levels are independently associated with higher nutritional risk in dialysis patients. After receiving Zn supplementation, serum Zn levels and oxidative stress state improve in dialysis patients. The patient’s hemoglobin and albumin levels also increase significantly, thereby, effectively improving the nutritional status of dialysis patients. Fukasawa et al. [Citation159] evaluated the nutritional status of dialysis patients by measuring abdominal fat mass and found that there was a significant positive correlation between serum Zn level and abdominal fat area in dialysis patients, and Zn level was an independent predictor of visceral fat area. Additionally, Zn affects the secretion of leptin [Citation160]. Zn deficiency increases the level of leptin in patients [Citation161], which affects the appetite of patients, and may lead to anorexia [Citation162]. Zn supplementation can improve taste abnormalities of dialysis patients [Citation163], and increase dietary intake and weight of patients [Citation164]. Gao et al. [Citation165] showed that Zn supplementation can reduce oxidative stress in peritoneal mesothelial cells (PMcs) to inhibit the effect of high glucose (HG) on epithelial-mesenchymal transition (EMT), thereby, improving peritoneal fibrosis (PF) during peritoneal dialysis. Zn deficiency is a predictor of cardiovascular death in dialysis patients [Citation166], and decrease of Zn levels in dialysis patients is significantly correlated with diastolic dysfunction [Citation167]. Latest research [Citation168–171] states that zinc supplementation also has a positive effect on the patients’ renal function, and low Zn levels in dialysis patients are independent predictors of mortality and infection-related hospitalization.
Trace element Se is a key component of various enzymes in the body and plays an important role in reducing oxidative stress and inflammation in dialysis patients [Citation172]. Se deficiency is common in dialysis patients [Citation146,Citation173,Citation174]. In the 1990s, it was reported that there is a certain correlation between Se and serum albumin levels in dialysis patients [Citation175,Citation176]. In 2011, a study [Citation177] demonstrated that serum Se concentrations were significantly lower in the hemodialysis group than in the control group (30 healthy volunteers) (p < 0.01), and that serum Se levels in patients in the hemodialysis group were significantly and positively correlated with their nutrition-related indicators such as albumin and high-density lipoprotein-cholesterol (HDL-C). The trial concluded that the low blood Se status in hemodialysis patients may be associated with malnutrition in patients. In 2012, Yang et al. [Citation170] recruited 111 patients on maintenance hemodialysis, measured serum levels of Se, Cu, and Zn and followed them up for two years; they found that patients with lower serum Se (p = 0.026) and Zn (p = 0.001) levels were more likely to be hospitalized for infectious diseases. In 2013, a randomized double-blind placebo trial by Salehi et al. [Citation178] found that treatment of hemodialysis patients with Se supplementation for 12 weeks significantly lowered the SGA scores and MIS in the Se group compared to the placebo group (p < 0.001), in addition to a significant decrease in IL-6 levels in the Se supplementation group (p = 0.016). Thereafter, in a prospective longitudinal study including 1278 hemodialysis patients [Citation179], blood concentrations of 25 trace elements were assessed and followed over time, showing that low blood Se levels in patients were significantly associated with their all-cause hospitalization and mortality rates. In 2021, a cross-sectional study by Liu et al. including 118 patients on hemodialysis treatment used the 2002 Nutritional Risk Screen (NRS 2002) to assess the nutritional status of patients and showed that lower blood Se levels were independently associated with high nutritional risk in maintenance hemodialysis patients [Citation153]. A recent report on peritoneal dialysis patients reported that 41.4% of 406 peritoneal dialysis patients had Se deficiency (< 0.8umol/L), and lower Se levels were associated with reduced dietary intake and increased weakness and inflammation [Citation174]. In addition, Se deficiency is also associated with the risk of all-cause mortality in dialysis patients [Citation180], severe sleep disorders [Citation181], and low response to erythropoietin (ESA) [Citation182].
Cu is also a basic trace element. It is a component of various Cu enzymes in the human body and participates in various physiological processes in the body. However, excessive blood Cu levels can induce oxidative stress in the body, leading to lipid peroxidation and protein oxidation [Citation183]. Compared with healthy people, the level of blood Cu in dialysis patients was significantly increased [Citation184,Citation185]. There was a significant correlation between high blood Cu levels and dyslipidemia, inflammation, cardiovascular disease, and high risk of death in dialysis patients [Citation179,Citation185,Citation186]. As Cu/Zn ratios are increasingly recognized as biomarkers of inflammation [Citation187], a greater number of studies [Citation153,Citation188] have shown that higher Cu/Zn ratios are independently associated with nutritional risk in dialysis patients. Moreover, a recent cross-sectional study by Zuo et al. [Citation189] reported that high Cu/Zn ratio was independently associated with anemia in dialysis patients. Although Zn supplementation can reduce the Cu/Zn ratio and improve anemia in dialysis patients, Takahashi’s results suggest that inappropriate Zn supplementation can lead to Cu deficiency, which directly affects the role of ceruloplasmin and lysine oxidase [Citation190]. Therefore, blood Cu levels should be monitored to avoid Cu deficiency during Zn supplementation in dialysis patients [Citation191].
In addition to the above three trace elements, the imbalance of other trace elements also affects the nutritional status of dialysis patients. Appropriate levels of chromium (Cr) can reduce oxidative stress and inflammation; however, very high levels of Cr have a negative impact on the human body. Cr can accumulate in the bones of patients with ESRD and blood Cr levels are significantly elevated in dialysis patients [Citation192]. Hsu et al. [Citation193] investigated the blood Cr level and nutritional status of 647 hemodialysis patients and found that blood Cr level was significantly negatively correlated with malnutrition. Manganese (Mn) is a cofactor of manganese superoxide dismutase (MnSOD) enzyme. Its deficiency can decrease MnSOD activity and aggravate oxidative damage in human body [Citation194,Citation195]. The level of blood Mn in dialysis patients decreased, and the level of blood Mn was independently correlated with the level of hemoglobin [Citation196,Citation197]. For iron deficiency in dialysis patients [Citation198], many studies have been conducted and their results are relatively clear; hence, we will not repeat them here.
Therefore, in order to monitor and prevent malnutrition in dialysis patients, we should pay attention to the intake of trace elements and their assessment.
6. Potential mechanisms by which Se may improve malnutrition
Patients with ESRD treated by dialysis have significantly lower blood Se levels than the healthy population due to decreased appetite, inadequate dietary intake, and its loss during dialysis [Citation199]. Se is an important trace element in the human body, which has a regulatory role in maintenance of metabolic activities of the body [Citation200]. In the body, it is involved in various physiological processes in the form of selenoproteins containing selenocysteine in the active center [Citation201,Citation202].
Till date, 25 selenoproteins have been identified in humans, including glutathione peroxidases (GPx1-GPx4 and GPx6), members of the thioredoxin reductase family (TXNRD1-TXNRD3), and methionine sulfoxide reductase (MSR B1) [Citation203–205]. Selenoprotein is a key component of the antioxidant defense system and plays an important role in maintaining redox homeostasis in the body. In contrast, a state of oxidative stress and inflammation is prevalent in patients treated with dialysis. A study by Salehi et al. [Citation178] demonstrated that Se supplementation group significantly reduced IL-6 production by inhibiting the activation of nuclear factor-κB (NF-κB) signaling pathway in dialysis patients and concluded that Se supplementation improves the nutritional status of dialysis patients probably by inhibiting oxidative damage and inflammation. Similarly, in a trial by Stockler et al. [Citation206], it was shown that treatment of dialysis patients with Se supplementation for 3 months led to a significant increase in their GPx activity and 8-hydroxytryptamine, 8-isoprostane, TNF-α and IL-6 levels were significantly decreased. This paper briefly summarizes the mechanism by which Se deficiency induces inflammation by activating the NF-κB pathway, as shown in .
Figure 2. A simple mechanism by which Se deficiency induces inflammation by activation of the NF-κB pathway. Abbreviations: Se: Selenium; GPX: glutathione peroxidases; TXNRD: thioredoxin reductase; ROS: Reactive oxygen species; IKK: IκB kinase; IκB: inhibitor of nuclear factor kappa B; NF-kB: nuclear factor kappa B; IL-1: interleukin-1; IL-6: interleukin-6; IL-8: interleukin-8; COX-2: cyclooxygenase-2; TNF-α: tumor necrosis factor alpha.
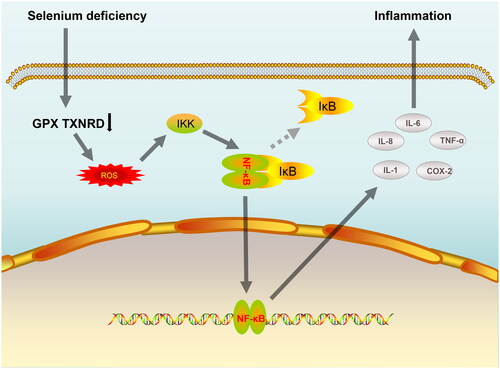
In addition to the fact that direct Se supplementation improves oxidative stress and inflammation in dialysis patients, the study by Xu et al. [Citation207] revealed the important role played by Se in oxidative stress and infection. His study showed that the application of Lactobacillus casei ATCC 393 (L. casei 393)-Se nanoparticles (Se-NPs) prevented pathogenic Escherichia coli K88 (ETEC K88)-induced intestinal epithelial barrier (IEB) dysfunction and ameliorated ETEC K88-induced oxidative stress. Se deficiency can also destroy the balance of intestinal flora and cause inflammatory reaction in intestinal tissue cells, leading to intestinal inflammation [Citation208,Citation209].
In addition to its anti-oxidant and anti-inflammatory properties, Se supplementation to improve malnutrition in patients may also be associated with its effects on fat digestion and absorption, nutrient utilization, reduction of ketone bodies, and improved insulin action [Citation210–212]. Therefore, additional pilot studies are needed to further explore the potential mechanisms by which Se may improve malnutrition in patients.
7. Summary and prospect
In conclusion, malnutrition is very common in CKD patients, especially in those on dialysis. Paying attention to the nutritional problems of CKD patients and integrating nutritional therapy throughout the treatment of CKD has great significance for improvement in the overall diagnosis and treatment of CKD, delayed disease progression, improved patient prognosis, and reduced medical expenses. Many of these studies have shown that blood Se levels in dialysis patients are significantly lower than those in the healthy population, and that blood Se levels are positively correlated with their nutrition-related indicators, while small sample trials have also shown that Se supplementation can improve the malnutrition status of patients. However, there is still a lack of prospective clinical studies with large samples, multicenter-based, and long duration to further prove this conclusion. There is also a lack of basic studies to describe the potential mechanisms by which Se can improve malnutrition in dialysis patients more comprehensively. Most of the current studies have been conducted in patients on maintenance hemodialysis, and data studying the relationship between nutritional status and blood Se in patients on peritoneal dialysis are currently scarce. Therefore, further research is needed to investigate the evidence of correlation between plasma Se and its nutritional status in patients with different dialysis modalities, to provide new diagnostic ideas for predicting and reducing the occurrence of malnutrition events in patients with ESRD treated with dialysis.
Author contributions
Writing—original draft preparation, Meiran Cao; writing—review & editing, Meiran Cao, Guicai Hu and Wenhua Zhang; visualization, Shuai Zheng and Meiran Cao; All authors have read and agreed to the published version of the manuscript.
Disclaimer
The author declare that the views expressed in the article are our own and not an official position of the institution or funder.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kalantar-Zadeh K, Jafar TH, Nitsch D, et al. Chronic kidney disease. Lancet. 2021;398(10302):1–17.
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733.
- Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr. 2018;28(6):380–392.
- Dekker MJE, Marcelli D, Canaud B, et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: results from the international MONDO initiative. Eur J Clin Nutr. 2016;70(7):779–784.
- Peng L, Gao Y, Lu R, et al. Efficacy of Omaha system-based nursing management on nutritional status in patients undergoing peritoneal dialysis: a randomized controlled trial protocol. Medicine (Baltimore). 2020;99(51):e23572.
- Visiedo L, Rey L, Rivas F, et al. The impact of nutritional status on health-related quality of life in hemodialysis patients. Sci Rep. 2022;12(1):3029.
- Li S, Zhao Q, Zhang K, et al. Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation. Metallomics. 2020;12(10):1576–1584.
- Oliveira EA, Zheng R, Carter CE, et al. Cachexia/protein energy wasting syndrome in CKD: causation and treatment. Semin Dial. 2019;32(6):493–499.
- Beddhu S, Pappas LM, Ramkumar N, et al. Malnutrition and atherosclerosis in dialysis patients. J Am Soc Nephrol. 2004;15(3):733–742.
- Obi Y, Qader H, Kovesdy CP, et al. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18(3):254–262.
- Maraj M, Kuśnierz-Cabala B, Dumnicka P, et al. Malnutrition, inflammation, atherosclerosis syndrome (MIA) and diet recommendations among end-stage renal disease patients treated with maintenance hemodialysis. Nutrients. 2018;10(1):69.
- Piccoli GB, Lippi F, Fois A, et al. Intradialytic nutrition and hemodialysis prescriptions: a personalized stepwise approach. Nutrients. 2020;12(3):785.
- Szeto CC, Chow KM. Metabolic acidosis and malnutrition in dialysis patients. Semin Dial. 2004;17(5):371–375.
- Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398.
- Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11(1):8–13.
- National Kidney Foundation (K/DOQI). Clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2000;35(Suppl. 2):S1–S140. Erratum in: American Journal of Kidney Disease 2001; 38:917)
- Chan M, Kelly J, Batterham M, et al. Malnutrition (subjective global assessment) scores and serum albumin levels, but not body mass index values, at initiation of dialysis are independent predictors of mortality: a 10-year clinical cohort study. J Ren Nutr. 2012;22(6):547–557.
- Knap B, Arnol M, Romozi K, et al. Malnutrition in renal failure: pleiotropic diagnostic approaches, inefficient therapy and bad prognosis. Ther Apher Dial. 2016;20(3):272–276.
- Sahathevan S, Khor B-H, Ng H-M, et al. Understanding development of malnutrition in hemodialysis patients: a narrative review. Nutrients. 2020;12(10):3147.
- Khor B-H, Tiong H-C, Tan SC, et al. Protein-energy wasting assessment and clinical outcomes in patients with acute kidney injury: a systematic review with meta-analysis. Nutrients. 2020;12(9):2809.
- Tennankore KK, Bargman JM. Nutrition and the kidney: recommendations for peritoneal dialysis. Adv Chronic Kidney Dis. 2013;20(2):190–201.
- Cooper BA, Bartlett LH, Aslani A, et al. Validity of subjective global assessment as a nutritional marker in end-stage renal disease. Am J Kidney Dis. 2002;40(1):126–132.
- Kirsch R, Matthews K, Williams V. Using global criteria to detect malnutrition: application in disease states. Nutr Clin Pract. 2020;35(1):85–97.
- Kurajoh M, Mori K, Miyabe M, et al. Nutritional status association with sarcopenia in patients undergoing maintenance hemodialysis assessed by nutritional risk index. Front. Nutr. 2022;9:896427.
- Riella MC. Nutritional evaluation of patients receiving dialysis for the management of protein-energy wasting: what is old and what is new? J Ren Nutr. 2013;23(3):195–198.
- Lopes AA. The malnutrition-inflammation score: a valid nutritional tool to assess mortality risk in kidney transplant patients. Am J Kidney Dis. 2011;58(1):7–9.
- Naeeni AE, Poostiyan N, et al. Assessment of severity of malnutrition in peritoneal dialysis patients via malnutrition: inflammatory score. Adv Biomed Res. 2017;6:128.
- Sá Martins V, Adragão T, Aguiar L, et al. Prognostic value of the malnutrition-inflammation score in hospitalization and mortality on long-term hemodialysis. J Ren Nutr. 2022;32(5):569–577.
- Prelevic V, Antunovic T, Radunovic D, et al. Malnutrition inflammation score (MIS) is stronger predictor of mortality in hemodialysis patients than waist-to-hip ratio (WHR)-4-year follow-up. Int Urol Nephrol. 2022;54(3):695–700.
- Kittiskulnam P, Chuengsaman P, Kanjanabuch T, et al. Protein-energy wasting and mortality risk prediction among peritoneal dialysis patients. J Ren Nutr. 2021;31(6):679–686.
- Rambod M, Bross R, Zitterkoph J, et al. Association of malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53(2):298–309.
- Ho L-C, Wang H-H, Chiang C-K, et al. Malnutrition-inflammation score independently determined cardiovascular and infection risk in peritoneal dialysis patients. Blood Purif. 2010;30(1):16–24.
- Spatola L, Finazzi S, Calvetta A, et al. Subjective global assessment-dialysis malnutrition score and cardiovascular risk in hemodialysis patients: an observational cohort study. J Nephrol. 2018;31(5):757–765.
- Spatola L, Finazzi S, Santostasi S, et al. Geriatric nutritional risk index is predictive of subjective global assessment and dialysis malnutrition scores in elderly patients on hemodialysis. J Ren Nutr. 2019;29(5):438–443.
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783.
- Yamada K, Furuya R, Takita T, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87(1):106–113.
- Nakagawa N, Maruyama K, Hasebe N, et al. Utility of geriatric nutritional risk index in patients with chronic kidney disease: a mini-review. Nutrients. 2021;13(11):3688.
- Yamada S, Yamamoto S, Fukuma S, et al. Geriatric nutritional risk index (GNRI) and creatinine index equally predict the risk of mortality in hemodialysis patients: J-DOPPS. Sci Rep. 2020;10(1):5756.
- Ren M, Sheng Q, Xie X, et al. Geriatric nutritional risk index is associated with mortality in peritoneal dialysis patients. Intern Med J. 2020;50(4):470–476.
- Takahashi H, Ito Y, Ishii H, et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J.Cardiol. 2014;64:32–36.
- Kuo I-C, Huang J-C, Wu P-Y, et al. A low geriatric nutrition risk index is associated with progression to dialysis in patients with chronic kidney disease. Nutrients. 2017;9(11):1228. pii
- Yajima T, Yajima K, Arao M, et al. Combined evaluation of geriatric nutritional risk index and modified creatinine index for predicting mortality in patients on hemodialysis. Nutrients. 2022;14(4):752.
- Fujioka H, Koike T, Imamura T, et al. Impact of geriatric nutritional risk index and modified creatinine index combination on mortality in hemodialysis patients. Nutrients. 2022;14(4):801.
- Kono K, Moriyama Y, Yabe H, et al. Relationship between malnutrition and possible sarcopenia in the AWGS 2019 consensus affecting mortality in hemodialysis patients: a prospective cohort study. BMC Nephrol. 2021;22(1):378.
- Nouri A, Mansour-Ghanaei R, Esmaeilpour-Bandboni M, et al. Geriatric nutritional risk index in prediction of muscular strength of elderly patients undergoing hemodialysis. Int Urol Nephrol. 2022;54(7):1575–1581.
- Tsai H-J, Tsai AC, Hung S-Y, et al. Comparing the predictive ability of population-specific mini-nutritional assessment with subjective global assessment for Taiwanese patients with hemodialysis: a cross-sectional study. Int J Nurs Stud. 2011;48(3):326–332.
- Tsai AC, Wang J-Y, Chang T-L, et al. A comparison of the full Mini Nutritional Assessment, short-form Mini Nutritional Assessment, and Subjective Global Assessment to predict the risk of protein-energy malnutrition in patients on peritoneal dialysis: a cross-sectional study. Int J Nurs Stud. 2013;50(1):83–89.
- Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–421.
- Li C, Xu L, Guan C, et al. Malnutrition screening and acute kidney injury in hospitalised patients: a retrospective study over a 5-year period from China. Br J Nutr. 2020;123(3):337–346.
- Buzby GP, Williford WO, Peterson OL, et al. Randomized clinical trial of total parenteral nutrition in malnourished surgical patients: the rationale and impact of previous clinical trials and pilot study on protocol design. Am J Clin Nutr. 1988;47(2):357–365.
- Kanda E, Kato A, Masakane I, et al. A new nutritional risk index for predicting mortality in hemodialysis patients: nationwide cohort study. PLoS One. 2019;14(3):e0214524.
- Kanno Y, Kanda E, Kato A, et al. Methods and nutritional interventions to improve the nutritional status of dialysis patients in Japan-a narrative review. Nutrients. 2021;13(5):1390.
- Camina Martín MA, de Mateo Silleras B, Redondo del Río MP. Body composition analysis in older adults with dementia. Anthropometry and bioelectrical impedance analysis: a critical review. Eur J Clin Nutr. 2014;68(11):1228–1233.
- Serón-Arbeloa C, Labarta-Monzón L, Puzo-Foncillas J, et al. Malnutrition screening and assessment. Nutrients. 2022;14(12):2392. Published Jun 9.
- Ostermann M, Lumlertgul N, Mehta R. Nutritional assessment and support during continuous renal replacement therapy. Semin Dial. 2021;34(6):449–456.
- Jackson HS, MacLaughlin HL, Vidal-Diez A, et al. A new renal inpatient nutrition screening tool (Renal iNUT): a multicenter validation study. Clin Nut. 2019;38(5):2297–2303.
- Badrasawi M, Zidan S, Sharif I, et al. Prevalence and correlates of malnutrition among hemodialysis patients at hebron governmental hospital, Palestine: cross-sectional study. BMC Nephrol. 2021;22(1):214.
- Komaba H, Fukagawa M. Secondary hyperparathyroidism and protein-energy wasting in end-stage renal disease. Ther Apher Dial. 2018;22(3):246–250.
- Gracia-Iguacel C, González-Parra E, et al. Defining protein-energy wasting syndrome in chronic kidney disease: prevalence and clinical implications. Nefrologia. 2014;34(4):507–519.
- Li C, Chen L, He L, et al. Study on the relationship between sarcopenia and its components and anorexia in elderly maintenance haemodialysis patients. Nurs Open. 2022;9(2):1096–1104.
- Bossola M, Tazza L, Giungi S, et al. Anorexia in hemodialysis patients: an update. Kidney Int. 2006;70(3):417–422.
- Carrero JJ. Identification of patients with eating disorders: clinical and biochemical signs of appetite loss in dialysis patients. J Ren Nutr. 2009;19(1):10–15.
- Iorember FM. Malnutrition in chronic kidney disease. Front. Pediatr. 2018;6:161.
- Gołębiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Influence of megestrol acetate on nutrition, inflammation and quality of life in dialysis patients. Int Urol Nephrol. 2012;44(4):1211–1222.
- Pascual López A, Roqué I Figuls M, Urrútia Cuchi G, et al. Systematic review of megestrol acetate in the treatment of anorexia-cachexia syndrome. J Pain Symptom Manage. 2004;27(4):360–369.
- Bolasco P, Cupisti A, Locatelli F, et al. Dietary management of incremental transition to dialysis therapy: once-weekly hemodialysis combined with low-protein diet. J Ren Nutr. 2016;26(6):352–359.
- Dwyer JP, Kelepouris E. New directions in phosphorus management in dialysis. J Ren Nutr. 2023;33(22):12–16.
- Piraino B. Recommendations for dietary protein intake in CAPD patients. Adv Perit Dial. 1996;12:275–279.
- Wright M, Woodrow G, O’Brien S, et al. Disturbed appetite patterns and nutrient intake in peritoneal dialysis patients. Perit Dial Int. 2003;23(6):550–556.
- Mamoun A-H, Anderstam B, Södersten P, et al. Influence of peritoneal dialysis solutions with glucose and amino acids on ingestive behavior in rats. Kidney Int. 1996;49(5):1276–1282.
- Ikizler TA, Wingard RL, Sun M, et al. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol. 1996;7(12):2646–2653.
- Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376.
- Bing C, Brown M, et al. Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Cancer Res. 2000;60(9):2405–2410.
- Cheung WW, Kuo H-J, Markison S, et al. Peripheral administration of the melanocortin-4 receptor antagonist NBI-12i ameliorates uremia-associated cachexia in mice. JASN. 2007;18(9):2517–2524.
- Cuppari L, de Carvalho AB, Avesani CM, et al. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol. 2004;15(11):2933–2939.
- Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–104.
- Disthabanchong S, Vantanasiri K, Khunapornphairote S, et al. Severe hyperparathyroidism is associated with nutritional impairment in maintenance hemodialysis patients. Front. Nutr. 2022;9:933918.
- Avesani CM, Carrero JJ, Axelsson J, et al. Inflammation and wasting in chronic kidney disease: partners in crime. Kidney Int Suppl. 2006;70:S8–S13.
- Kourtellidou SI, Ashby DR, Johansson LR, et al. Oral sodium bicarbonate in people on haemodialysis: a randomised controlled trial. BMC Nephrol. 2021;22(1):346.
- Szczecińska K, Wajdlich M, Nowicka M, et al. Effects of oral bicarbonate supplementation on the cardiovascular risk factors and serum nutritional markers in non-dialysed chronic kidney disease patients. Medicina (Kaunas). 2022;58(4):518.
- Chiu Y-W, Kopple JD, Mehrotra R, et al. Correction of metabolic acidosis to ameliorate wasting in chronic kidney disease: goals and strategies. Semin Nephrol. 2009;29(1):67–74.
- Mehrotra R, Bross R, Wang H, et al. Effect of high-normal compared with low-normal arterial pH on protein balances in automated peritoneal dialysis patients. Am J Clin Nutr. 2009;90(6):1532–1540.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20(9):2075–2084.
- Bailey JL, Wang X, England BK, et al. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Invest. 1996;97(6):1447–1453.
- Zha Y, Qian Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients. 2017;9(3):208.
- Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. IJMS. 2021;22(9):4496.
- Graterol Torres F, Molina M, Soler-Majoral J, et al. Evolving concepts on inflammatory biomarkers and malnutrition in chronic kidney disease. Nutrients. 2022;14(20):4297.
- DePalma RG, Hayes VW, O’Leary TJ, et al. Optimal serum ferritin level range: iron status measure and inflammatory biomarker. Metallomics. 2021;13(6):mfab030.
- Erdem E, Karatas A, Ecder T, et al. The relationship between serum ferritin levels and 5-year all-cause mortality in hemodialysis patients. Blood Purif. 2022;51(1):55–61.
- Wang AY-M, Sanderson J, Sea MM-M, et al. Important factors other than dialysis adequacy associated with inadequate dietary protein and energy intakes in patients receiving maintenance peritoneal dialysis. Am J Clin Nutr. 2003;77(4):834–841.
- Ikizler TA. Optimal nutrition in hemodialysis patients. Adv Chron Kidney Dis. 2013;20(2):181–189.
- DeBoer MD, Scarlett JM, Levasseur PR, et al. Administration of IL-1beta to the 4th ventricle causes anorexia that is blocked by agouti-related peptide and that coincides with activation of tyrosine-hydroxylase neurons in the nucleus of the solitary tract. Peptides. 2009;30(2):210–218.
- DeFronzo RA, Alvestrand A, Smith D, et al. Insulin resistance in uremia. J. Clin. Invest. 1981;67(2):563–568.
- Nishikawa H, Fukunishi S, Asai A, et al. Pathophysiology and mechanisms of primary sarcopenia. Int J Mol Med. 2021;48(2):156.
- Pérez-Cruz E, Castro-Martínez D, González-Guzman OP, et al. Association between sarcopenic obesity with insulin resistance and metabolic syndrome. Asociación entre obesidad sarcopénica con resistencia a la insulina y síndrome metabólico. Med Clin (Barc). 2022;159(1):1–5.
- Wang X, Hu Z, Hu J, et al. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147(9):4160–4168.
- Sun-Wang JL, Yarritu-Gallego A, Ivanova S, et al. The ubiquitin-proteasome system and autophagy: self-digestion for metabolic health. Trends Endocrinol Metab. 2021;32(8):594–608.
- Landau D, Assadi MH, Abu Hilal R, et al. SOCS2 silencing improves somatic growth without worsening kidney function in CKD. Am J Nephrol. 2020;51(7):520–526.
- Gungor O, Ulu S, Hasbal NB, et al. Effects of hormonal changes on sarcopenia in chronic kidney disease: where are we now and what can we do?. J Cachexia Sarcopenia Muscle. 2021;12(6):1380–1392.
- TöNshoff B, Schaefer F, Mehls O, et al. Disturbance of growth hormone–insulin-like growth factor axis in uraemia. Implications for recombinant human growth hormone treatment. Pediatr Nephrol. 1990;4(6):654–662.
- Coimbra S, Reis F, Nunes S, et al. The protective role of adiponectin for lipoproteins in end-stage renal disease patients: relationship with diabetes and body mass index. Oxid Med Cell Longev. 2019;2019:1–11.
- Lim P-S, Chen S-L, Wu M-Y, et al. Association of plasma adiponectin levels with oxidative stress in hemodialysis patients. Blood Purif. 2007;25(4):362–369.
- Risović I, Vlatković V, Popović-Pejičić S, et al. Relationship between leptin level, inflammation, and volume status in maintenance hemodialysis patients. Ther Apher Dial. 2019;23(1):59–64.
- Navaneethan SD, Kirwan JP, Remer EM, et al. Adiposity, physical function, and their associations with insulin resistance, inflammation, and adipokines in CKD. Am J Kidney Dis. 2021;77(1):44–55.
- Małgorzewicz S, Aleksandrowicz-Wrona E, Owczarzak A, et al. Adipokines and nutritional status for patients on maintenance hemodialysis. J Ren Nutr. 2010;20(5):303–308.
- Qin Z, Yang Q, Yang M, et al. Serum leptin concentration can predict cardiovascular outcomes and all-cause death in maintenance hemodialysis patients. Clin Chim Acta. 2021;520:87–94.
- Mitch WE. Cachexia in chronic kidney disease: a link to defective Central nervous system control of appetite. J Clin Invest. 2005;115(6):1476–1478.
- Oner-Iyidogan Y, Gurdol F, Kocak H, et al. Appetite-regulating hormones in chronic kidney disease patients. J Ren Nutr. 2011;21(4):316–321.
- Abi N, Xu X, Yang Z, et al. Association of serum adipokines and resting energy expenditure in patients with chronic kidney disease. Front Nutr. 2022;9:828341.
- Drechsler C, Krane V, Winkler K, et al. Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int. 2009;76(5):567–575.
- Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10(5):524–529.
- Kaynar K, Kural BV, Ulusoy S, et al. Is there any interaction of resistin and adiponectin levels with protein-energy wasting among patients with chronic kidney disease. Hemodial Int. 2014;18(1):153–162.
- Carrero JJ, Witasp A, Stenvinkel P, et al. Visfatin is increased in chronic kidney disease patients with poor appetite and correlates negatively with fasting serum amino acids and triglyceride levels. Nephrol Dial Transplant. 2010;25(3):901–906.
- Jiang S, Xie S, Lv D, et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep. 2017;7(1):2870.
- Zhou J, Yang C, Lei W, et al. Exploration of the correlation between intestinal flora and peritoneal dialysis-related peritonitis. Clin Exp Nephrol. 2022;26(10):1030–1038.
- Luo D, Zhao W, Lin Z, et al. The effects of hemodialysis and peritoneal dialysis on the gut microbiota of end-stage renal disease patients, and the relationship between gut microbiota and patient prognoses. Front Cell Infect Microbiol. 2021;11:579386.
- Salles Junior LD, Santos PR, dos Santos AA, et al. Dyspepsia and gastric emptying in end-stage renal disease patients on hemodialysis. BMC Nephrol. 2013;14:275.
- Sabatino A, Regolisti G, Brusasco I, et al. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. 2015;30(6):924–933.
- Noce A, Marchetti M, et al. Link between gut microbiota dysbiosis and chronic kidney disease. Eur Rev Med Pharmacol Sci. 2022;26(6):2057–2074.
- Pan Y, Yang L, Dai B, et al. Effects of probiotics on malnutrition and health-related quality of life in patients undergoing peritoneal dialysis: a randomized controlled trial. J Ren Nutr. 2021;31(2):199–205.
- Ives D, Brown S. Gastroparesis in the CKD patient: clinical management and implications for practice intended audience: chronic kidney disease patients. J Ren Nutr. 2022 Mar 12:S1051-2276(22)00035-8.
- Stompór T, Hubalewska–Hola A, Staszczak A, et al. Association between gastric emptying rate and nutritional status in patients treated with continuous ambulatory peritoneal dialysis. Perit Dial Int. 2002;22(4):500–505.
- Limketkai BN, LeBrett W, Lin L, et al. Nutritional approaches for gastroparesis. Lancet Gastroenterol Hepatol. 2020;5(11):1017–1026.
- Soliman H, Mariano G, Duboc H, et al. Gastric motility disorders and their endoscopic and surgical treatments other than bariatric surgery. J Visc Surg. 2022;159(1S):S8–S15.
- Silang R, Regalado M, Cheng TH, et al. Prokinetic agents increase plasma albumin in hypoalbuminemic chronic dialysis patients with delayed gastric emptying. Am J Kidney Dis. 2001;37(2):287–293.
- Schoonjans R, Van Vlem B, Vandamme W, et al. Gastric emptying of solids in cirrhotic and peritoneal dialysis patients: influence of peritoneal volume load. Eur J Gastroenterol Hepatol. 2002;14(4):395–398.
- Van Vlem BA, Schoonjans RS, Struijk DG, et al. Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Perit Dial Int. 2002;22(1):32–38.
- Lee SW, Song JH, Kim GA, et al. Effect of dialysis modalities on gastric myoelectrical activity in end-stage renal disease patients. Am J Kidney Dis. 2000;36(3):566–573.
- Gregg LP, Bossola M, Ostrosky-Frid M, et al. Fatigue in CKD: epidemiology, pathophysiology, and treatment. Clin J Am Soc Nephrol. 2021;16(9):1445–1455.
- Bossola M, Picca A, Marzetti E, et al. Post-dialysis fatigue and serum S100B protein in patients on chronic hemodialysis. A pilot study. Ther Apher Dial. 2022 Oct 22.
- Buehring B, Belavy DL, Michaelis I, et al. Changes in lower extremity muscle function after 56 days of bed rest. J Appl Physiol (1985). 2011;111(1):87–94.
- Deligiannis A, D’Alessandro C, Cupisti A, et al. Exercise training in dialysis patients: impact on cardiovascular and skeletal muscle health. Clin Kidney J. 2021;14(Suppl 2):ii25–ii33.
- Zelko A, Rosenberger J, Skoumalova I, et al. The effects of an intradialytic resistance training on lower extremity muscle functions. Disabil Rehabil. 2022;44(2):275–281.
- Johansen KL. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18(6):1845–1854.
- Moraes C, Marinho SM, da Nobrega AC, et al. Resistance exercise: a strategy to attenuate inflammation and protein-energy wasting in hemodialysis patients? Int Urol Nephrol. 2014;46(8):1655–1662.
- Do JY, Kim AY, Kang SH, et al. Peritoneal protein loss is not associated with sarcopenia in peritoneal dialysis patients. Front. Med. 2021;8:653807.
- Hendriks FK, Smeets JSJ, Broers NJH, et al. End-stage renal disease patients lose a substantial amount of amino acids during hemodialysis. J Nutr. 2020;150(5):1160–1166.
- Tiranathanagul K, Khemnark N, Takkavatakarn K, et al. Comparative efficacy between hemodialysis using super high-flux dialyzer with hemoperfusion and high-volume postdilution online hemodiafiltration in removing protein bound and middle molecule uremic toxins: a cross-over randomized controlled trial. Artif Organs. 2022;46(5):775–785.
- Blumenkrantz MJ, Gahl GM, Kopple JD, et al. Protein losses during peritoneal dialysis. Kidney Int. 1981;19(4):593–602.
- Westra WM, Kopple JD, Krediet RT, et al. Dietary protein requirements and dialysate protein losses in chronic peritoneal dialysis patients. Perit Dial Int. 2007;27(2):192–195.
- van Gelder MK, Abrahams AC, Joles JA, et al. Albumin handling in different hemodialysis modalities. Nephrol Dial Transplant. 2018;33(6):906–913.
- Salame C, Eaton S, Grimble G, et al. Protein losses and urea nitrogen underestimate total nitrogen losses in peritoneal dialysis and hemodialysis patients. J Ren Nutr. 2018;28(5):317–323.
- Ikizler TA, Pupim LB, Brouillette JR, et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002;282(1):E107–E116.
- Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84(6):1096–1107.
- López-Cisneros S, González-Ortiz A, et al. Is there a relationship between oral hygiene and nutritional status in peritoneal dialysis patients? ¿Existe alguna relación entre la higiene bucal y el estado nutricional de los pacientes en diálisis peritoneal? Nutr Hosp. 2022;39(2):355–364.
- Almeida A, Gajewska K, Duro M, et al. Trace element imbalances in patients undergoing chronic hemodialysis therapy - report of an observational study in a cohort of Portuguese patients. J Trace Elem Med Biol. 2020;62:126580.
- Dizdar OS, Yıldız A, Gul CB, et al. The effect of hemodialysis, peritoneal dialysis and renal transplantation on nutritional status and serum micronutrient levels in patients with end-stage renal disease; multicenter, 6-month period, longitudinal study. J Trace Elem Med Biol. 2020;60:126498.
- Stojsavljević A, Ristić-Medić D, Krstić Đ, et al. Circulatory imbalance of essential and toxic trace elements in pre-dialysis and hemodialysis patients. Biol Trace Elem Res. 2022;200(7):3117–3125.
- Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12(6):646–652.
- Walker FC, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24(1):255–275.
- Elgenidy A, Amin MA, Awad AK, et al. Serum zinc levels in chronic kidney disease patients, hemodialysis patients, and healthy controls: systematic review and meta-analysis. J Ren Nutr. 2023;33(1):103–115. published online ahead of printX.
- Şahin H, Uyanik F, İnanç N, et al. Serum zinc, plasma ghrelin, leptin levels, selected biochemical parameters and nutritional status in malnourished hemodialysis patients. Biol Trace Elem Res. 2009;127(3):191–199.
- Liu Y, Wang L, Li S, et al. Associations between blood trace element levels and nutritional status in maintenance hemodialysis. J Ren Nutr. 2021;31(6): 661–668.
- Garagarza C, Valente A, Caetano C, et al. Zinc deficient intake in hemodialysis patients: a path to a high mortality risk. J Ren Nutr. 2022;32(1):87–93.
- Mazani M, Argani H, Rashtchizadeh N, et al. Effects of zinc supplementation on antioxidant status and lipid peroxidation in hemodialysis patients. J Ren Nutr. 2013;23(3):180–184.
- Guo CH, Wang CL. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int J Med Sci. 2013;10(1):79–89.
- Wang L-J, Wang M-Q, Hu R, et al. Effect of zinc supplementation on maintenance hemodialysis patients: a systematic review and meta-analysis of 15 randomized controlled trials. Biomed Res Int. 2017;2017:1–11.
- Guo C-H, Chen P-C, Hsu G-S, et al. Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: a pilot study. Nutrients. 2013;5(4):1456–1470.
- Fukasawa H, Niwa H, Ishibuchi K, et al. The impact of serum zinc levels on abdominal fat mass in hemodialysis patients. Nutrients. 2020;12(3):656.
- Konukoglu D, Turhan MS, Ercan M, et al. Relationship between plasma leptin and zinc levels and the effect of insulin and oxidative stress on leptin levels in obese diabetic patients. J Nutr Biochem. 2004;15(12):757–760.
- Lee S-L, Kwak E-H, Kim Y-H, et al. Leptin gene expression and serum leptin levels in zinc deficiency: implications for appetite regulation in rats. J Med Food. 2003;6(4):281–289.
- Lobo JC, Aranha LN, Moraes C, et al. Linking zinc and leptin in chronic kidney disease: future directions. Biol Trace Elem Res. 2012;146(1):1–5.
- Manley KJ, Haryono RY, et al. Taste changes and saliva composition in chronic kidney disease. Renal Soc Australas J. 2012;8(2):56–60.
- Abdollahi S, Toupchian O, Jayedi A, et al. Zinc supplementation and body weight: a systematic review and dose-response meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(2):398–411.
- Gao L, Fan Y, Zhang X, et al. Zinc supplementation inhibits the high glucose induced EMT of peritoneal mesothelial cells by activating the Nrf2 antioxidant pathway. Mol Med Rep. 2019;20(1):655–663.
- Lobo J C, Stockler-Pinto MB, Farage NE, et al. Reduced plasma zinc levels, lipid peroxidation, and inflammation biomarkers levels in hemodialysis patients: implications to cardiovascular mortality. Renal Failure. 2013;35(5):680–685.
- Huang J-C, Huang Y-C, Wu P-Y, et al. Association between reduced serum zinc and diastolic dysfunction in maintenance hemodialysis patients. Nutrients. 2021;13(6):2077.
- Hosseini R, Montazerifar F, Shahraki E, et al. The effects of zinc sulfate supplementation on serum copeptin, C-reactive protein and metabolic markers in zinc-deficient diabetic patients on hemodialysis: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2022;200(1):76–83.
- Chen C-Y, Chiu C-H, Wu I-W, et al. Micronutrients and renal outcomes: a prospective cohort study. Nutrients. 2022;14(15):3063.
- Yang C-Y, Wu M-L, Chou Y-Y, et al. Essential trace element status and clinical outcomes in long-term dialysis patients: a two-year prospective observational cohort study. Clin Nutr. 2012;31(5):630–636.
- Saka Y, Naruse T, Matsumoto J, et al. Low serum zinc concentration is associated with infection particularly in patients with stage 5 chronic kidney disease medicated with proton pump inhibitors. J Ren Nutr. 2021;31(6):579–585.
- Iglesias P, Selgas R, Romero S, et al. Selenium and kidney disease. JN. 2013;26(2):266–272.
- Ji M, Bae E, Kim HW, et al. Status of four trace elements in elderly patients with online hemodiafiltration. Clin Lab. 2021;67(6). DOI: 10.7754/Clin.Lab.2020.201026.
- Beligaswatta C, Sudusinghe D, De Silva S, et al. Prevalence and correlates of low plasma selenium concentrations in peritoneal dialysis patients. J Trace Elem Med Biol. 2022;69:126899.
- Bonomini M, Forster S, et al. Effects of selenium supplementation on immune parameters in chronic uraemic patients on haemodialysis. Nephrol Dial Transplant. 1995;10(9):1654–1661.
- Dworkin B, Weseley S, Rosenthal WS, et al. Diminished blood selenium levels in renal failure patients on dialysis: correlations with nutritional status. Am J Med Sci. 1987;293(1):6–12.
- Liu M-L, Xu G, Huang Z-Y, et al. Euthyroid sick syndrome and nutritional status are correlated with hyposelenemia in hemodialysis patients. Int J Artif Organs. 2011;34(7):577–583.
- Salehi M, Sohrabi Z, Ekramzadeh M, et al. Selenium supplementation improves the nutritional status of hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Nephrol Dial Transplant. 2013;28(3):716–723.
- Tonelli M, Wiebe N, Bello A, et al. Concentrations of trace elements and clinical outcomes in hemodialysis patients: a prospective cohort study. CJASN. 2018;13(6):907–915.
- Anadón Ruiz A, Martín Jiménez E, Bermejo-Barrera P, et al. Selenium and all-cause mortality in end-stage renal disease. Retrospective observational cohort study. J Ren Nutr. 2020;30(6):484–492.
- Xu S, Zou D, Tang R, et al. Levels of trace blood elements associated with severe sleep disturbance in maintenance hemodialysis patients. Sleep Breath. 2021;25(4):2007–2013.
- Yasukawa M, Arai S, Nagura M, et al. Selenium associates with response to erythropoiesis-stimulating agents in hemodialysis patients. Kidney Int Rep. 2022;7(7):1565–1574.
- Tapiero H, Townsend DM, Tew KD, et al. Trace elements in human physiology and pathology. Copper. Biomed Pharmacother. 2003;57(9):386–398.
- Tonelli M, Wiebe N, Hemmelgarn B, et al. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med. 2009;7(1):25.
- Ikee R, Tsunoda M, Sasaki N, et al. Clinical factors associated with serum copper levels and potential effect of sevelamer in hemodialysis patients. Int Urol Nephrol. 2013;45(3):839–845.
- Ari E, Kaya Y, Demir H, et al. The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol Trace Elem Res. 2011;144(1–3):351–359.
- Karahan SC, Deger O, et al. The effects of impaired trace element status on polymorphonuclear leukocyte activation in the development of vascular complications in type 2 diabetes mellitus. Clin Chem Lab Med. 2001;39:109–115.
- Liu Y, Tang R, Xu Q, et al. High blood Cu/Zn ratio is associated with nutritional risk in patients undergoing maintenance hemodialysis. Biol Trace Elem Res. 2022;200(12):4977–4987.
- Zuo S, Liu M, Liu Y, et al. Association between the blood copper-zinc (Cu/Zn) ratio and anemia in patients undergoing maintenance hemodialysis. Biol Trace Elem Res. 2022;200(6):2629–2638.
- Osaki S, Johnson DA, Frieden E, et al. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241(12):2746–2751.
- Takahashi A. Role of zinc and copper in erythropoiesis in patients on hemodialysis. J Ren Nutr. 2022;32(6):650–657.
- Prodanchuk M, Makarov O, et al. Disturbances of trace element metabolism in ESRD patients receiving hemodialysis and hemodiafiltration. Cent European J Urol. 2014;66(4):472–476.
- Hsu C-W, Weng C-H, Lee C-C, et al. Association of serum chromium levels with malnutrition in hemodialysis patients. BMC Nephrol. 2019;20(1):302.
- Li C, Zhou H-M. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res. 2011,2011;387176.
- Malecki E, Greger J. Manganese protects against heart mitochondrial lipid peroxidation in rats fed highconcentrations of dietary polyunsaturated fatty acids. J. Nutr. 1996;126(1):27–33.
- Kim M, Koh ES, Chung S, et al. Altered metabolism of blood manganese is associated with low levels of hemoglobin in patients with chronic kidney disease. Nutrients. 2017;9(11):1177.
- Liu Y, Hu J, Tang R, et al. Association between the blood manganese (Mn) and hemoglobin in patients undergoing maintenance hemodialysis. J Trace Elem Med Biol. 2022;71:126947.
- Bazeley JW, Wish JB. Recent and emerging therapies for iron deficiency in anemia of CKD: a review. Am J Kidney Dis. 2022;79(6):868–876.
- Rucker D, Thadhani R, Tonelli M, et al. Trace element status in hemodialysis patients. Semin Dial. 2010;23(4):389–395.
- Niu R, Yang Q, Dong Y, et al. Selenium metabolism and regulation of immune cells in immune-associated diseases. J Cell Physiol. 2022;237(9):3449–3464.
- Tsuji PA, Santesmasses D, Lee BJ, et al. Historical roles of selenium and selenoproteins in health and development: the good, the bad and the ugly. Int J Mol Sci. 2021;23(1):5.
- Minich WB. Selenium metabolism and biosynthesis of selenoproteins in the human body. Biochemistry (Mosc). 2022;87(Suppl 1):S168–S102.
- Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–3303.
- Arnér ESJ. Selective evaluation of thioredoxin reductase enzymatic activities. Methods Mol Biol. 2018;1661:301–309.
- Huang J, Xie L, et al. Selenium status and its antioxidant role in metabolic diseases. Oxid Med Cell Longev. 2022;2022:7009863.
- Stockler-Pinto MB, Malm O, Moraes C, et al. A follow-up study of the chronic kidney disease patients treated with Brazil nut: focus on inflammation and oxidative stress. Biol Trace Elem Res. 2015;163(1–2):67–72.
- Xu C, Guo Y, Qiao L, et al. Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. Front Microbiol. 2018;9:1129.
- Wang F, Sun N, Zeng H, et al. Selenium deficiency leads to inflammation, autophagy, endoplasmic reticulum stress, apoptosis and contraction abnormalities via affecting intestinal flora in intestinal smooth muscle of mice. Front Immunol. 2022;13:947655.
- Yang L, Cui Y, Liang H, et al. Multifunctional selenium nanoparticles with different surface modifications ameliorate neuroinflammation through the gut Microbiota-NLRP3 Inflammasome-Brain axis in APP/PS1 mice. ACS Appl Mater Interfaces. 2022;14(27):30557–30570.
- Agbor GA, Vinson JA, Patel S, et al. Effect of selenium- and glutathione-enriched yeast supplementation on a combined atherosclerosis and diabetes hamster model. J Agric Food Chem. 2007;55(21):8731–8736.
- Bunk MJ, Combs GF, Jr. Effect of selenium on appetite in the selenium-deficient chick. J Nutr. 1980;110(4):743–749.
- Olsson U. Impaired ketone body metabolism in the selenium deficient rat. Possible implications. Metabolism. 1985;34(11):993–998.
