Abstract
Background & Aims
Individuals with high blood pressure (BP) have varying risks of cardiovascular events due to other coexisting factors. We aimed to identify the predictors of long-term absence of coronary artery calcium (CAC) in individuals with high BP, which is an indicator of healthy arterial aging and can guide preventive strategies.
Methods
We analyzed data from participants with high BP (≥120/80 mm Hg) in the Multi-Ethnic Study of Atherosclerosis who had baseline CAC = 0 and underwent a second CAC scanning after 10 years. We used multivariable logistic regression to evaluate the association between various risk factors for atherosclerotic cardiovascular disease (ASCVD) and long-term CAC = 0. We also calculated the area under the receiver operating characteristic curve (AUC) to predict the phenotype of healthy arterial aging in this population.
Results
We included 830 participants (37.6% male, mean ± SD age of 59.4 ± 8.7 years). During follow-up, 46.5% of participants (n = 386) had CAC = 0, and they were younger and had fewer metabolic syndrome components. Adding ASCVD risk factors to the demographic model (age, sex, and ethnicity) moderately increased the predictive value for long-term CAC = 0 (AUC: demographic model + ASCVD risk factors vs. demographic model alone, 0.653 vs. 0.597, p < .001; category net reclassification improvement = 0.104, p = .044; integrated discrimination improvement = 0.040, p < .001).
Conclusion
In individuals with high BP and initial CAC = 0, over 40% maintained CAC = 0 during a 10-year follow-up, which was associated with fewer ASCVD risk factors. These findings may have implications for preventive strategies in individuals with high BP.
Clinical Trial registration number: The MESA was registered at clinical trials. gov as NCT 00005487.
Nearly half (46.5%) of individuals with high blood pressure (BP) maintained a long-term absence of coronary artery calcium (CAC) during a 10-year follow-up, and this was associated with a 66.6% lower risk of atherosclerotic cardiovascular disease (ASCVD) events compared to those who developed incident CAC.
Individuals with high BP, who are usually assumed to have an increased risk of ASCVD, exhibit significant heterogeneity in their ASCVD risk; those who maintain CAC = 0 have a lower ASCVD risk.
Adding overall ASCVD risk factors to demographic information resulted in a moderate improvement in predicting long-term CAC = 0.
KEY MESSAGES
Introduction
High blood pressure (BP) is a major modifiable risk factor for atherosclerotic cardiovascular disease (ASCVD) [Citation1,Citation2], accounting for an estimated 8.5 million deaths from stroke, coronary artery disease, other vascular diseases, and chronic kidney disease worldwide each year [Citation3,Citation4]. Coronary artery calcium (CAC) assessed by computed tomography is a highly specific feature of coronary atherosclerosis and a powerful tool for ASCVD risk stratification, with a reliable prognostic value for cardiovascular morbidity and mortality [Citation5,Citation6]. On the other hand, arterial aging can be classified into two categories: biological arterial aging and chronological arterial aging. Biological aging is characterized by a decline in arterial function, while chronological aging, also known as healthy arterial aging, simply refers to the passage of time [Citation7,Citation8]. The absence of CAC is considered a marker of healthy arterial aging, and is associated with a very low risk of ASCVD events and mortality [Citation9–11]. In contrast, a high CAC score identifies an increased risk even in individuals with a low burden of traditional risk factors [Citation12]. Additionally, elderly individuals without CAC have a higher survival rate than young individuals with a high CAC score [Citation13]. CAC score has been shown to be independently associated with a 10-year ASCVD risk across different age, sex, and race/ethnic groups [Citation5]. Although the risk factors contributing to CAC progression are well-established [Citation14], predictors of healthy arterial aging as measured by long-term CAC = 0 remain largely unexplored, especially in individuals with traditional ASCVD risk factors such as age, dyslipidemia, diabetes mellitus, hypertension, etc. Long-term CAC = 0 confers a very low risk for future ASCVD events and mortality [Citation10,Citation11], and it can inform the safe avoidance of certain preventive pharmacotherapies [Citation15]. Therefore, there is a need to better understand strategies for maintaining healthy arterial aging characterized by long-term absence of CAC in individuals with high BP and/or other risk factors, which may add to our understanding of primary prevention of ASCVD.
To better understand these relationships, this study aims to investigate the predictors of long-term absence of CAC in participants of the Multi-Ethnic Study of Atherosclerosis (MESA) with baseline BP levels of 120/80 mm Hg or higher. This will shed light on (1) the predictors of healthy arterial aging in individuals at intermediate-high risk for ASCVD and (2) which individuals with high BP are more likely to maintain healthy arterial aging over an extended period.
Materials and methods
Study design and participants
The MESA study design and examination procedures have been previously described [Citation16]. In summary, from July 2000 to September 2002, 6 communities in the United States enrolled 6,814 multi-ethnic participants aged 45–84 years who did not have overt ASCVD at baseline. All participants provided written informed consent, and the study protocol was approved by the Institutional Review Boards at each participating institution.
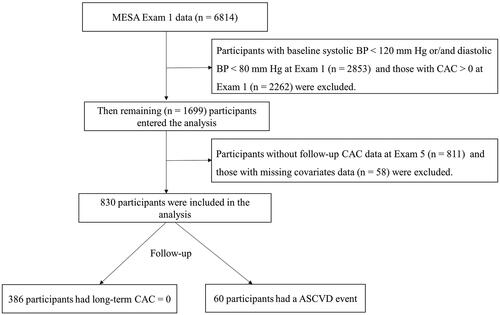
Epidemiological evidence from numerous cohort studies suggests that ASCVD risk increases progressively with higher levels of baseline BP above the typical level of 115/75 mm Hg, which is associated with minimal ASCVD risk at the population level [Citation17,Citation18]. In this analysis, all participants who were enrolled at baseline (Exam 1: 2000–2002) were included. As shown in , participants were excluded if they had a baseline systolic BP < 120 mm Hg or diastolic BP < 80 mm Hg (n = 2853), baseline CAC score > 0 (n = 2262), without a repeat CAC scan at Exam 5 (n = 811), or without other covariates of interest (n = 58). Finally, the analysis included 830 participants.
Definition of high BP
Resting BP was measured in a seated position on the participant’s right arm after a 5-minute rest period using a Dinamap Pro-100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL). The mean of the last 2 measurements was used for the analysis, consistent with previous studies [Citation19,Citation20].
Following the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for high BP in adults, BP was categorized as normal (<120/80 mm Hg), elevated (120–129/80 mm Hg), and stages 1 or 2 hypertension (130–139/80–89 mm Hg and ≥ 140/90 mm Hg, respectively) [Citation1]. High BP in this study was defined as elevated BP, stage 1 hypertension, and stage 2 hypertension (≥120/80 mm Hg).
Measurement of CAC
Depending on the study site, the MESA used either a multi-detector computed tomography or a cardiac-gated electron-beam computed tomography to assess CAC, which has been shown to be comparable [Citation21]. All MESA participants underwent a CAC scan at Exam 1 (2000–2002), but only 50% of them repeated a CAC scan at Exam 5 (2010–2011). The severity of each calcified lesion was evaluated independently by 2 blinded analysts using the Agatston method, and the scores were summed across all lesions in all coronary artery branches (left main, left anterior descending, circumflex, and right coronary) [Citation21,Citation22]. In this study, long-term CAC = 0 was defined as maintaining CAC = 0 at baseline and at Exam 5. Incident CAC was defined as CAC > 0 at Exam 5 in participants who had CAC = 0 at baseline.
ASCVD events
The participants in the study were followed from their baseline examination until October 2012. ASCVD events were examined and independently categorized by 2 physicians who were blinded to the study data. In cases of disagreement, a mortality and morbidity committee made the final classification. ASCVD events were categorized as incident coronary artery disease, stroke, or other incident ASCVD according to the protocol established by the MESA [Citation5]. Exam 5 was used as the follow-up time for all ASCVD event analyses to compare event rates between individuals with and without long-term persistent CAC = 0.
Measurement of other covariates
Participant characteristics were collected during the baseline examination of the MESA cohort using standardized survey methods to obtain demographic and clinical information. Waist circumference (WC) was measured at the level of the umbilicus using a Gullick II 150 cm anthropometric steel measuring tape with 4-ounce tension (Sammons Preston, Chicago, IL). Body mass index was calculated as weight divided by the square of height (kg/m2). A 10-year ASCVD risk was calculated for each participant using the Pooled Cohort Equations based on their baseline demographics and relevant risk factor information [Citation23]. The presence of carotid artery atherosclerotic plaque was determined by a distinct, focal wall thickening > 1.5 cm or focal thickening > 50% than the surrounding intima-media thickness and was measured with an M12L transducer (General Electric Medical Systems, Waukesha, WI; common carotid artery frequency, 13 MHz) [Citation24].
Blood samples were collected after a 12-hour fast, processed and stored at −80 °C for analysis. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, and estimated glomerular filtration rate (eGFR) were measured as previously described [Citation14,Citation25]. Diabetes was defined as either fasting glucose ≥ 126 mg/dL, self-reported diabetes, or the use of medication for lowering glucose. Metabolic risk factors were based on metabolic syndrome (MetS) diagnostic criteria [Citation26], which included: (1) WC ≥102 cm for men and ≥88 cm for women; (2) triglycerides ≥150 mg/dL or receiving triglyceride-lowering therapy; (3) HDL-C < 40 mg/dL for men and < 50 mg/dL for women, or on drug treatment for reduced HDL-C; (4) systolic BP ≥130 mm Hg or diastolic BP ≥ 85 mm Hg, or on antihypertensive treatment; and 5) fasting glucose ≥ 100 mg/dL or being treated for diabetes. We also defined the individual MetS components as a dichotomy using the respective cutoffs mentioned above.
Statistics analysis
The values were expressed as mean ± SD, median (IQR), or numbers (percentages) depending on the types of data. Differences between individuals with long-term CAC = 0 and those with incident CAC were evaluated using the Student t test or Wilcoxon signed-rank for continuous variables and the χ2 test for categorical variables. We performed multivariate logistic regression and calculated odd ratios (ORs) and 95% confidence intervals (CIs) to access predictors of persistent CAC = 0. The multivariate model was adjusted for age, diabetes, fasting glucose, LDL-C, lipid-lowering medication use, race, sex, and smoking status. We also investigated the incidence of ASCVD stratified by the status of long-term CAC = 0. In addition, a sensitivity analysis was performed in participants with high BP who did not take anti-hypertensive medication. We created receiver operating characteristic (ROC) curves and estimated the area under the curve (AUC) to indicate the predictive power of different models for the persistent CAC = 0 phenotype in participants with high BP. The C-statistic was examined using Delong’s test to determine whether adding ASCVD risk factors and imaging data to the baseline risk model increased the predictive value. The incremental predictive values were further assessed using the net reclassification improvement (NRI) and integrated differentiation improvement (IDI) risk models [Citation27]. All analyses were performed using R 4.2.1 or SPSS 23 (SPSS, Inc., Chicago, IL). Statistical significance was defined as a 2-sided p-value less than .05.
Results
The baseline characteristics of 830 participants (mean ± SD age of 59.4 ± 8.7 years, 37.6% male; 39.2% African American, 27.8% Caucasian, 23.3% Hispanic and 9.8% Chinese) with high BP and no detectable CAC at the start of the study are presented in . During a follow-up of 9.4 ± 0.5 years, 46.5% of participants (n = 386) maintained persistent CAC = 0. Compared with those with incident CAC, participants with long-term CAC = 0 were younger and had fewer ASCVD risk factors, such as lower levels of systolic BP and fasting glucose, more favorable lipid profiles, and lower 10-year ASCVD risk scores. They also used fewer antihypertensive and lipid-lowering medications and had lower carotid intima-media thickness and a lower presence of carotid plaques. However, there were no significant differences between the 2 groups in terms of sex, ethnicity, diastolic BP, heart rate, body mass index, WC, physical activity, smoking status, diabetes, glucose-lowing medication use, C-reactive protein, urinary albumin, and eGFR (all p > .05). Similar results were obtained when the analysis was limited to participants not on antihypertensive medication use ().
Table 1. Characteristics of included participants with high BP.
Table 2. Characteristics of 487 high-BP participants free of anti-hypertensive medication use.
The participants with baseline CAC = 0 who developed incident CAC during follow-up had a median CAC score of 25.6. As shown in , in the fully adjusted model, individuals with younger age (OR 1.324 [95% CI 1.144–1.533], p < .001), lower levels of LDL-C (OR 1.239 [95% CI 1.071–1.434], p = .004), and systolic BP (OR 1.186 [95% CI 1.022–1.377], p = .025) were more likely to maintain long-term CAC = 0. Specifically, the incidence of CAC at Exam 5 increased significantly with graded elevations of systolic BP (< 120 mm Hg vs 120–129 mm Hg vs 130–139 mm Hg vs > 140 mm Hg: 41.2% vs 50.4% vs 51.8% vs 59.2%, p = .064 for high-BP participants, 40.0% vs 45.4% vs 47.1% vs 60.1%, p = .030 for high-BP participants free of anti-hypertensive use, respectively) but was similar across participants with different diastolic BP (Supplementary online resource Figure 1). In addition, participants with < 3 MetS components were more likely to maintain persistent CAC = 0 than those with ≥ 3 MetS components (OR 1.373 [95% CI 1.005–1.875], p = .046). Likewise, participants who did not have carotid plaques were more likely to maintain CAC = 0 than those who had carotid plaques (OR 1.589 [95% CI 1.153–2.188], p = .005). However, there was no significant difference in the probability of persistent CAC = 0 regarding smoking status, WC, fasting glucose, HDL-C, triglycerides, eGFR and urinary albumin in the fully adjusted model (all p > .05). The results remained consistent when the analysis was limited to participants not on antihypertensive medication use ().
Table 3. Multivariate logistic regression analysis of potential influencing factors for long-term CAC = 0 among high-BP participants (n = 830).
Table 4. Multivariate logistic regression analysis of potential influencing factors for long-term CAC = 0 among high-BP participants free of anti-hypertensive medication use (n = 487).
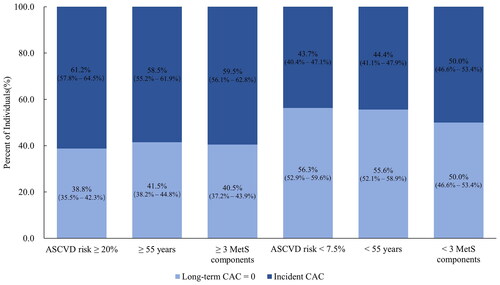
A significant heterogeneous ASCVD risk was detected in individuals with high BP who were traditionally categorized as having an intermediate-high or low risk of ASCVD. Among those considered at intermediate-high risk of developing ASCVD, 38.8% of participants with a high 10-year ASCVD risk (≥ 20%), 41.5% of participants aged ≥ 55 years, and 40.5% of participants with ≥ 3 MetS components had long-term CAC = 0. In contrast, among those traditionally categorized as low risk for ASCVD, 43.7% with a low 10-year ASCVD risk (< 7.5%), 44.4% of participants aged < 55 years, and 50.0% of participants with < 3 MetS components did develop CAC ( and Supplementary online resource Table 1). Like incident CAC, there was a tendency for the incidence of ASCVD to increase with a graded elevation of systolic BP rather than diastolic BP (Supplementary online resource Figure 2). We also found a tendency towards an association between long-term CAC = 0 and incident ASCVD events in this high-BP sample (Supplementary online resource Table 2). A lower incidence of ASCVD events was observed in high-BP participants with long-term CAC = 0 compared to those with incident CAC (5.7% versus 8.6%, Supplementary online resource Figure 3). We observed similar patterns in individuals who were not taking antihypertensive medication (Supplementary online resource Table 2 and Figure 3).
Figure 2. Percentage of participants with and without long-term coronary artery calcium (CAC) stratified by baseline risk factor profile. ASCVD: atherosclerotic cardiovascular disease.
To predict healthy arterial aging in the high-BP population, we conducted ROC curve analyses to assess the predictive ability of different models. As shown in , including ASCVD risk factors in addition to the demographic model (age, ethnicity, and sex) had a modest incremental effect on the AUC for predicting persistent CAC = 0 (AUC: demographic model + ASCVD risk factors vs. demographic model alone, 0.653 vs. 0.597; p < .001). Moreover, the inclusion of ASCVD risk factors in the demographic model significantly improved the ability for discrimination and reclassification (category INR = 0.104, p = .044; IDI = 0.040, p < .001; and ). However, adding the absence of carotid plaques did not improve the ability of the demographic model to predict long-term CAC = 0 (AUC: demographic model + ASCVD risk factors 0.620, vs. demographic model, 0.597; p = .061; ), nor did it have a significant incremental effect on the ability of reclassification and discrimination (; category INR = 0.059, p = .093; IDI = 0.016, p < .001; ).
Figure 3. Receiver-operating characteristic curve for evaluating the incremental effects of ASCVD risk factors, absence of carotid plaques beyond the baseline risk model. ASCVD: atherosclerotic cardiovascular disease; AUC: the area under the curve.
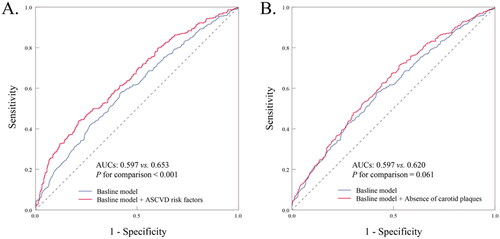
Table 5. Category-free NRI and IDI for the incremental values of different models for predicting long-term coronary artery calcium = 0.
Discussion
In this analysis of healthy arterial aging in participants with high BP from the MESA cohort, we demonstrate 3 main findings: (1) About 30% MESA participants with high BP have a baseline CAC = 0, among whom 46.5% maintain long-term CAC = 0 after a follow-up of 10 years. (2) Except for a younger age, only fewer overall ASCVD risk factors, including a lower number of individual MetS components, are associated with an increased probability of healthy arterial aging. (3) Optimal management of cardiometabolic profiles may help to maintain healthy arterial aging (Supplementary online resource Figure 4).
High BP is an important contributor cardiovascular morbidity and mortality and has a high prevalence of exposure, necessitating rigorous BP management aimed at reducing future ASCVD events [Citation1,Citation2]. Previous studies demonstrated different roles of BP components and measure in the development of vascular calcification [Citation28–32]. A routine health maintenance screening of 9510 patients has indicated that pulse pressure and isolated systolic hypertension are strong risk factors of calcified atherosclerosis in various vascular beds [Citation28]. Analysis of ambulatory BP has demonstrated that higher asleep systolic BP and higher awake and asleep diastolic BP were associated with higher CAC scores [Citation29]. However, a post hoc analysis of data from Coronary Artery Risk Development in Young Adults study detected no correlations among early adulthood long-term systolic, diastolic BP variability and CAC burden [Citation30]. These discrepancies across the studies may be due to the differences in the selected population and BP patterns. In the present study on the MESA cohort enrolling individuals aged 45–84 years, we demonstrate that baseline systolic BP levels are positively associated with follow-up CAC severity, which supports the hypothesis that systolic BP is a better predictor of events in individuals over 50 years old, compared to diastolic BP [Citation31–33]. Based on the current American guideline for BP management, we selected participants with high BP (defined as systolic BP ≥ 120 mm Hg and/or diastolic BP ≥ 80 mm Hg) and investigated the primary prevention strategies for healthy artery aging.
Previous studies have demonstrated that incident CAC, defined as progression to CAC > 0 from a baseline CAC = 0, was associated with age, diabetes, and smoking [Citation34]. CAC scores are strongly and progressively associated with the 10-year risk of incident ASCVD [Citation5]. However, maintaining healthy arterial aging (i.e. a zero CAC score) has a high negative predictive value for incident ASCVD and a good prognosis [Citation9,Citation35]. Similarly, our results suggest that maintaining long-term CAC = 0 over a 10-year follow-up tends to have a lower incidence of ASCVD. Although it is well-established that age, hypertension, smoking, C-reactive protein, and total cholesterol are risk factors for incident CAC [Citation36], the potential influencing factors for maintaining long-term CAC = 0 is largely unknown. Lehmann et al. demonstrated that there is an inverse relationship between the number of cardiovascular risk factors and healthy arterial aging, as assessed by Doppler ultrasound measurements of aortic compliance [Citation37]. Although BP has an incremental effect on CAC progression, our results indicate that well management of cardiometabolic profiles may have an additional beneficial effect on healthy arterial aging over a 10-year follow-up. Our results fit into an exploratory study that showed considerable heterogeneity in risk among individuals with hypertension [Citation38]. The study suggests that the risks associated with high BP can vary widely among individuals, emphasizing the need for personalized approaches to reduce cardiovascular risk in high-BP population.
The study of 5445 participants from the MESA cohort who did not have clinical ASCVD found that carotid artery plaque detected by ultrasound was associated with a 37% increased likelihood of CAC progression, independent of traditional ASCVD risk factors [Citation39]. Despite the similarities in characteristics and underlying mechanisms, only 24.1% of participants with carotid artery plaques developed CAC, suggesting differences in the pathobiology of atherosclerosis between these two locations [Citation39]. Although the carotid and coronary arteries experience vastly different hemodynamics, carotid atherosclerosis is still an important part of systemic atherosclerotic burden and can even occur earlier than coronary atherosclerosis [Citation40]. Further studies are needed to determine whether carotid artery plaque evaluation could be incorporated into routine cardiovascular risk assessment. Previous studies have demonstrated a significant association between MetS components and calcification in various vascular beds [Citation41,Citation42]. Our analysis extends these findings by showing that among high BP individuals, a lower number of MetS components, including systolic BP, LDL-C, and fasting glucose, are positively associated with long-term CAC = 0. These results suggest that assessing the overall cardiometabolic profile can be a powerful predictive tool for healthy arterial aging and identifying early-stage ASCVD in high-BP populations. This can inform targeted screening and intervention of ASCVD risk factors to reduce the risk of future ASCVD events [Citation1,Citation43].
One strength of our study is its prospective design, which allowed us to focus on predictors of healthy arterial aging in individuals with high BP over a 10-year follow-up period. Additionally, our study utilized standardized data collection processes and strict quality control measures. However, it is important to acknowledge potential limitations of our study. The AUC of our final predictive model for long-term CAC = 0 was modest, indicating that other factors, including genetics, may also contribute to healthy arterial aging in clinical practice [Citation44,Citation45]. Furthermore, our sample size was relatively small, and the exclusion of certain participants at baseline may have influenced our findings. Despite our comprehensive correction for putative risk factors, residual confounders may still exist given the observational nature of our study.
Conclusion
The post hoc analysis of the MESA cohort reveals that over 40% of individuals with high BP and no detectable CAC at baseline remained CAC = 0 over a decade of follow-up. These findings underscore the importance of promoting an optimal cardiometabolic profile in the hypertensive population to prevent or delay the development of CAC and subsequent ASCVD events.
Ethics approval and consent to participate
The Multi-Ethnic Study of Atherosclerosis (MESA) is a publicly available dataset and all participants in the MESA provided written informed consent. The study protocol was approved by the Institutional Review Boards at each participating institution.
Author contributions
The authors’ contributions to the paper are as follows: conception and design of the work: Jing-Wei Gao and Pin-Ming Liu; analysis and interpretation of the data: Jing-Wei Gao and Si You; the drafting of the paper: Jing-Wei Gao and Si You; data collection, revising it critically for intellectual content: Si You, Zhuo-Chao Xiong, Hai-Feng Zhang, Qing-Yun Hao, Jia-Jin Han, Jing-Feng Wang, Shao-Ling Zhang; the final approval of the version to be published: Jing-Wei Gao, Si You, Zhuo-Chao Xiong, Hai-Feng Zhang, Qing-Yun Hao, Jia-Jin Han, Jing-Feng Wang, Shao-Ling Zhang and Pin-Ming Liu. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (10.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study are publicly available on the BioLINCC website (https://biolincc.nhlbi.nih.gov/home/).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;138(17):1–11.
- Nguyen TN, Chow CK. Global and national high blood pressure burden and control. Lancet. 2021;398(10304):932–933.
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–980.
- Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388(10060):2665–2712.
- Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–2408.
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345.
- Hamczyk MR, Nevado RM, Barettino A, et al. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):919–930.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging. 2015;8(12):1393–1400.
- Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imag. 2009;2(6):692–700.
- Shareghi S, Ahmadi N, Young E, et al. Prognostic significance of zero coronary calcium scores on cardiac computed tomography. J Cardiovasc Comput Tomogr. 2007;1(3):155–159.
- Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imag. 2009;2(6):675–688.
- Silverman MG, Blaha MJ, Krumholz HM, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the multi-ethnic study of atherosclerosis. Eur Heart J. 2014;35(33):2232–2241.
- Tota-Maharaj R, Blaha MJ, McEvoy JW, et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33(23):2955–2962.
- Gassett AJ, Sheppard L, McClelland RL, et al. Risk factors for long-term coronary artery calcium progression in the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2015;4(8):e001726.
- Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ. 2021;373:n776.
- Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881.
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913.
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292.
- Inoue K, Goldwater D, Allison M, et al. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi-ethnic study of atherosclerosis. Hypertension. 2020;76(1):113–120.
- Harding BN, Norby FL, Heckbert SR, et al. Longitudinal measures of blood pressure and subclinical atrial arrhythmias: the MESA and the ARIC study. J Am Heart Assoc. 2021;10(11):e020260.
- Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of multi-ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology. 2005;234(1):35–43.
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73.
- Tattersall MC, Gassett A, Korcarz CE, et al. Predictors of carotid thickness and plaque progression during a decade: the multi-ethnic study of atherosclerosis. Stroke. 2014;45(11):3257–3262.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–2752.
- Lin XL, Li QY, Zhao DH, et al. Serum glycated albumin is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents: an observational study. Front Cardiovasc Med. 2022;9:943185.
- Jensky NE, Criqui MH, Wright MC, et al. Blood pressure and vascular calcification. Hypertension. 2010;55(4):990–997.
- Zhang Y, Schwartz JE, Jaeger BC, et al. Association between ambulatory blood pressure and coronary artery calcification: the JHS. Hypertension. 2021;77(6):1886–1894.
- Nwabuo CC, Yano Y, Moreira HT, et al. Long-term blood pressure variability in young adulthood and coronary artery calcium and carotid intima-media thickness in midlife: the CARDIA study. Hypertension. 2020;76(2):404–409.
- Vishram JK, Borglykke A, Andreasen AH, et al. Impact of age on the importance of systolic and diastolic blood pressures for stroke risk: the MOnica, risk, genetics, archiving, and monograph (MORGAM) project. Hypertension. 2012;60(5):1117–1123.
- Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation. 1999;100(4):354–360.
- Williams B, Lindholm LH, Sever P. Systolic pressure is all that matters. Lancet. 2008;371(9631):2219–2221.
- Min JK, Lin FY, Gidseg DS, et al. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: What is the “warranty period” for remaining normal? J Am Coll Cardiol. 2010;55(11):1110–1117.
- Wang X, Le EPV, Rajani NK, et al. A zero coronary artery calcium score in patients with stable chest pain is associated with a good prognosis, despite risk of non-calcified plaques. Open Heart. 2019;6(1):e000945.
- Wu YJ, Mar GY, Wu MT, et al. A LASSO-derived risk model for subclinical CAC progression in Asian population with an initial score of zero. Front Cardiovasc Med. 2020;7:619798.
- Lehmann ED, Hopkins KD, Rawesh A, et al. Relation between number of cardiovascular risk factors/events and noninvasive doppler ultrasound assessments of aortic compliance. Hypertension. 1998;32(3):565–569.
- Yang DY, Nie ZQ, Liao LZ, et al. Phenomapping of subgroups in hypertensive patients using unsupervised data-driven cluster analysis: an exploratory study of the SPRINT trial. Eur J Prev Cardiol. 2019;26(16):1693–1706.
- Polak JF, Tracy R, Harrington A, et al. Carotid artery plaque and progression of coronary artery calcium: the multi-ethnic study of atherosclerosis. J Am Soc Echocardiogr. 2013;26(5):548–555.
- Liu R, Shao J. Research progress on risk factors related to intracranial artery, carotid artery, and coronary artery stenosis. Front Cardiovasc Med. 2022;9:970476.
- Yamazoe M, Hisamatsu T, Miura K, et al. Relationship of insulin resistance to prevalence and progression of coronary artery calcification beyond metabolic syndrome components: Shiga epidemiological study of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(8):1703–1708.
- Razavi AC, Wong N, Budoff M, et al. Predicting long-term absence of coronary artery calcium in metabolic syndrome and diabetes: the MESA study. JACC Cardiovasc Imaging. 2021;14(1):219–229.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104.
- Grootaert MOJ, Finigan A, Figg NL, et al. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ Res. 2021;128(4):474–491.
- Dumor K, Shoemaker-Moyle M, Nistala R, et al. Arterial stiffness in hypertension: an update. Curr Hypertens Rep. 2018;20(8):72.