Abstract
Aim: To investigate the treatment of intractable epistaxis after radiotherapy for nasopharyngeal carcinoma (NPC).Methods: This review focuses on the anatomy and pathophysiology, mechanism, and clinical treatments of epistaxis after NPC radiotherapy.Results: For treating NPC, radiation therapy is the primary therapeutic modality. However, radiotherapy can lead to varied degrees of harm to the neighboring tissues and is correlated with numerous complications. Among these complications, epistaxis is a common occurrence after NPC radiotherapy, owing to damage to the surrounding tissues caused by radiotherapy. Unfortunately, epistaxis, particularly carotid blowout, can have a dangerous course and a high mortality rate. Accurate understanding of epistaxis following radiotherapy, prompt bleeding cessation, and reduction of bleeding volume are key considerations. Nasal tamponade is a crucial rescue treatment, while tracheotomy is an active and effective method. Intravascular balloon embolization is a reliable and effective treatment method for ICA hemorrhage, and vascular embolization is the primary approach for treating external carotid artery maxillary bleeding. Implantation of a covered stent can achieve hemostasis without altering hemodynamics.Conclusion: A comprehensive approach utilizing these methods can improve the success rate of treating nosebleeds following NPC radiotherapy.
The mortality rate for carotid blowout following radiotherapy for NPC is high.
Radiation therapy and tumor condition are correlated with epistaxis in NPC.
Treatment methods for NPC-related epistaxis include posterior nostril tamponade, endoscopic hemostasis, DSA, selective vascular embolization, and stent implantation.
The use of a covered stent for NPC-related carotid blowout achieves hemostasis without altering blood perfusion.
Effective and timely application of various hemostasis methods is key to improving the success rate of rescue, considering the characteristics of NPC-related epistaxis.
Highlights
1. Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor that is highly prevalent in southern China, with an annual incidence rate of nearly 30/100,000 [Citation1]. Due to its unique anatomical location, extensive invasion, and sensitivity to radiation, radiotherapy is often the primary treatment for NPC [Citation2]. However, as the cure rate improves, the adverse effects of radiation have become a significant concern. Nasal and nasopharyngeal bleeding are among the most severe side effects of NPC radiotherapy [Citation3]. According to Zheng et al.’s statistics [Citation4], the incidence of fatal carotid blowout after NPC radiotherapy is approximately 2%, leading to rapid patient death. The mortality rate can reach up to 40%. Late complication rates of 48–73% and mortality rates of 2–4% after five years were observed with previous 2D and 3D conformal radiotherapy [Citation5,Citation6]. Fortunately, the development of intensity-modulated radiotherapy (IMRT) has led to a lower recurrence rate and reduced side effects in the head and neck region [Citation7]. However, carotid blowout remains one of the most fatal complications of head and neck irradiation, especially in patients receiving re-irradiation after relapse. In studies analyzing 1,554 patients who underwent a second-course radiation treatment, the incidence of carotid blowout was found to be 2.6 and 76% of cases were fatal [Citation8]. The incidence of carotid blowout was also higher in patients who received accelerated hyperfractionation during re-radiation, with a daily dose of over 2.5 Gy [Citation9]. Researches show that the incidence of massive epistaxis ranged from 1 to 22% in [Citation10–29]. While the incidence is low, traditional rescue methods have demonstrated a high mortality rate due to the dangerous course, necessitating increased attention to this issue.
2. Anatomy and pathophysiology
2.1. Pathophysiology of epistaxis
After NPC radiotherapy, epistaxis is closely associated with radiation treatment and tumor status. Several factors contribute to the development of this symptom: (1) Radiation therapy is the primary treatment for NPC, which involves high doses of radiation to eliminate tumor cells. However, normal tissues and organs in the surrounding area are also exposed to radiation, resulting in various side effects such as soft tissue fibrosis, tissue necrosis ulcers, nasal adhesions, and restricted mouth opening. The risk of carotid blowout increases when necrotic tissue falls off, and late-stage tumor erosion exposes the blood vessels [Citation30]. (2) During radiation therapy, bone tissues absorb a significant amount of radiation, leading to inflammatory reactions in the intraosseous arteries, vascular embolism, local blood supply and nutritional disorders, which can cause aseptic radiation osteonecrosis. If skull base bone necrosis occurs, it can further damage the peripheral vascular of the bone and lead to bleeding [Citation31]. (3) NPC recurrence often involves blood vessels, which can also result in bleeding [Citation32]. (4) Patients who undergo NPC radiotherapy frequently experience dry nasopharyngeal mucosa, poor oral hygiene, and restricted mouth opening. These factors make it difficult to clean the mouth and nasopharynx and discharge secretions, increasing the risk of nasopharyngeal mucosal infections. Poor repair ability of the nasopharyngeal mucosa after radiotherapy can lead to complications such as mucosal erosion, ulcers, and major carotid blowout [Citation33]. (5) The presence of recurrent tumors in the nasopharynx, paranasal sinus, or nasal cavity is another important cause of bleeding. In summary, epistaxis after NPC radiotherapy is a multifactorial issue, and it is essential to consider all potential causes in diagnosis and treatment.
2.2. Vessels related to epistaxis
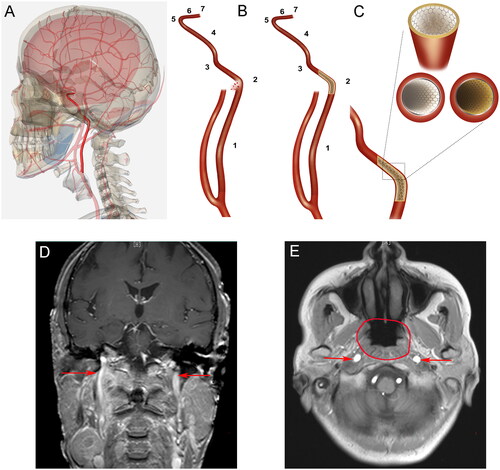
According to reports, the determination of the bleeding site is based on clinical manifestations and previous MRI or CT scans of the affected area [Citation34]. The nasopharynx has a rich blood supply, with bilateral symmetrical vessels supplying the area and lateral anastomosis being abundant. The main components of the nasopharyngeal blood vessels are the internal maxillary arteries [Citation35]. Nasopharyngeal hemorrhage after NPC treatment is mainly caused by the rupture of blood vessels, with internal carotid artery (ICA) rupture being the primary cause of rapid bleeding [Citation36]. provides a schematic diagram of the segmentation of the internal carotid artery and its positional relationship with the nasopharyngeal area.
Figure 1. (A) Schematic diagram of the internal carotid artery in the human body (highlighted: internal carotid artery); (B) The seven segments of the internal carotid artery are shown with different marks. The left image shows the rupture and bleeding of the C2 segment of the blood vessels. The image on the right shows bleeding stopped after stent implantation; (C) A local magnification shows the use of a stent to treat an internal carotid rupture. The lower right image shows a cross-sectional view of an ordinary stent implant. The lower left image shows a cross-sectional view after the covered stent is implanted, and a white membrane is visible between the vessel wall and the stent; (D) Coronal plane and (E) axial plane: The carotid arteries and neighboring anatomic structures are shown (arrows indicate the internal carotid artery; circle shows the nasopharyngeal area).
2.2.1. Bleeding from the externzal carotid artery
The primary blood supply to the posterior wall of the nasopharynx and pharyngeal recess area is provided by the ascending pharyngeal artery. This artery arises from the external carotid artery, with some branches originating from the ICA or occipital artery [Citation37]. The pterygoid artery, a secondary branch of the pharyngeal artery from the internal maxillary artery, supplies blood to the front of the nasopharynx, front of the pharyngeal crypt, and cartilage of the Eustachian tube. If the ascending iliac artery is absent, it can be replaced by a descending branch of the ascending pharyngeal artery. The branches of the maxillary artery from the external carotid artery are distributed on the top and posterior walls of the nasopharynx, the side walls of the nasopharynx, and the nasal floor near the posterior nostril of the nasal cavity [Citation38]. The superficial blood supply of the pharyngeal recess of the nasopharynx is primarily through the branches of the external carotid artery and ascending pharyngeal artery. Nasopharyngeal bleeding can occur due to rupture of the ascending pharyngeal recess.
2.2.2. Bleeding from the ICA
Based on the anatomy of the nasopharynx, it can be concluded that the main arterial blood supply comes from the internal maxillary arteries. When the lesion in the pharyngeal recess is deep, it can invade the foramen lacerum and the ICA, resulting in uncontrolled carotid blowout, which can be harmful to patients. During DSA examination, internal maxillary angiography can reveal small blood vessels and a few bleeding points [Citation39]. This may be due to the existence of the pharyngeal recess that is adjacent to the cervical segment of the ICA. Since the ICA extends to the skull via the foramen lacerum, rupture of its walls can lead to fatal bleeding. The pharyngeal recess and the areas outside the sphenoid sinus are considered the apical regions of the ICA. High-dose radiotherapy can cause blood vessels to become hard and fibrotic, with stage IV patients often experiencing skull-base bone destruction. Regeneration of tumor tissues and nasopharyngeal ulcers compresses the bone and surrounding tissues in the apical region of the ICA, which can cause the formation of a pseudoaneurysm (PSA) if the invaded ICA ruptures initially [Citation40]. The rupture of PSA can cause nasopharyngeal hemorrhage due to increased blood pressure. In case of a major hemorrhage, temporary hemostasis may be observed clinically after the blood pressure drops. However, when the blood pressure returns to normal, blood volume recovery can lead to fatal hemorrhage. The apical vessels of the ICA are considered nasopharyngeal hemorrhage-prone areas after radiotherapy in patients with NPC [Citation41]. Lesions in the pharyngeal recess area that invade the foramen lacerum and ICA often result in uncontrolled major bleeding. In addition, bleeding caused by PSA in the neck vessels after NPC radiotherapy can be fatal if left untreated or mistreated, accounting for approximately 1% of patients with significant bleeding.
3. Mechanism
The formation of intracranial aneurysms as a result of radiation therapy is a recent phenomenon that has been observed with the increasing lifespan of patients after treatment. The mechanism behind the formation of pseudoaneurysms (PSA) in the internal carotid artery (ICA) might be due to the formation of hematomas around the vascular breach after arterial rupture and bleeding caused by nasopharyngeal carcinoma (NPC) radiotherapy. These hematomas slowly liquefy over time, and the continuous pulsation of aneurysms and arteries causes irregular contraction of the medial smooth muscle, leading to thin or even missing medial smooth muscle. This causes liquefaction of vascular wall hematoma and aneurysmal communication through the breach, forming a pulsatile hematoma [Citation42]. Once the PSA is created, it causes rupturing and bleeding due to its extremely thin wall, and the bleeding can stop on its own when the blood pressure drops, and the process repeats afterward.
Numerous studies have described the location and timing of radiation-induced aneurysms. These aneurysms are typically located in the previously irradiated field, in the non-branch segment of the intracranial artery, and are identified at least 5 years after radiotherapy. Previous reports have suggested that aneurysms are caused by the peripheral segments of the intracranial arteries, which are usually congenital, traumatic, fungal, or associated with arteriovenous malformations. The incidence of aneurysms in the surrounding areas without the above-mentioned related lesions is very low, leading researchers to try to determine the causal relationship between aneurysm formation and radiation exposure. In one study, cats were irradiated with a dose of 100 or 300 Gy to the basilar artery, and the researchers detected degenerative hyalinization of the artery wall [Citation43]. Another study found that irradiating rats’ carotid arteries with a dose of 30 or 60 Gy caused significant changes 4, 12, and 24 weeks after irradiation [Citation44]. The radiation dose seems to affect the type of lesions; 30 Gy radiation dose leads to proliferative vascular hyperplasia, and 60 Gy mainly causes degenerative vascular wall hyalinization. In addition, elastic lamina fragmentation was observed in both scenarios, and the changes were significantly more significant 24 weeks later than four weeks.
4. Treatment
Epistaxis is a severe and unique complication that occurs after NPC radiotherapy, characterized by rapid, repeated, and variable bleeding, which requires different treatment principles from general bleeding. To manage epistaxis in irradiated NPC patients, a standard protocol has been established (), and various treatments can be given after identifying the bleeding cause. The commonly used methods include posterior nostril tamponade, endoscopic hemostasis, digital subtraction angiography (DSA), selective vascular embolization, and stent implantation [Citation45].
Figure 2. An algorithm for the diagnosis and treatment of carotid blowout in NPC patients after radiotherapy.
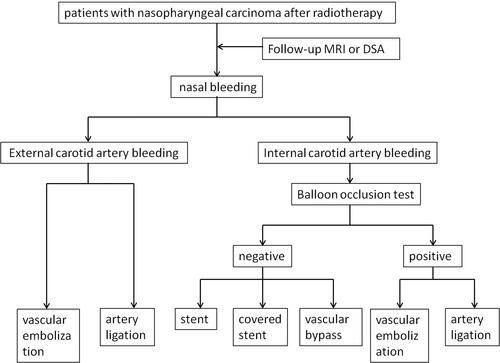
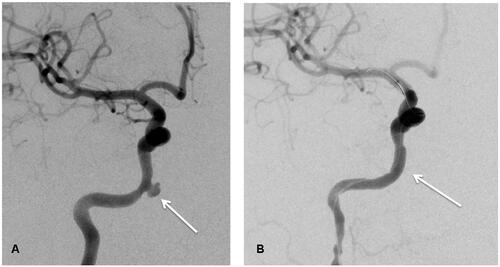
Figure 3. (A) Image before Willis covered stent implantation (white arrow shows the bleeding site); (B) Image after Willis covered stent implantation.
4.1. Ordinary packing
After NPC radiotherapy, the bleeding site is often concealed and associated with a wide range of lesions located at the back of the nasal cavity or nasopharynx. Nasal packing is currently the preferred hemostatic method to treat epistaxis [Citation46,Citation47]. This method is effective for patients with a small amount of bleeding, and it involves simple operation without advanced equipment. A suitable and effective packing method can be chosen according to the different bleeding sites of the patient, which significantly improves the hemostatic effects. However, patients with various degrees of restricted mouth opening, nasal adhesions, and increased embrittlement of nasal mucosal blood vessels after radiotherapy often need nasal tamponade, which causes great pain for patients and often induces new bleeding. Furthermore, nasal packing can cause nasal infections, reduce nasopharyngeal tissue oxygen supply, and blood circulation, leading to further necrosis of nasopharyngeal tissue and exacerbating the possibility of nasal bleeding, requiring close observation. The packing period should not exceed 3-4 days.
4.2. Nasal endoscopy
Nasal endoscopy provides a clear and wide vision, enabling comprehensive inspection of the nasal cavity and nasopharynx, helping locate the bleeding site with the suction device. Bipolar electrocoagulation, laser, plasma, and other equipment can be used to achieve hemostasis under direct vision, avoiding the blindness of nasal packing and the pain caused to patients. This method not only achieves a hemostatic effect but also protects the normal tissues of the nasal cavity. Studies have reported that nasal endoscopic electrocoagulation has achieved satisfactory hemostatic effects with less nasal tissue damage [Citation48], and some patients have healed by debridement of necrotic tissue [Citation49].
4.3. DSA and vascular embolization
DSA, a new technology developed at the end of the twentieth century, allows for selective vascular embolization and covered stenting of the ICA or external carotid artery and its branches using materials such as a gelatin sponge, spring steel ring, detachable balloon, and others [Citation50]. Traditional methods of achieving surgical hemostasis and oral intubation have low success rates due to fibrosis of the temporomandibular joint and masseter muscle caused by radiotherapy, making DSA’s advantages even more apparent [Citation51]. DSA can clearly demonstrate and detect new abnormal blood vessels and deal with damaged parts simultaneously, making it the preferred treatment for pseudoaneurysm after NPC radiotherapy [Citation2]. Endovascular treatment can also prevent acute carotid bursts in patients undergoing reirradiation for recurrent head and neck malignancies and can successfully treat recurrent NPC epistaxis [Citation34,Citation52]. Furthermore, DSA examination and selective arterial embolization have significant hemostatic effects and are considered a safe and effective method for the clinical diagnosis of major bleeding after NPC radiotherapy and the first choice to control it [Citation40,Citation45].
However, endovascular treatment is not without risks. Traditional endovascular treatment for aneurysmal rupture often uses microparticles to embolize responsible blood vessels, but embolic agent displacement is often the most alarming complication, especially in the ICA. Vascular embolism can increase the risk of complications related to an enlarged thrombus, embolic agent displacement, and internal carotid artery embolism [Citation53]. Complications during pure diagnostic angiography occur in 8.5% of cases, with neurological complications occurring in 2.6%, of which 0.33% are permanent. Using more than one stent can increase the incidence of neurological and other complications [Citation54], including death, hemiplegia, speech impairment, blindness, hematomas at the site of stent implantation, infection, arterial blockages, and transient hypertension and hypotension.
4.4. Stent and covered stent
The covered stent, in addition to balloon or coil embolization, can be used to treat major hemorrhage in NPC intravascularly [Citation55]. However, there is still a high risk of immediate or delayed cerebral ischemia or diplopia, with a complication rate of 15–20% [Citation56]. These complications may arise from incomplete repair of the Willis ring, making angiography essential for assessing the bleeding site. Before stent implantation, the collateral flow needs to be evaluated using a balloon occlusion test (BOT) [Citation57]. The stent can be implanted when the BOT test result is negative, and the use of a covered stent is recommended to minimize the risk of ischemic stroke. The Willis-covered stent has undergone technical improvements and has accumulated vast experience in treating intracranial PSA [Citation58]. Our team has previously treated five patients with NPC who suffered from hemorrhage in the intracranial ICA caused by radiation therapy [Citation59,Citation60]. All five patients’ intracranial ICA bleeding was immediately stopped after Willis-covered stent implantation without any adverse effects during follow-up. We hereby show the angiograms before and after the treatment of one of the patients we treated in . Retrospective analyses have indicated that the covered stent can increase the average survival time in patients with head and neck cancer [Citation55]. Endovascular covered stent has been emphasized as a practical and versatile rescue treatment for life-threatening carotid burst syndrome [Citation61]. The self-expanding covered stent is a safe, effective, and minimally invasive option for treating carotid hemorrhage [Citation62]. However, the long-term efficacy of covered stents needs further observation.
DSA technology has advantages in treating NPC hemorrhage by identifying the bleeding site and responsible blood vessels through angiography and selecting ICA or external carotid artery for branch vessel embolization and covered stent implantation. It has reliable hemostatic effects, small trauma, and a low recurrence rate. Prompt DSA inferior arterial embolization is necessary for critical conditions after radiotherapy of NPC to rescue patients [Citation63]. However, the equipment and cooperation from related departments are imperative, and other rescue measures such as anti-shock and anti-asphyxia are essential. Treatment costs and consumables, such as vascular embolism and covered stents, are expensive and require high economic conditions for patients’ families. The placement of a stent in the infected tissue around the pseudoaneurysm formation area can lead to bacterial colonization and eventual stent thrombosis [Citation63]. We need to pay attention to the risk of thrombosis and stent extrusion in NPC patients. Corresponding complications associated with Willis-covered stents, such as thrombosis, infections, and brain abscesses, have also been reported. Some studies have reported that implantation of covered stents is not conducive to long-term prognosis due to persistent contamination and neoplastic diseases [Citation64,Citation65].
4.5. Vascular bypass
In cases of bleeding in the main and large branch vascular artery, ligation of the external carotid artery and internal maxillary artery has been identified as a viable method for achieving hemostatic effects [Citation66]. However, as internal and external carotid artery ligation can significantly impede blood supply and repair of nasopharyngeal tissue, thereby worsening tissue necrosis, avoiding this method for hemostasis after radiotherapy for NPC is necessary. In such cases, it is crucial to evaluate the tolerance of ICA occlusion and ICA invasion in patients who have relapsed after NPC radiation therapy. For patients who cannot tolerate these procedures, intracranial and external vascular bypass with embolization of the affected ICA can be combined as an alternative method. Successful use of bypass grafting in treating a patient with NPC hemorrhage has been reported, thus avoiding complications caused by vascular ligation [Citation67]. In addition, retrospective analysis of data from 9 patients with nasopharyngeal carcinoma carotid burst syndrome who underwent extracranial/intracranial vascular bypass grafting without perioperative death or stroke showed a 1-year survival rate of 75%. The study concluded that patients with internal carotid burst hemorrhage after radiotherapy for nasopharyngeal carcinoma could achieve longer survival with extracranial/intracranial bypass surgery [Citation32]. For patients with recurrent NPC and positive ICA occlusion, intracranial and external vascular bypass grafting combined with internal arterial interventional embolization has been shown to be a precise and safe treatment if relevant imaging examination shows a close association of NPC with ICA. However, the disadvantage of this method is that it changes the original anatomy and interferes with blood perfusion in the brain [Citation68].
4.6. Others
Patients with NPC and comorbid systemic diseases, such as hypertension and coagulopathy, should proactively manage their blood pressure and improve their coagulation function. In the event of an emergency requiring patient rescue, ensuring the patency of the respiratory tract should also be prioritized. Comprehensive and effective treatment measures should be employed to address shock prevention and treatment, as well as local hemostasis. Notably, He et al. [Citation69] demonstrated that timely prophylactic tracheotomy before the onset of major bleeding can effectively improve the success rate of rescuing patients with major bleeding after NPC radiotherapy.
5. Prevent
Given the potentially fatal consequences of an aneurysm rupture, we emphasize the importance of assessing the pathophysiology of aneurysm formation following radiation therapy. We recommend monitoring the development of iatrogenic aneurysms during the follow-up period after treatment. Magnetic resonance imaging (MRI) is a crucial tool for detecting and monitoring intracranial and extracranial vascular lesions of various etiologies. In addition to evaluating vascular cavity information, MRI can assess the condition of the vascular wall, allowing for the differentiation of various vascular lesions. Compressed black blood MRI has the advantages of high image quality and relatively short acquisition time, making it a promising modality for routine vascular wall imaging in clinical settings [Citation34]. Guggenberger et al. demonstrated the efficacy of a high-resolution compressed-sensing black-blood 3D T1-weighted fast spin-echo technique for evaluating various vascular conditions in radiology [Citation70]. While continuous angiography has been recommended for evaluating vascular conditions, it is a traumatic examination. The MRI black blood sequence can be used to assess the condition of the blood vessel wall. If PSA formation is possible, a covered stent can be implanted to prevent bleeding, and further DSA examination is recommended to determine the condition of the vessel wall.
6. Conclusions
Radiation therapy is considered the primary treatment method for NPC, and while it effectively controls the primary nasopharyngeal tumor, it is crucial to emphasize the prevention, treatment, and management of radiation-induced nosebleeds to enhance the quality of life for NPC patients. Accurate understanding of epistaxis following radiotherapy, prompt bleeding cessation, and reduction of bleeding volume are key considerations. Nasal tamponade is a crucial rescue treatment, while tracheotomy is an active and effective method. Intravascular balloon embolization is a reliable and effective treatment method for ICA hemorrhage, and vascular embolization is the primary approach for treating external carotid artery maxillary bleeding. Implantation of a covered stent can achieve hemostasis without altering hemodynamics. A comprehensive approach utilizing these methods can improve the success rate of treating nosebleeds following NPC radiotherapy.
Author contributions
Xiaojing Yang and Hanru Ren wrote the manuscript. Minghua Li, Yueqi Zhu, Weitian Zhang, and Jie Fu reviewed and edited the manuscript. All authors have read and approved the manuscript. All the authors meet the criteria for authorship as per the ICMJE criteria.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Table 1. Recent reports on radiotherapy for recurrent nasopharyngeal carcinoma.
Additional information
Funding
References
- Chang ET, Ye W, Zeng YX, et al. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1–11.
- Zeng L, Wan W, Luo Q, et al. Retrospective analysis of massive epistaxis and pseudoaneurysms in nasopharyngeal carcinoma after radiotherapy. Eur Arch Otorhinolaryngol. 2022;279(6):2973–2980.
- Li J, Yi X, He G, et al. Analysis of the risk of death and clinical management for nasal or nasopharyngeal bleeding occurring after radiotherapy for nasopharyngeal carcinoma. Auris Nasus Larynx. 2022;49(4):703–708.
- Zheng L, Yan S, Yan D, et al. Fatal bleeding in a nasopharyngeal carcinoma patient after concurrent chemoradiation plus cetuximab: a case report. Onco Targets Ther. 2013;6:703–706.
- Yu KH, Leung SF, Tung SY, Hong Kong Nasopharyngeal Carcinoma Study G, et al. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong nasopharyngeal carcinoma study group. Head Neck. 2005;27(5):397–405.
- Hsiung CY, Yorke ED, Chui CS, et al. Intensity-modulated radiotherapy versus conventional three-dimensional conformal radiotherapy for boost or salvage treatment of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2002;53(3):638–647.
- Yao Z, Zhang B, Huang J, et al. Radiation-induced acute injury of intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in induction chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a prospective cohort study. Sci Rep. 2021;11(1):7693.
- Mcdonald MW, Moore MG, Johnstone PA. Risk of carotid blowout after reirradiation of the head and neck: a systematic review. Int J Radiat Oncol Biol Phys. 2012;82(3):1083–1089.
- Kong L, Lu JJ. Reirradiation of locally recurrent nasopharyngeal cancer: history, advances, and promises for the future. Chin Clin Oncol. 2016;5(2):26.
- Hu J, Huang Q, Gao J, et al. Clinical outcomes of carbon-ion radiotherapy for patients with locoregionally recurrent nasopharyngeal carcinoma. Cancer. 2020;126(23):5173–5183.
- King WW, Ku PK, Mok CO, et al. Nasopharyngectomy in the treatment of recurrent nasopharyngeal carcinoma: a twelve-year experience. Head Neck. 2000;22(3):215–222.
- Fee WE, Jr., Moir MS, Choi EC, et al. Nasopharyngectomy for recurrent nasopharyngeal cancer: a 2- to 17-year follow-up. Arch Otolaryngol Head Neck Surg. 2002;128(3):280–284.
- Hao SP, Tsang NM, Chang KP, et al. Nasopharyngectomy for recurrent nasopharyngeal carcinoma: a review of 53 patients and prognostic factors. Acta Otolaryngol. 2008;128(4):473–481.
- Ko JY, Wang CP, Ting LL, et al. Endoscopic nasopharyngectomy with potassium-titanyl-phosphate (KTP) laser for early locally recurrent nasopharyngeal carcinoma. Head Neck. 2009;31(10):1309–1315.
- Seo Y, Yoo H, Yoo S, et al. Robotic system-based fractionated stereotactic radiotherapy in locally recurrent nasopharyngeal carcinoma. Radiother Oncol. 2009;93(3):570–574.
- Chua DT, Wu SX, Lee V, et al. Comparison of single versus fractionated dose of stereotactic radiotherapy for salvaging local failures of nasopharyngeal carcinoma: a matched-cohort analysis. Head Neck Oncol. 2009;1:13.
- Vlantis AC, Chan HS, Tong MC, et al. Surgical salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma: a multivariate analysis of prognostic factors. Head Neck. 2011;33(8):1126–1131.
- Wei WI, Chan JY, Ng RW, et al. Surgical salvage of persistent or recurrent nasopharyngeal carcinoma with maxillary swing approach – critical appraisal after 2 decades. Head Neck. 2011;33(7):969–975.
- Ozyigit G, Cengiz M, Yazici G, et al. A retrospective comparison of robotic stereotactic body radiotherapy and three-dimensional conformal radiotherapy for the reirradiation of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e263-8–e268.
- Qiu S, Lin S, Tham IW, et al. Intensity-modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;83(2):676–683.
- Chen HY, Ma XM, Ye M, et al. Effectiveness and toxicities of intensity-modulated radiotherapy for patients with locally recurrent nasopharyngeal carcinoma. PLOS One. 2013;8(9):e73918.
- Tian YM, Zhao C, Guo Y, et al. Effect of total dose and fraction size on survival of patients with locally recurrent nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: a phase 2, single-center, randomized controlled trial. Cancer. 2014;120(22):3502–3509.
- Kong L, Wang L, Shen C, et al. Salvage Intensity-Modulated radiation therapy (IMRT) for locally recurrent nasopharyngeal cancer after definitive IMRT: a novel scenario of the modern era. Sci Rep. 2016;6:32883.
- Liu J, Yu H, Sun X, et al. Salvage endoscopic nasopharyngectomy for local recurrent or residual nasopharyngeal carcinoma: a 10-year experience. Int J Clin Oncol. 2017;22(5):834–842.
- Tian YM, Huang WZ, Yuan X, et al. The challenge in treating locally recurrent T3-4 nasopharyngeal carcinoma: the survival benefit and severe late toxicities of re-irradiation with intensity-modulated radiotherapy. Oncotarget. 2017;8(26):43450–43457.
- Chan OS, Sze HC, Lee MC, et al. Reirradiation with intensity-modulated radiotherapy for locally recurrent T3 to T4 nasopharyngeal carcinoma. Head Neck. 2017;39(3):533–540.
- Lee VH, Kwong DL, Leung TW, et al. Hyperfractionation compared to standard fractionation in intensity-modulated radiation therapy for patients with locally advanced recurrent nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2017;274(2):1067–1078.
- Ng WT, Ngan RKC, Kwong DLW, et al. Prospective, multicenter, phase 2 trial of induction chemotherapy followed by Bio-Chemoradiotherapy for locally advanced recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(3):630–638.
- Ng WT, Lee MC, Fung NT, et al. Dose volume effects of re-irradiation for locally recurrent nasopharyngeal carcinoma. Head Neck. 2020;42(2):180–187.
- Hirose K, Sato M, Kato T, et al. Profile analysis of adverse events after boron neutron capture therapy for head and neck cancer: a Sub-analysis of the JHN002 study. J Radiat Res. 2022;63(3):393–401.
- Leonetti JP, Weishaar JR, Gannon D, et al. Osteoradionecrosis of the skull base. J Neurooncol. 2020;150(3):477–482.
- Wu P, Yuan G, Lu Z, et al. Extracranial/intracranial vascular bypass to control carotid artery blowout in postirradiated nasopharyngeal carcinoma patients. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;35(5):448–452.
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80.
- Ay N, Todt I, Sudhoff H. Nasopharyngeal coil dislocation of an embolized internal carotid artery pseudoaneurysm. Case Rep Otolaryngol. 2021;2021:4270441.
- Gargula S, Saint-Maurice JP, Labeyrie MA, et al. Embolization of internal carotid artery branches in juvenile nasopharyngeal angiofibroma. Laryngoscope. 2021;131(3):E775–E780.
- Zhao Z, Huang L, Chen J, et al. Comprehensive treatment strategy for internal carotid artery blowout syndrome caused by nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2021;164(5):1058–1064.
- Escoffier C, Hage D, Tanaka T, et al. Ascending palatine branch from the lingual artery with multiple other variations of the external carotid artery. Folia Morphol. 2023;82(1):205–210.
- Gao Z, Chi FL. Anatomy relationship around internal carotid artery in the endoscopic surgery of nasopharynx: a study based on computed tomography angiography. J Neurol Surg B Skull Base. 2015;76(3):176–182.
- Ha HD, Duc NM, Christine JA, et al. A case series of ethmoidal dural arteriovenous fistulas treated by endovascular embolization with onyx. Med Arch. 2020;74(2):139–141.
- Zhao ZY, Huang LJ, Chen JH, et al. [Evaluation and embolization strategy by ASITN/SIR grade for injured internal carotid artery of nasopharyngeal carcinoma]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55(7):671–676.
- Zhan J, Zhang S, Wei X, et al. Etiology and management of nasopharyngeal hemorrhage after radiotherapy for nasopharyngeal carcinoma. Cancer Manag Res. 2019;11:2171–2178.
- Lam JW, Chan JY, Lui WM, et al. Management of pseudoaneurysms of the internal carotid artery in postirradiated nasopharyngeal carcinoma patients. Laryngoscope. 2014;124(10):2292–2296.
- Nilsson A, Wennerstrand J, Leksell D, et al. Stereotactic gamma irradiation of basilar artery in cat. Preliminary experiences. Acta Radiol Oncol Radiat Phys Biol. 1978;17(2):150–160.
- Mayberg MR, Luo Z, London S, et al. Radiation inhibition of intimal hyperplasia after arterial injury. Radiat Res. 1995;142(2):212–220.
- Wang Y, Dong X, Zhou C, et al. Endovascular management of intractable nasopharyngeal hemorrhage in patients irradiated for nasopharyngeal carcinoma: a twelve-year experience. Cancer Manag Res. 2020;12:11945–11952.
- Yau S. An update on epistaxis. Aust Fam Physician. 2015;44(9):653–656.
- Sahan M, Beydilli H, Deveer M, et al. Is nasal packing required in epistaxis? Clin Ter. 2014;165(1):e24-7.
- Tsai HM, Shu CH. Transnasal sphenopalatine artery electrocautery for posterior epistaxis. Zhonghua Yi Xue Za Zhi. 2002;65(11):529–533.
- Lan G, Huang B, Si Y, et al. The clinical experience of transnasal endoscopic approach for skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51(5):367–371.
- Wang X, Li L, Wang C, et al. Application of digital subtraction angiography in canine hindlimb arteriography. Vascular. 2022;30(3):474–480.
- Merkel H, Lindner D, Gaber K, et al. Standardized classification of cerebral vasospasm after subarachnoid hemorrhage by digital subtraction angiography. J Clin Med. 2022;11(7):2011.
- Alterio D, Turturici I, Volpe S, et al. Carotid blowout syndrome after reirradiation for head and neck malignancies: a comprehensive systematic review for a pragmatic multidisciplinary approach. Crit Rev Oncol Hematol. 2020;155:103088.
- Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386(14):1303–1313.
- Algra AM, Lindgren A, Vergouwen MDI, et al. Procedural clinical complications, Case-Fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis. JAMA Neurol. 2019;76(3):282–293.
- Jong MA, Candanedo C, Gross M, et al. Intervening in the acute phase of postradiation carotid blowout syndrome. Int Arch Otorhinolaryngol. 2019;23(2):172–177.
- Song X, Wang N. The "hand as foot" teaching method in Willis ring anatomy. Asian J Surg. 2022;45(7):1478–1479.
- Rothman A, Ciccolo ML, Galindo A, et al. Left subclavian artery test balloon occlusion before covered stent for recoarctation and aneurysm. World J Pediatr Congenit Heart Surg. 2020;11(4):NP235–NP238.
- Li MH, Gao BL, Wang YL, et al. Management of pseudoaneurysms in the intracranial segment of the internal carotid artery with covered stents specially designed for use in the intracranial vasculature: technical notes. Neuroradiology. 2006;48(11):841–846.
- Wang H, Yang HJ, Zeng S, et al. Willis covered stent for paraclinoid aneurysms:efficacy and mid-long-term follow-up results. Zhonghua Yi Xue Za Zhi. 2022;102(15):1119–1122.
- Gu Y, Chen L, Zhang Y, et al. Reconstructive treatment of symptomatic vertebral artery dissecting aneurysms with Willis covered stent: initial experience. J Interv Med. 2020;3(4):184–191.
- Wong DJY, Donaldson C, Lai LT, et al. Safety and effectiveness of endovascular embolization or stent-graft reconstruction for treatment of acute carotid blowout syndrome in patients with head and neck cancer: case series and systematic review of observational studies. Head Neck. 2018;40(4):846–854.
- Yuan B, Xin HN, Yuan K, et al. Heparin-bonded stent graft placement for treatment of massive epistaxis from ruptured radiation-induced internal carotid artery pseudoaneurysm: a case report. Radiol Case Rep. 2022;17(6):2129–2132.
- Moulakakis KG, Alexiou VG, Sfyroeras GS, et al. Endovascular management of infected iliofemoral pseudoaneurysms – a systematic review. Vasa. 2017;46(1):5–9.
- Zhao Y, Liu Z, Sun R, et al. The clinical efficacy analysis of treatment with a Willis covered stent in traumatic pseudoaneurysm of the internal carotid artery. Front Neurol. 2021;12:739222.
- Ma L, Feng H, Yan S, et al. Endovascular treatment of complex vascular diseases of the internal carotid artery using the Willis covered stent: preliminary experience and technical considerations. Front Neurol. 2020;11:554988.
- Morofuji Y, Matsunaga Y, Izumo T. Carotid-Carotid bypass for a carotid artery aneurysm. World Neurosurg. 2020;138:7–8.
- Zhao Z, Huang L, Chen J, et al. Clinical efficacy of bypass grafting in recurrent nasopharyngeal carcinoma patients with internal carotid artery invasion. Am J Otolaryngol. 2021;42(3):102860.
- Chan JYW, Wong STS, Wei WI. Surgical salvage of recurrent T3 nasopharyngeal carcinoma: prognostic significance of clivus, maxillary, temporal and sphenoid bone invasion. Oral Oncol. 2019;91:85–91.
- He CC, Si YF, Yu L, et al. Management of intractable epistaxis and bleeding points localization of post-therapy nasopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;47(3):191–195.
- Guggenberger K, Krafft AJ, Ludwig U, et al. High-resolution compressed-sensing T1 black-blood MRI: a new multipurpose sequence in vascular neuroimaging? Clin Neuroradiol. 2021;31(1):207–216.
