Abstract
Objective
Proton pump inhibitor (PPI) use has been associated with reduced diversity of the gut microbiome and may lead to worse clinical outcomes in inflammatory bowel disease (IBD). We aimed to evaluate whether PPI use affects clinical outcomes in a real-world setting.
Design
Healthcare claims data of adult IBD patients were obtained from the IBM MarketScan Database. Multivariable analysis and propensity score-matched analysis were performed to assess associations between PPI use and new biologic start, and IBD-related hospitalizations and surgeries.
Results
A total of 46,234 IBD patients were identified (6,488 (14%) and 39,746 (86%) patients with and without PPI, respectively). Patients on PPI were more likely to be older, female, and smokers and less likely to be on immunomodulators. Multivariable analyses demonstrated that PPI use was associated with new biologic start (odds ratio (OR) 1.11, 95% confidence interval (CI) 1.04–1.18), and IBD-related admissions (OR 1.95, 95% CI 1.74–2.19) and surgeries (OR 1.46, 95% CI 1.26–1.71). Following propensity score matching, patients on PPI remained more likely to start a new biologic (23% vs 21%, p = 0.011), and have IBD-related admissions (8% vs 4%, p < 0.001) and surgeries (4% vs 2%, p < 0.001). Subgroup analyses stratified by age, smoking, and glucocorticoid use showed similar results. There was a dose-response relationship between the number of PPI prescriptions and the risk of new biologic use (p < 0.001) and IBD-related admissions (p < 0.001).
Conclusion
PPI use was associated with worse clinical outcomes in patients with IBD in the real-world setting. Further studies are warranted to validate these findings, but caution may be needed when prescribing a PPI to IBD patients.
Proton pump inhibitors (PPIs) are one of the most prescribed therapies in the United States (US).
Reduction of gastric acid secretion by PPI use increases the risk of imbalance in gut microbiota composition and may increase the risk of enteric infections.
Recent studies have reported that the use of PPI was associated with development of inflammatory bowel disease (IBD) and reduced rates of remission in patients on infliximab therapy, which may be due to alterations of intestinal microbiota.
Study highlights WHAT IS KNOWN
In a large real-world US healthcare database study, IBD patients with PPI use were more likely to have a new biologic medication started, have an IBD-related surgery, and have an IBD-related hospitalization, which remained significant after adjusting for confounders by multivariable analysis, propensity-score matched analysis, and subgroup analysis.
Appropriate clinical review of PPI necessity may need to be performed in patients with IBD when considering prescribing a PPI or who are already on PPI therapy.
WHAT IS NEW HERE
Introduction
Inflammatory bowel diseases (IBD), consisting of ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, inflammatory diseases that affect the gastrointestinal tract and requires lifelong therapy. Although this diagnosis initially carried a very poor prognosis, there have been significant improvements in both our understanding of the disease process as well as our therapeutic approach in the last few decades. While there remains no curative therapy, the mainstay of medical therapy involves using immunomodulatory medications to induce remission and improve quality of life [Citation1,Citation2]. Given the improved medical and surgical control of IBD leading to its widened prevalence in a range of ages, an increasing number of patients are treated with medications for other medical comorbidities [Citation3,Citation4]. The effect of additional concomitant medications on the overall disease course and response to IBD medications is still largely unknown.
Proton pump inhibitors (PPIs) are the most widely used agents for the suppression of gastric acid. They have demonstrated success in the treatment of gastroesophageal reflux disease (GERD), Helicobacter pylori (H. pylori) infections and peptic ulcers [Citation5,Citation6]. They are also widely used in the treatment of general dyspeptic symptoms and in patients with functional dyspepsia and more recently in eosinophilic esophagitis [Citation7–9]. PPIs remain one of the most used medications in the outpatient setting, and data from surveys in the United States (US) outpatient setting show that approximately 8–10% of all ambulatory adults have been prescribed a PPI within the last thirty days [Citation10,Citation11]. They are overall safe medications; however, in recent literature, there have been multiple associations between PPI use and adverse events such as risks of Clostridioides difficile infections, vitamin B12 deficiency, small intestinal bacterial overgrowth, kidney dysfunction, and bacterial enteritis in the general population [Citation12–14].
More specifically within the realm of IBD, a few recent studies have begun to show links between PPI use and outcomes associated with IBD. A nested-case control study from US demonstrated a link between early-life PPI use and subsequent development of pediatric IBD [Citation15]. Similarly, a recent pooled analysis of the Nurses’ Health Study and the United Kingdom (UK) Biobank showed that regular use of PPIs had a significantly positive association with IBD risk as compared with nonusers [Citation16]. In adult populations, a claims database study from Canada showed PPIs were associated with medication changes in UC patients and a case-control study using the Veteran Affairs database showed increased rates of hospitalization and surgeries in patients with IBD who were on concomitant PPIs [Citation17]. Lastly, a recent patient-level meta-analysis of randomized controlled studies looking at the effect of PPI on patient response to infliximab therapy showed IBD patients taking a PPI were less likely to achieve remission with infliximab when compared with patients who were not on a PPI [Citation18].
Given these initial findings and the possibility that PPI use alters the richness of the gut microbiome [Citation19], further investigation into the effects of PPI on IBD-specific outcomes and therapies is needed. To this end, we sought to investigate the effect of PPI use on IBD-related outcomes and changes in treatment in the wider general IBD population in the US using a large claims database.
Methods
Study design
We performed a longitudinal, retrospective cohort analysis of IBD patients (CD and UC) in the Truven MarketScan Commercial Claims and Encounters Database from 2017 to 2018. The Truven MarketScan Database consists of de-identified outpatient, inpatient, and pharmaceutical claims of approximately 40–50 million privately insured patients each year [Citation20,Citation21]. These claims originate from more than 150 large employer-sponsored health insurance plans with patient coverage in all 50 states. The database includes patient-level data such as patient characteristics (age, sex, geographic region), financial variables (inpatient, outpatient, and pharmaceutical costs), and pharmacy-level data (National Drug Code (NDC), days’ supply, strength, administration method).
Patient identification
Patient inclusion criteria comprised patients who were ≥18 years of age, were continuously enrolled in the MarketScan database between the years of 2016–2018, had an initial diagnosis of IBD in the index year 2017 followed by a subsequent repeat diagnosis within one year after the index encounter date (defined under the International Classification of Diseases, Tenth Revision [ICD-10] as K50.xx for CD and K51.xx for UC, respectively), and had at least one outpatient drug claim in both the years 2017 and 2018 ().
Variable classification:
PPI use was determined using dispensed prescription drug claims during the years 2017–2018. PPI medications included in the study were omeprazole, esomeprazole, pantoprazole, lansoprazole, dexlansoprazole, and rabeprazole, as identified by their NDC numbers. A patient was classified as meeting the criteria for PPI use if there were PPI claims on distinct dates during the study period (defined as starting on or during the year after the index service date). Separately, PPI use after the index service date and before the index service data were also collected. Additionally, outpatient biologic use as denoted by prescription drug claims for infliximab, adalimumab, certolizumab pegol, vedolizumab, and ustekinumab were collected for the year prior to the 2017 index service date as well as during the study period. Immunomodulator use as denoted by prescription drug claims for azathioprine, mercaptopurine, and methotrexate was also collected for the year prior to the 2017 index service date as well as during the study period.
Covariates of interest were classified using patient data during the study period. Demographic information included age and gender. Conditions that are indications for PPI use and common comorbidities were defined a priori based on clinical judgement and past research with population-based databases and the MarketScan database using ICD-10 codes in the year prior to the index encounter in 2017 [Citation22–25]. These included GERD, esophagitis, gastritis, gastric ulcer, duodenal ulcer, H. pylori, essential hypertension, hypertensive heart disease, hypertension, chronic kidney disease (CKD), secondary hypertension, smoking, congestive heart failure, obesity, diabetes, chronic obstructive pulmonary disease, cerebrovascular diseases, and dementia (see Supplementary Table 1 for a full list of ICD-10 codes).
Table 1. Baseline characteristics of IBD patients with and without concurrent PPI therapy.
Outcomes of interest
Outcomes of interest included (1) new biologic use, (2) IBD-related admissions, and (3) IBD-related surgery. New biologic use was defined as a new prescription of a biologic drug started during the study period or if any alternative biologic drug for the treatment of IBD was started. This was determined through new claims for any biological drug during the study period. Subsequent IBD-related hospital admissions were defined as admissions with a primary diagnosis of IBD including K50.xx and K51.xx during the study period. IBD-related surgeries were defined as any surgeries for IBD during the study period including small bowel resection, ileocolic resection, colonic resection, etc. (Supplementary Table 1).
Statistical analysis
Descriptive statistical analyses were performed to assess the effect of PPI use on new biologic use, admissions, and surgery in IBD. Univariate and multivariate analysis were performed and logistic regression was used to estimate odds ratios (the ratio of odds of an event in the PPI group versus the odds of the event in the non-PPI group). We performed backward selection to reduce the set of independent variables in multivariate models to those that are statistically significant at the level of 0.05.
Propensity score-matched analysis was performed between the group of patients with PPI use and the group without PPI use during the study period to adjust for differences in baseline covariates between the two groups. The patients were matched on covariates including patient age, gender, and comorbidities. We used a caliper width of 0.1 of the standard deviation of the logit of the propensity score and a matching ratio of 1:2. Outcome variables was then compared between the two matched groups using simple t-test and Chi-square tests.
In order to assess for potential effect modifiers, we performed subgroup analyses stratified by clinical variables such as age, sex, smoking, as well as comorbidities and PPI indications that were found to be influential on multivariate analyses. Estimated effects were based on the multivariable-adjusted logistic regression model adjusted for age, sex, smoking, chronic kidney disease, obesity, diabetes, gastritis, gastroesophageal reflux disease, and immunomodulator use.
To assess the dose-response relationship, we analyzed the association between the number of PPI prescriptions and IBD outcomes events by univariate and multivariate models. The number of PPI prescriptions was the total cumulative number of PPI prescriptions during the study period. Dosage or dosing regimen were not taken into account due to limited data.
A two-sided p value of <0.05 was considered statistically significant. All analyses were performed with STATA version 17 (College Station, Texas, USA) and GraphPad Prism (San Diego, CA, USA).
Patient and public involvement
We did not directly include patient and public involvement in this study. Patients were not invited to comment on the study design and were not consulted to interpret the results. Patients were not invited to contribute to the writing or editing of this manuscript.
Results
Patient characteristics
We identified a total of 46,234 patients with IBD during the study period, with 6,488 patients on PPI therapy compared with 39,746 patients not on PPI therapy. The baseline patient characteristics are listed in for all patients combined and for patients with CD and UC shown separately (Supplementary Table 2). For all patients combined, patients on PPI therapy were significantly older, more likely to be female and smoking as compared with patients who were not on PPI therapy. The proportion of patients with comorbidities such as hypertension, obesity, diabetes, GERD, and H. Pylori also differed between the two groups. With regard to IBD therapy, there was a significantly smaller number of patients on immunomodulators that were on PPI therapy compared with those without PPI therapy. These baseline characteristics were overall similar when examined separately in CD and in UC (Supplementary Table 2).
Table 2. Propensity score-matched analysis: characteristics of IBD patients with and without concurrent PPI therapy.
Effect of PPI therapy on IBD outcomes
New biologic prescription
On univariate analysis, IBD patients on PPI more frequently started a new biologic prescription (OR 1.10, 95% CI 1.04–1.18) (Supplementary Table 3). Multivariable analysis demonstrated that amongst all patients with IBD, those on PPI were significantly more likely to start a new biologic therapy (OR 1.11, 95% CI 1.04–1.18) ( and Supplementary Table 3). Additionally, patients on immunomodulators (OR 1.18, 95% CI) and glucocorticoids (OR 1.21, 95% CI 1.13–1.31) as well as CD patients (OR 3.00, 95% CI 2.86–3.15) were more likely to start a new biologic therapy. Conversely, older patients (OR 0.98, 95% CI 0.98-0.98), female sex (OR 0.86, 95% CI 0.83–0.91), and patients with diabetes (OR 0.87, 95% CI 0.80-0.95) were less likely to have started a new biologic. The difference in the new biologic start was seen in patients on PPI with UC (OR 1.28, 95% CI 1.14-1.44) but not in CD with multivariable analyses (Supplementary Table 3).
Figure 2. Multivariate analyses of outcomes of IBD patients with and without concurrent PPI therapy. (A) Multivariate analysis of new biologic use. (B) Multivariate analysis of IBD-related admission. (C) Multivariate analysis of IBD-related surgery.
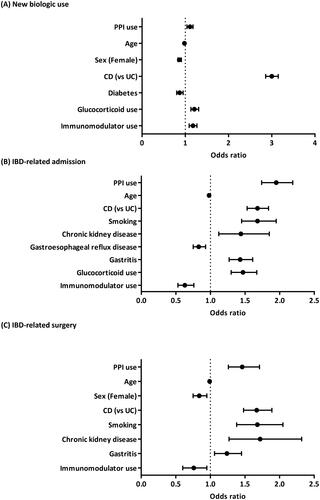
Table 3. Association between PPI prescription count and the risk of new biologic use, IBD- related admission, and IBD-related surgery.
IBD-related admissions
On univariate analysis, IBD patients on PPI more frequently had IBD-related admissions (OR 1.93, 95% CI 1.74–2.14) (Supplementary Table 4). Multivariable analysis demonstrated that amongst all patients with IBD, those on PPI were significantly more likely to have an IBD-related admission (OR 1.95, 95% CI 1.74–2.19) ( and Supplementary Table 4). Additionally, patients who were smokers (OR 1.68, 95% CI 1.45–1.95), had CD (OR 1.68, 95% CI 1.53–1.84), had CKD (OR 1.44, 95% CI 1.12–1.85), had gastritis (OR 1.43, 95% CI 1.27–1.61), and taking glucocorticoids (OR 1.47, 95% CI 1.30–1.67) were more likely to have IBD-related admissions. In comparison, older patients (OR 0.98, 95% CI 0.97–0.98), patients with GERD (OR 0.83, 95% CI 0.75–0.93) and patients on immunomodulators (OR 0.63, 95% CI 0.53–0.76) were less likely to have IBD-related admissions. This difference in admission was seen in both subsets of CD and UC patients on PPI therapy with multivariable analyses (OR 1.62, 95% CI 1.42–1.86 and OR 2.40, 95% CI 1.99–2.91, respectively) (Supplementary Table 4).
IBD-related surgeries
On univariate analysis, IBD patients on PPI more frequently had IBD-related surgeries (OR 1.56, 95% CI 1.35–1.81) (Supplementary Table 5). Multivariable analysis demonstrated that amongst all patients with IBD, those on PPI were significantly more likely to have an IBD-related surgery (OR 1.46, 95% CI 1.26–1.71) ( and Supplementary Table 5). Patients who were smokers (OR 1.68, 95% CI 1.38–2.05), with CD (OR 1.67, 95% CI 1.48–1.89), with CKD (OR 1.72, 95% CI 1.27–2.32), and with gastritis (OR 1.24, 95% CI 1.06–1.45) also had higher likelihood of having an IBD-related surgery. Older patients (OR 0.99, 95% CI 0.98–0.99), female sex (OR 0.84, 95% CI 0.75–0.95), and immunomodulator use (OR 0.76, 95% CI 0.60–0.95) were associated with a lower likelihood of having IBD-related surgeries. This difference was seen in both subsets of CD (OR 1.25, 95% CI 1.03–1.52) and UC (OR 1.93, 95% CI 1.52–2.45) patients taking PPI therapy with multivariable analyses (Supplementary Table 5).
Propensity score matched analyses
Propensity score-matched analysis with a 1:2 match with each PPI user to non-user was performed, in order to control for variables between the two cohorts that could affect IBD outcomes. As shown in , the characteristics of the two groups were similar except for the proportion of patients with obesity, diabetes, esophagitis, gastritis, and glucocorticoid use which had very minor differences. In this propensity score-matched cohort analysis, patients on a PPI were significantly more likely to start a new biologic (23% vs 21%, p = 0.011), have an IBD-related admission (8% vs 4%, p < 0.001) and have an IBD-related surgery (4% vs 2%, p < 0.001).
Subgroup analyses
The risk of new biologic use, IBD-related admissions, and IBD-related surgeries were consistently elevated among PPI users compared to non-users among almost all subgroup analyses (age, smoking, GERD, glucocorticoid use, etc.) supporting the robustness of our results (Supplementary Table 6-8).
Dose-response relationship of PPI prescription and IBD outcomes
In order to make treatment-effect inferences, we assessed the dose-response relationship between the number of PPI prescriptions and IBD outcomes event. As demonstrated in , there was a small but statistically significant association between PPI prescription count and the risk of new biologic use (OR 1.03, 95% CI 1.02-–.04) and IBD-related admissions (OR 1.04, 95% CI 1.02–1.06), but not IBD-related surgery on multivariable analyses.
Discussion
In this large US claims database analysis evaluating patients with IBD, concurrent PPI therapy was associated with a significantly higher risk of undesirable clinical outcomes as compared with patients not on PPI therapy. This included an increased likelihood to start a new biologic medication, undergoing IBD-related hospital admission and IBD-related surgeries. This association was significant for all patients with IBD combined and showed a similar trend when UC and CD were analyzed separately. Due to the large sample size, there were differences in clinical characteristics between IBD patients with or without PPI therapy, but we undertook propensity score-matched analysis and subgroup analyses to confirm the robustness of our results. Furthermore, sensitivity analysis according to the number of PPI prescriptions demonstrated a dose-response relationship supporting a potential causal effect.
A prior case-control study of patients with IBD in the Veterans Health System demonstrated PPI exposure was associated with IBD-related hospitalizations and surgeries [Citation17]. In a patient-level meta-analysis of prospective randomized controlled studies, IBD patients taking PPI were less likely to achieve remission while on infliximab or placebo treatments [Citation18]. Our study confirms these findings in a larger and more generalizable US population by utilizing one of the largest longitudinal overviews of both inpatient and outpatient claims data, including pharmaceutical data. Additionally, we showed that there is an increased risk for three important clinical outcomes, namely new biologic start, admissions, and surgery, indicating that PPI use is associated with worsening disease at various levels. We also showed that the number of PPI prescriptions was associated with a greater risk of new biologic use and IBD-related admissions (dose-response relationship), suggesting a possible causal inference. The results of our study suggest that physicians should take caution when prescribing PPIs to IBD patients and confirm that there is a clinical indication for prescribing it.
Although the underlying mechanisms of worse clinical outcomes associated with PPI use are still unknown, there are a few possible hypothesized mechanisms. One includes the effect of ongoing gastric acid suppression on intestinal microbiome alterations. Prolonged use of PPI reduces microbial diversity, which have been shown to be associated with increased risks of gastrointestinal infections [Citation19,Citation26,Citation27]. PPIs have also been shown to reduce anti-inflammatory bacteria such as Faecalibacterium which could also potentially lead to poor outcomes in patients with IBD [Citation28,Citation29]. Further research elucidating the influence of PPIs on intestinal inflammation is warranted.
Our study has several limitations. First, there were statistically significant differences in the baseline characteristics (age, gender, smoking status, etc.) between patients with or without PPI use. This was addressed by performing multivariable analyses. We further performed baseline characteristics matching of patients without and on concomitant PPI therapy through propensity score matching as well as subgroup analyses. Despite the comprehensive adjustment for confounders and dose-response relationships, causal relationships cannot be established through this observational study. The use of the Truven MarketScan database also has certain limitations. This includes reliance upon the accuracy of claims and prescription data, lack of data on uninsured patients, inability to obtain endoscopic findings, and limited demographic information. Data on diet patterns, which may influence the use of PPI or symptoms of IBD, are also unavailable.0 It may contain biases and may not generalize well to other populations due to unmeasured clinical information. The lack of phenotypic data such as perianal and upper gastrointestinal tract CD limited us to assess whether they are over-represented in the PPI group. PPI drugs are also available over-the-counter, however, we were unable to assess the proportion of patients taking over-the-counter drugs as it cannot be recorded in the prescription database. Similarly, the influence of non-steroidal anti-inflammatory drugs (NSAIDs) could not be analyzed as a majority of them are purchased over-the-counter. Statistical significance was present in many analyses including the dose-response relationship due to a large number of participants, but clinical significance needs to be carefully considered.
In conclusion, concurrent PPI therapy use was associated with increased risks of new biologic use representing possible need for treatment escalation as well as increased risks of IBD-related hospital admissions and surgeries. Further prospective studies are needed to validate these findings and explore potential underlying mechanisms, but caution may be needed in prescribing PPIs to IBD patients.
Ethics approval
Patient consent for publication not required.
Author contribution
TC; drafting of manuscript and analysis of data, HZ; data extraction and analysis, drafting of the manuscript, AS; concept of study, data analysis, and manuscript preparation.
Supplemental Material
Download MS Word (54.1 KB)Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the author(s). All authors have no conflict of interest directly relevant to the content of this article.
Data availability statement
The data that support the findings of this study are available from IBM Truven MarketScan Database. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of IBM Truven MarketScan Database.
Additional information
Funding
References
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):1–9.
- Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517.
- Tran V, Limketkai BN, Sauk JS. IBD in the elderly: management challenges and therapeutic considerations. Curr Gastroenterol Rep. 2019;21(11):60.
- Windsor JW, Kaplan GG. Evolving epidemiology of IBD. Curr Gastroenterol Rep. 2019;21(8):40.
- Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239.
- Laine L, Barkun AN, Saltzman JR, et al. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. 2021;116(5):899–917.
- Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–692; quiz 693.
- Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117(1):27–56.
- Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112(7):988–1013.
- Targownik L. Discontinuing long-term PPI therapy: why, with whom, and how? Am J Gastroenterol. 2018;113(4):519–528.
- Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLOS One. 2013;8(2):e56060.
- Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017;23(35):6500–6515.
- Schnoll-Sussman F, Niec R, Katz PO. Proton pump inhibitors: the good, bad, and ugly. Gastrointest Endosc Clin N Am. 2020;30(2):239–251.
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American gastroenterological association. Gastroenterology. 2017;152(4):706–715.
- Schwartz NRM, Hutfless S, Herrinton LJ, et al. Proton pump inhibitors, H2 blocker use, and risk of inflammatory bowel disease in children. J Pediatr Pharmacol Ther. 2019;24(6):489–496.
- Xia B, Yang M, Nguyen LH, et al. Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: pooled analysis of 3 prospective cohorts. Gastroenterology. 2021;161(6):1842–1852 e1810.
- Shah R, Richardson P, Yu H, et al. Gastric acid suppression is associated with an increased risk of adverse outcomes in inflammatory bowel disease. Digestion. 2017;95(3):188–193.
- Lu TX, Dapas M, Lin E, et al. The influence of proton pump inhibitor therapy on the outcome of infliximab therapy in inflammatory bowel disease: a patient-level meta-analysis of randomised controlled studies. Gut. 2021;70(11):2076–2084.
- Reveles KR, Ryan CN, Chan L, et al. Proton pump inhibitor use associated with changes in gut microbiota composition. Gut. 2018;67(7):1369–1370.
- IBM. IBM Marketscan Research Databases. 2021. https://www.ibm.com/products/marketscan-research-databases/databases.
- Kulaylat AS, Schaefer EW, Messaris E, et al. Truven health analytics MarketScan databases for clinical research in colon and rectal surgery. Clin Colon Rectal Surg. 2019;32(1):54–60.
- Howden CW, Manuel M, Taylor D, et al. Estimate of refractory reflux disease in the United States: economic burden and associated clinical characteristics. J Clin Gastroenterol. 2021;55(10):842–850.
- Yuan J, He Q, Nguyen LH, et al. Regular use of proton pump inhibitors and risk of type 2 diabetes: results from three prospective cohort studies. Gut. 2021;70(6):1070–1077.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238–246.
- Ortiz-Guerrero G, Amador-Munoz D, Calderon-Ospina CA, et al. Proton pump inhibitors and dementia: physiopathological mechanisms and clinical consequences. Neural Plast. 2018;2018:5257285.
- Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(3):225–233.
- McDonald EG, Milligan J, Frenette C, et al. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784–791.
- Fujimoto T, Imaeda H, Takahashi K, et al. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of crohn’s disease. J Gastroenterol Hepatol. 2013;28(4):613–619.
- Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283.