Abstract
Background
The connection between vitamin D to non-alcoholic fatty liver disease (NAFLD) is still unclear. Herein, the relationship of vitamin D with NAFLD and liver fibrosis (LF) detected by vibration controlled transient elastography was investigated in US adults.
Methods
The National Health and Nutrition Examination Survey of 2017–2018 was employed for our analysis. Participants were categorized as having either vitamin D deficiency (<50 nmol/L) or vitamin D sufficiency (≥50 nmol/L). A controlled attenuation parameter score of ≥ 263 dB/m was employed to define NAFLD. Significant LF was identified by the liver stiffness measurement value of ≥ 7.9 kPa. Multivariate logistic regression was adopted to explore the relationships.
Results
Among the 3407 participants, the prevalence of NAFLD and LF was 49.63% and 15.93% respectively. Compared to participants without NAFLD, no significant difference in serum vitamin D was observed in NALFD participants (74.26 vs. 72.24 nmol/L; p = 0.21). Using multivariate logistic regression analysis, no obvious connection of vitamin D status to NAFLD (sufficiency vs. deficiency, OR 0.89, 95%CI 0.70–1.13) was discovered. However, among NAFLD participants, the sufficiency of vitamin D represents a lower LF risk (OR 0.56, 95%CI 0.38–0.83). When evaluated in quartiles, in comparison to the lowest quartile, high vitamin D represents low LF risk in a dose-dependent manner (Q2 vs. Q1, OR 0.65, 95%CI 0.37–1.14; Q3 vs. Q1, OR 0.64, 95%CI 0.41–1.00; Q4 vs. Q1, OR 0.49, 95%CI 0.30–0.79).
Conclusions
No relationship was found between vitamin D and CAP-defined NAFLD. However, a positive connection of the high serum vitamin D to the reduced LF risk was found among NAFLD subjects.
Our study found no relationship between vitamin D and CAP-defined NAFLD in US adults.
High serum vitamin D was inversely associated with liver fibrosis in a dose-dependent manner among NAFLD participants.
Key messages:
Introduction
As a most frequently identified liver disorder worldwide, non-alcoholic fatty liver disease (NAFLD) affects about a quarter of the whole population [Citation1]. Considering the epidemic of obesity and diabetes, NAFLD prevalence is projected to continue increasing. NAFLD is considered as a widely types of liver disorder, including simple liver steatosis and non-alcoholic steatohepatitis, which can potentially progressed to liver cirrhosis and fibrosis [Citation2]. NAFLD is characterized as a metabolic disease associated with liver, and frequently accompanied by hypertension, dyslipidaemia, insulin resistance, and obesity. Thus, the NAFLD is renamed as metabolic dysfunction-associated fatty liver disease (MAFLD) [Citation3]. For the quantitation and diagnosis of fatty liver, biopsy is the gold standard. However, because of the possible sampling error, elevated costs, and invasive nature, it is unsuitable to use it in a broad population. Thus, in population studies, the serum biomarkers and ultrasonography are frequently employed. However, ultrasonography has limited sensitivity and does not reliably detect steatosis when the degree of steatosis <20% [Citation4]. As a non-invasive and accurate method, the vibration controlled transient elastography (VCTE) is frequently employed to examine liver steatosis and fibrosis severity of NAFLD patients, and simultaneously record the values of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) [Citation5]. Meanwhile, it is also commonly used for the evaluation of hepatic fibrosis and steatosis in general population [Citation6].
Vitamin D is a sterol hormone synthesized in the skin through a chemical reaction dependent on ultraviolet radiation and is later activated by hydroxylation in liver and kidneys. Except its classical functions in regulating calcium homeostasis and the bone metabolism [Citation7], vitamin D has extensive extra-skeletal effects such as anti-inflammatory [Citation8], anti-fibrotic [Citation9], and immunomodulatory [Citation10] properties. Vitamin D deficiency, characterized by low 25-hydroxy vitamin D (25(OH)D) in serum, has been correlated with increasing risks of cardiovascular diseases [Citation11], metabolic syndrome [Citation12], and type 2 diabetes [Citation13]. The contradictory results about the association between NAFLD and the deficiency of vitamin D were obtained previously [Citation14–16]. In a health check-up Korean population, connection of the high serum vitamin D to the reduced NAFLD risk was observed by Heo et al. [Citation14]. However, another study in South Korea found that serum vitamin D concentration is not connected to MAFLD [Citation15]. A recent bi-directional mendelian randomization study also supported no association between low vitamin D and ultrasound-diagnosed NAFLD [Citation16].
Therefore, according to the 2017–2018 National Health and Nutrition Examination Survey (NHANES), we conducted this cross-sectional study to examine the connection of serum vitamin D to NAFLD and liver fibrosis (LF) detected by VCTE, providing novel ideal for liver steatosis and fibrosis diagnosis at an early stage.
Methods
Study population
As a survey program based on national population, NHANES is performed every 2 years and used to evaluate the nutritional and health status of the non-institutionalized US civilian general population. Herein, we employed the NHANES of the 2017–2018 cycle for our analysis because of the data of VCTE was specifically included.
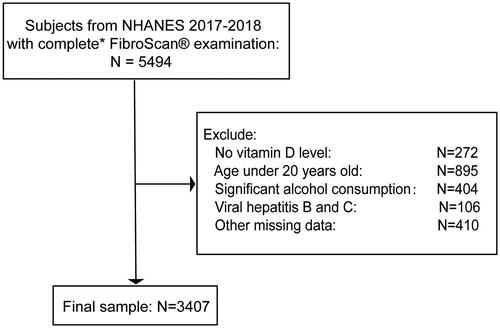
A total of 9254 participants were enrolled in this cycle. Among them, 5494 participants had elastography exam with complete status. We excluded 272 participants with missing vitamin D data and 895 participants aged below 20 years. For NAFLD, 404 participants with significant alcohol intake (>20 g/day for females and >30 g/day for males) were excluded [Citation17]. We also excluded 106 hepatitis B or C-infected participants (characterized as the presence of hepatitis C antibody or the hepatitis B surface antigen) and 410 participants with other missing data. Finally, we enrolled 3407 participants in our present study (). According to the hepatic steatosis determined by CAP and any of the three metabolic conditions, such as diabetes mellitus, overweight/obesity, or metabolic dysregulation, MAFLD was identified [Citation3].
Figure 1. Flow chart for study population selection. *Complete transient elastography (FibroScan®) examination was defined as a fasting time of ≥3 h, ≥10 complete liver stiffness measures, and a liver stiffness interquartile range/median <30%.
The original NHANES study protocol was approved by the Ethics Review Board of the National Center for Health Statistics Research. The signed written informed consents were provided by all the enrolled individuals.
Measurement of serum vitamin D
In our present study, the total 25(OH)D in serum was set as the concentration of 25(OH)D2 plus 25(OH)D3. For measuring the 25(OH)D concentrations, the high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) was conducted by the National Center for Environmental Health. According to the 25(OH)D concentrations in serum, we separated the participants to two groups: the vitamin D deficiency group, whose participants had less than 50 nmol/L vitamin D in serum and the vitamin D sufficiency group, whose participants had no less than 50 nmol/L vitamin D in serum [Citation14,Citation18]. In addition, we also defined the quartile 1, 2, 3, and 4 of vitamin D in serum as 9.96–47.05 nmol/L, 47.05–65.00 nmol/L, 65.00–85.65 nmol/L, and 85.65–372.00 nmol/L, respectively. The vitamin D level quartile 1 was employed as the reference group.
Vibration controlled transient elastography (VCTE)
The examination of transient elastography was carried out to record the data and diagnose NAFLD and LF. A XL or M probe equipped FibroScan® model 502 V2 Touch (Echosens, Paris, France) was employed to conduct the elastography exam in the NHANES Mobile Examination Center. For each participant, no less than 10 measurements were obtained, and the values of median CAP and LSM were calculated by the device along with the interquartile range. Exams were considered complete if participants fasted at least 3 h before the exam, there were 10 or more complete liver stiffness measures, and the interquartile range/median of the LSM was < 30%. In this study, CAP value of 263 dB/m or higher was used to define NAFLD [Citation19,Citation20] and we also set the LSM ≥ 7.9 kPa as significant LF [Citation21].
Covariates
Based on the previous report [Citation22], the NAFLD-associated covariates, such as total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), estimated glomerular filtration rate (eGFR), diabetes, hypertension, body mass index (obesity: ≥30 kg/m2, overweight: 25–30 kg/m2, and under/normal weight: < 25 kg/m2), smoking status (never smoker, former smoker, or current smoker), education level (> high school or ≤ high school), race/ethnicity (Asian, Mexican-American, Non-Hispanic Black, Non-Hispanic White, and Other Races), sex, and age, were included. Dietary vitamin D intake and supplemental vitamin D intake were also adjusted to account for the potential impact of dietary factors. We computed eGFR using the Chronic Kidney Disease Epidemiology Collaborative equation [Citation23]. Diabetes was defined by the following criteria: no less than 6.5% haemoglobin A1c level, self-reported diagnosis of diabetes, or taking diabetic medications. The participants with more than 80 mmHg diastolic blood pressure, or more than 130 mmHg systolic blood pressure, or taking hypertension medications, or high blood pressure told by a doctor, were defined as hypertension [Citation24].
Statistical analysis
Continuous variables were expressed as mean ± SE and categorical variables were expressed as number and percentage. The two sample t-test and chi-square test were carried out for comparing the continuous and categorical variables between groups, respectively. For exploring the connections of vitamin D to NAFLD and LF, we conducted the multivariate logistic regression analyses. Three different logistic models, such as non-adjusted model 1, ethnicity-, sex-, and age-adjusted model 2, and TC-, HLD-C-, eGFR-, diabetes-, hypertension-, BMI group-, dietary vitamin D intake-, supplemental vitamin D intake-, smoking status-, education level-, and model 2 covariates-adjusted model 3, were employed in our present study. As suggested by NHANES, sample weights used for accounting for unequal selection probabilities and nonresponse were utilized in all analyses. Statistical analysis was conducted with R 4.2.0 software and the value of p less than 0.05 was considered statistically significant.
Results
Characteristics of study population
According to the exclusion criteria, we enrolled 3407 participants in this analysis. The mean age of our study population was 48.54 ± 0.79 years, and 47.78% of the participants were male. The weighted prevalence was 49.63% for NAFLD, and the weighted prevalence of significant fibrosis among NAFLD participants was 15.93%. Mean serum vitamin D level was 73.25 ± 1.66 nmol/L, with 21.25% of participants below 50 nmol/L. Mean dietary vitamin D intake and supplemental vitamin D intake were 4.24 ± 0.12 mcg/day and 21.82 ± 2.81 mcg/day, respectively. Based on the status of vitamin D, summarizes the clinical characteristics of participants. The vitamin D deficient participants were younger and had lower education level. They showed higher BMI and lower HDL-C levels. They also had less dietary and supplemental intakes of vitamin D. Between the participants with deficient and sufficient vitamin D, no significant difference in the prevalence of hypertension, diabetes, and NAFLD defined by CAP ≥ 263 dB/m were discovered. In comparison to vitamin D sufficient participants, high percentage of participants with LSM ≥ 7.9 kPa was observed in the vitamin D deficiency group.
Table 1. Characteristics of the study population according to vitamin D status.
Association of vitamin D level with NAFLD/MAFLD
In comparison to individuals without NAFLD, no significant difference in serum vitamin D was observed in NALFD participants (74.26 ± 1.30 vs. 72.24 ± 2.25 nmol/L; p = 0.21). After adjusting for TC, HDL-C, eGFR, diabetes, hypertension, BMI group, dietary vitamin D intake, supplemental vitamin D intake, smoking status, education level, race/ethnicity, sex, and age, no significant associations of vitamin D status or quartiles with NAFLD were observed in the multivariate logistic regression analysis. Among the enrolled 3407 subjects, 48.42% of the participants were MAFLD. Additionally, the correlation of vitamin D with MAFLD were similar to that of vitamin D with NAFLD ().
Table 2. Multivariate analysis for the association between vitamin D and NAFLD/MAFLD.
Association of vitamin D level with LF in NAFLD participants
Among the subset of people with NAFLD, significantly fibrotic participants showed low vitamin D concentrations in the serum (67.40 ± 2.97 vs. 73.15 ± 2.20, p = 0.01). For evaluating the adjusted connection of vitamin D level to LF, the multivariate logistic regression analysis was conducted. In the unadjusted model, vitamin D status (sufficiency vs. deficiency) was inversely correlated to LF (OR 0.56, 95%CI 0.41–0.78). And this inverse association was also present in adjusted model 2 (OR 0.49, 95%CI 0.33–0.72) and 3 (OR 0.56, 95%CI 0.38–0.83). When we evaluated vitamin D levels in quartiles, a dose-dependent relationship between LF and higher vitamin D levels was observed in comparison to those with lowest quartile after adjusting multiple confounding factors (Q2 vs. Q1, OR 0.65, 95%CI 0.37–1.14; Q3 vs. Q1, OR 0.64, 95%CI 0.41–1.00; Q4 vs. Q1, OR 0.49, 95%CI 0.30–0.79) ().
Table 3. Multivariate analysis for the association between vitamin D and liver fibrosis.
Discussion
Using a large general US population, our study found that serum vitamin D is not connected to the CAP-defined NAFLD, however, among NAFLD participants, low vitamin D represents high risk of developing significant LF.
The contradictory conclusions of the correlation between NAFLD and vitamin D have been obtained from several studies [Citation16,Citation25–29]. According to the data from NHANES III, previous studies concluded that relatively low vitamin D in serum is significantly connected to elevated NAFLD [Citation25] or MAFLD risk [Citation26]. In their studies, hepatic steatosis was confirmed by ultrasonography, and their mean vitamin D levels were lower than the levels of our study. Similar to our result, using the data from the Korea NHANES, researchers also observed similar vitamin D concentrations in serum from 409 NAFLD subjects and 1403 control subjects without NAFLD [Citation27]. Similarly, no significant association of the reduced vitamin D with the hepatic steatosis was found in general Portuguese population [Citation28]. Apart from conventional case-control and cross-sectional studies, other researchers conducted bidirectional Mendelian randomization study, which used genetic variants in non-experimental data to make causal inferences and could avoid problems in conventional epidemiological studies such as reverse causation and residual confounding. Using three European descent populations, the researchers observed an association of genetically predicted higher vitamin D levels with the reduced NAFLD risk [Citation29]. However, another Mendelian randomization study using 4568 healthy controls and 4614 NAFLD subjects from east Chinese population found that the four genetic variants-instrumented vitamin D is not associated with the NAFLD risk [Citation16]. In an animal study, deficient vitamin D could even cause alleviation of hepatic lipid accumulation induced by high-fat diet through inhibiting hepatic PPARγ and up-regulating carnitine palmitoyltrans 2, the major enzyme for fatty acid β-oxidation [Citation30].
As a hallmark characteristic of liver injury, the advanced LF can comprise severe risks for development of hepatocellular carcinoma and hepatic failure. In consistent with our current conclusion, the negative correlation of vitamin D with LF determined by NAFLD fibrosis score was observed by other researchers [Citation18,Citation31]. In the biopsy-proven NAFLD patients, Arai et al. also demonstrated an inversely association of advanced LF with vitamin D [Citation32]. However, a study based on NHANES III revealed that advanced fibrosis identified by non-invasive scores are not connected to the low 25(OH)D in serum [Citation26]. The inconsistent conclusions may result from the different measurements of LF and various vitamin D concentrations.
Currently, why vitamin D could effectively suppress the LF in NAFLD patients remains unclear. During the development of LF, hepatic stellate cell activation plays important effect. Activated hepatic stellate cells can effectively induce cell growth and transformation from a quiescent vitamin A-storing cell into an activated myofibroblast-like cell, increasing the production of extracellular matrix and tissue inhibitors of metalloproteinases [Citation33]. Through inhibiting the activation of TGF-β/Smad signalling pathway in hepatic stellate cells, vitamin D can effectively impair the fibrosis of liver [Citation34]. A recent study further indicated that vitamin D reduced hepatic stellate cells and TGF-β/Smad signalling activation through negative regulation of histidine-rich calcium binding protein [Citation35].
No effective treatment strategies for hepatic fibrosis are available so far. Based on previous research, we believe that the supplementation of vitamin D may be used as new widely available and cost-effective treatment for LF prevention and therapy. A randomized double-blind placebo-controlled trial, including 311 NAFLD patients, found that 12-month usage of vitamin D (1000 IU/day) can significantly attenuate the transient elastography indices of liver steatosis and fibrosis [Citation36]. Another trial failed to show an effect of vitamin D on the hepatic fat fraction measured by magnetic resonance and on biochemical markers of fibrosis after 6 months treatment of type 2 diabetes patients with NAFLD [Citation37]. Nevertheless, for complete revealing the regulation of vitamin D in the fibrogenesis of liver and assessing the efficiency and safety of vitamin D for LF treatment in NAFLD patients, further trials with longer term are warranted.
The strength of this study is that a nationally representative data is included. Using this dataset, we could analyse the data from healthy individuals and are not restricted to certain kinds of patients such as diabetes. However, there are also some limitations present in our study. First, due to the cross-sectional design, the verification of causality was limited, and the impact of vitamin D over time was not evaluated. Second, there are no consensual cut-offs for CAP and LSM. Third, the vitamin D seasonal variations, as well as the usage of supplement containing calcium were not investigated in this study.
Conclusions
In summary, based on a large general US population, this study demonstrated that the CAP-defined NAFLD is not associated with the deficiency of vitamin D. On the other hand, low serum vitamin D was closely connected to increased LF risk among NAFLD participants. Thus, the role of vitamin D in prevention and treatment of NAFLD warrants further research.
Ethical approval
The study was approved by the Ethics Review Board of the National Center for Health Statistics Research and written informed consents were obtained from each participant.
Author contributions
LLH: conception and design of the study. YJ, CBW, WG and LLH: acquisition, analysis and interpretation of the data. YJ and CBW: the drafting of the paper. LLH and WG: revising it critically for intellectual content. All authors read and approved the final version of the manuscript. And that all authors agreed to be accountable for all aspects of the work.
Acknowledgment
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The survey data are publicly available on the internet for data users and researchers throughout the world (https://www.cdc.gov/nchs/nhanes/).
Additional information
Funding
References
- Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1–7.
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1): S99–S112.
- Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750.
- Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730.
- Zhang X, Heredia NI, Balakrishnan M, et al. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: results from NHANES 2017-2018. PLoS One. 2021;16(6):e0252164.
- Goltzman D, Miao D, Panda DK, et al. Effects of calcium and of the vitamin D system on skeletal and calcium homeostasis: lessons from genetic models. J Steroid Biochem Mol Biol. 2004;89-90(1-5):485–489.
- El-Sharkawy A, Malki A. Vitamin D signaling in inflammation and cancer: molecular mechanisms and therapeutic implications. Molecules (Basel, Switzerland). 2020;25(14):3219.
- Komolmit P, Kimtrakool S, Suksawatamnuay S, et al. Vitamin D supplementation improves serum markers associated with hepatic fibrogenesis in chronic hepatitis C patients: a randomized, double-blind, placebo-controlled study. Sci Rep. 2017;7(1):8905.
- von Essen MR, Kongsbak M, Schjerling P, et al. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11(4):344–349.
- de la Guía-Galipienso F, Martínez-Ferran M, Vallecillo N, et al. Vitamin D and cardiovascular health. Clin Nutr. 2021;40(5):2946–2957.
- Barchetta I, De Bernardinis M, Capoccia D, et al. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS One. 2013;8(7):e68689.
- Abouzid M, Główka AK, Karaźniewicz-Łada M. Trend research of vitamin D receptor: bibliometric analysis. Health Informatics J. 2021;27(4):14604582211043158.
- Heo NJ, Park HE, Yoon JW, et al. The association between vitamin D and nonalcoholic fatty liver disease assessed by controlled attenuation parameter. JCM. 2021;10(12):2611.
- Lee HK, Shin SR, Han AL. Metabolic dysfunction associated fatty liver disease (MAFLD) and serum vitamin D concentration in South Korea. Asia Pac J Clin Nutr. 2022;31(2):201–207.
- Wang N, Chen C, Zhao L, et al. Vitamin D and nonalcoholic fatty liver disease: bi-directional mendelian randomization analysis. EBioMedicine. 2018;28:187–193.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–357.
- Kim HS, Rotundo L, Kothari N, et al. Vitamin D is associated with severity and mortality of non-alcoholic fatty liver disease: a US population-based study. J Clin Transl Hepatol. 2017;5(3):185–192.
- Petroff D, Blank V, Newsome PN, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(3):185–198.
- Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-Controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156–163 e2.
- Tao MH, Fulda KG. Association of magnesium intake with liver fibrosis among adults in the United States. Nutrients. 2021;13(1):142.
- Liu X, Shen H, Chen M, et al. Clinical relevance of vitamins and carotenoids with liver steatosis and fibrosis detected by transient elastography in adults. Front Nutr. 2021;8:760985.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):2199–2269.
- Liu S, Liu Y, Wan B, et al. Association between vitamin D status and Non-Alcoholic fatty liver disease: a Population-Based study. J Nutr Sci Vitaminol (Tokyo). 2019;65(4):303–308.
- Wan B, Gao Y, Zheng Y, et al. Association between serum 25-hydroxy vitamin D level and metabolic associated fatty liver disease (MAFLD)-a population-based study. Endocr J. 2021;68(6):631–637.
- Ha Y, Hwang SG, Rim KS. The association between vitamin D insufficiency and nonalcoholic fatty liver disease: a Population-Based study. Nutrients. 2017;9(8):806.
- Leitão J, Carvalhana S, Silva AP, et al. No evidence for lower levels of serum vitamin D in the presence of hepatic steatosis. A study on the portuguese general population. Int J Med Sci. 2018;15(14):1778–1786.
- Yuan S, Larsson SC. Inverse association between serum 25-Hydroxyvitamin D and nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol.. 2023;21(2):398–405.e4.
- Liu XJ, Wang BW, Zhang C, et al. Vitamin d deficiency attenuates high-fat diet-induced hyperinsulinemia and hepatic lipid accumulation in male mice. Endocrinology. 2015;156(6):2103–2113.
- Yang BB, Chen YH, Zhang C, et al. Low vitamin D status is associated with advanced liver fibrosis in patients with nonalcoholic fatty liver disease. Endocrine. 2017;55(2):582–590.
- Arai T, Atsukawa M, Tsubota A, et al. Association of vitamin D levels and vitamin D-related gene polymorphisms with liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Dig Liver Dis. 2019;51(7):1036–1042.
- Udomsinprasert W, Jittikoon J. Vitamin D and liver fibrosis: molecular mechanisms and clinical studies. Biomed Pharmacother. 2019;109:1351–1360.
- Beilfuss A, Sowa JP, Sydor S, et al. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut. 2015;64(5):791–799.
- Lu W, Li X, Liu N, et al. Vitamin D alleviates liver fibrosis by inhibiting histidine-rich calcium binding protein (HRC). Chem Biol Interact. 2021;334:109355.
- Lukenda Zanko V, Domislovic V, Trkulja V, et al. Vitamin D for treatment of non-alcoholic fatty liver disease detected by transient elastography: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(11):2097–2106.
- Barchetta I, Del Ben M, Angelico F, et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. BMC Med. 2016;14:92.
