Abstract
Background
The global prevalence of nonalcoholic steatohepatitis (NASH) is rising. Despite this, NASH is underdiagnosed and does not yet have approved pharmacological treatments. We sought to understand the path to diagnosis, patient interactions with healthcare professionals, treatment regimens, and disease management for patients with NASH.
Methods
Cross-sectional online surveys of patients with a self-reported diagnosis of NASH and healthcare professionals treating patients with NASH were conducted from 10th November 2020, to 1st January 2021. This manuscript focuses on responses from 152 patients with NASH and 101 primary care physicians (PCPs).
Results
Patients (n = 152, mean age = 40, SD = 11) and healthcare professionals (n = 226) were located throughout the US. In the most common patient journey, 72% of patients had initial discussions about symptoms with a PCP but only 30% report receiving their NASH diagnosis from a PCP. Almost half of PCPs (47%) were not aware of any clinical practice guidelines for diagnosis and management of NASH. For ongoing management of NASH, PCPs most frequently prescribed lifestyle changes such as exercise (89%), lifestyle changes focused on diet (79%), and/or metformin (57%). Other healthcare professionals rarely referred patients to PCPs for treatment, but when they did, the primary reasons were patients struggling with lifestyle modifications (58%), needing to lose weight (46%), and needing treatment of comorbidities (42%).
Conclusions
PCPs may benefit from greater awareness of NASH and guidelines for its diagnosis and treatment. Given the absence of pharmacological treatments approved for NASH, PCPs can offer support in obesity management, comorbidity management, and risk stratification for liver disease progression.
PLAIN LANGUAGE SUMMARY
Nonalcoholic steatohepatitis (NASH) is a form of nonalcoholic fatty liver disease (NAFLD) with a higher risk of more severe liver disease. Patients with NASH have too much fat deposited in their liver with associated liver inflammation, scarring, and, in some patients, liver failure. Patients with NASH may not experience symptoms until their disease reaches a dangerous point. We wanted to understand how patients with NASH are diagnosed, how they interact with doctors, and how doctors manage their disease. We surveyed 101 primary care doctors and 152 patients with NASH to ask them about their experiences with NASH. Most patients (72%) report having initial discussions about potential NASH symptoms with a primary care doctor, but only 30% receive their NASH diagnosis from a primary care doctor. Almost half of primary care doctors were not aware of guidelines for the diagnosis and management of NASH. To manage patients’ NASH, most primary care doctors prescribed lifestyle changes such as exercise (89%), lifestyle changes focused on diet (79%), or metformin (57%). Other types of doctors rarely referred their patients with NASH to primary care doctors for treatment; when they did the main reasons were that their patients were struggling with lifestyle modifications (58%), needed to lose weight (46%), or needed treatment of one of their other conditions (42%). In conclusion, primary care doctors may benefit from greater awareness of guidelines for the diagnosis and treatment of NASH. Primary care doctors can play an important role in supporting patients with lifestyle change and management of patients’ other conditions that may be related to their NASH.
Primary care physicians (PCPs) are the most common initial touchpoint for patients with NASH.
PCPs lack awareness of guidelines for the diagnosis and treatment of NASH.
Other physicians believe that PCPs can help patients with lifestyle changes, weight loss, and management of comorbidities.
Key messages
Background
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis. Patients are diagnosed with NAFLD when secondary causes are not apparent (e.g. excessive alcohol consumption, medications, Wilson disease, or disorders of lipid metabolism) [Citation1]. Some patients with NAFLD progress to nonalcoholic steatohepatitis (NASH), the more severe, progressive form of NAFLD. NASH (defined by >5% hepatic steatosis, the presence of inflammation, and hepatocellular injury) increases risk of hepatocellular carcinomas (HCC) and can progress to cirrhosis, and the need for liver transplant [Citation1].
NAFLD is the most common cause of liver disease globally with global prevalence estimated between 25-38% [Citation1–4]. In 2015, approximately 20% of NAFLD cases were classified as NASH, estimated to rise to 27% by 2030 [Citation5]. Global prevalence of NASH is estimated at 2–6% [Citation6]. NASH is the leading cause of liver transplantation for women and those older than 65, the second leading cause for men, and is expected to become the overall leading cause of liver transplantation in the United States [Citation7].
NASH is often characterized as an asymptomatic condition; however, some patients experience non-specific symptoms such as abdominal pain and fatigue. NAFLD diagnosis requires radiographic or histologic identification of >5% hepatic steatosis in the absence of excessive alcohol consumption. Per current American Association for the Study of Liver Diseases’ (AASLD) guidelines, a definitive diagnosis of NASH requires a liver biopsy with histologic analysis identifying hepatocyte ballooning degeneration, hepatic lobular inflammation, and >5% hepatic steatosis [Citation8]. However, liver biopsies are painful, expensive, and can cause serious complications [Citation9]. The European Association of the Liver (EASL) recently updated their clinical practice guidelines (CPGs) prioritizing non-invasive tests (NITs) for the evaluation of liver disease severity and prognosis [Citation10].
The objective of NASH treatment is to delay, halt, or reverse progression of liver disease. However, there are currently no Food and Drug Administration (FDA) approved pharmacological treatments for NASH. Consequently, treatment focuses on managing related comorbidities. Lifestyle therapy is recommended and changes resulting in weight loss can be very effective, with higher degrees of weight loss being associated with greater improvement in NASH and fibrosis [Citation11]. Among patients with NASH who lost ≥10% body weight, 90% had resolution of NASH and 45% had regression of fibrosis, though the majority of patients in that study did not have advanced fibrosis [Citation11]. However, clinical trials set in tertiary centers, community-based interventions and internet-based interventions show that most patients struggle to attain the goal of ≥10% body weight loss and may require additional support [Citation12,Citation13].
There is a lack of scientific literature on the experiences of, and interactions between, patients and healthcare professionals related to NASH diagnosis and management. Several studies have investigated health-related quality of life in patients with NASH [Citation14,Citation15]. Despite the perception of NASH as a mostly asymptomatic condition, patients with NASH and advanced fibrosis were found to have worse physical health-related scores than matched patients with chronic hepatitis C [Citation14]. A recent study investigated differences in NASH management from the perspective of clinicians, but did not capture patient experiences [Citation16].
This research seeks to map the medical journeys of patients with NASH, the role of primary care physicians (PCPs) in diagnosis and treatment of NASH, and gaps in diagnosis and treatment of NASH. Understanding how patients with NASH interact with healthcare professionals can highlight delayed or inadequate diagnosis and treatment. Importantly, it also helps focus interventions to disseminate best practice via emerging guidelines and educational activities such as targeted reviews in PCP-facing journals and educational endeavors.
Materials and methods
Study design and participants
A cross-sectional quantitative study using an anonymous national online survey was conducted among patients with a self-reported diagnosis of NASH and healthcare professionals treating patients with NASH in the US. Email survey responses were gathered from 10th November 2020, to 1st January 2021. Respondents were recruited from online survey panel companies. Potential participants had given their permission to be contacted by the survey panel companies to participate in relevant surveys, which would be used in research. Eligible participants completing the entire survey received a modest monetary incentive.
The surveys (See Supplementary Additional file Citation1: HCP Survey and Supplementary Additional file Citation2: Patient Survey, which demonstrate the surveys used) were developed by the research team involved in the project, based on an extensive literature review and qualitative interviews with patients and healthcare professionals. The surveys were not adapted from any previously published sources and were not validated, as they were intended to be descriptive in nature. Separate surveys were used for each audience to measure attitudes and experiences with NASH prior to diagnosis; experience with the diagnosis process; management and treatment of NASH; NASH management guidelines; attitudes toward obesity and its management; and to identify informational sources used to educate patients about NASH and obesity. The quantitative surveys consisted of a variety of yes/no, multiple-choice, and 5–7-point Likert-scale questions (i.e. respondents could select the degree to which they agreed with a statement on a 5-point scale where 1 = ‘strongly disagree’ and 5 = ‘strongly agree’), which were not validated. Patients and healthcare professionals surveyed were not matched pairs.
The survey was conducted in accordance with the principles and guidelines established by the Office for Human Research Protections and the Insights Association Code of Standards and Ethics. The study protocol was submitted to the Western Institutional Review Board for review and was determined to be exempt because the research includes survey procedures with adequate provisions to protect the privacy of participants and maintain data confidentiality. After reviewing information about the purpose and nature of the survey, respondents selected a yes/no option indicating consent to participate in the study prior to screening. Once consented, respondents continued to the survey, and could opt out at any time.
Patients included were US residents over the age of 18 who had a self-reported NASH diagnosis within the past 10 years, were currently seeing a healthcare professional to treat and manage NASH, and were aware of diagnostic screens having been completed. Healthcare professionals included were employed in US facilities (except Maine and Vermont to comply with Sunshine reporting requirements), were practicing PCPs, gastroenterologists, hepatologists, or endocrinologists. PCPs specialized in internal medicine, general practice, or family practice. PCPs were required to treat at least five patients with NASH in the past month while gastroenterologists, hepatologists, endocrinologists were required to treat at least 20 patients with NASH in the past month. Physicians required board certification or eligibility in their chosen specialty, 3–25 years in practice, and could not be based in a government facility or an ambulatory surgical center. Due to the prevalence of NASH, the lack of available analyses and knowing HCPs had to treat a minimum number of patients with NASH, a sample size of 150 was deemed to be broadly generalizable to patients diagnosed with NASH in the US, and a sample size of 225 was deemed to be sufficient for HCPs in the US who are knowledgeable about the condition. This was determined with an acceptable margin of error based on feasibility. In this paper we report results from the patient survey and PCP-focused results from the healthcare professional survey; hepatologist- and gastroenterologist-focused results from the healthcare professional survey have been previously reported [Citation17].
Statistical analyses
Descriptive statistical analyses (means, frequencies) and tests of differences (chi square, t-tests) within respondent types were performed using SPSS Statistics for Windows 23 (SPSS, Chicago, Illinois). Statistical significance was set at p < 0.05 using 2-tailed tests.
The final patient sample was weighted to representative racial demographic targets for the population of US adults with NASH, which was derived from published literature [Citation18]. All reported statistics are weighted accordingly except for demographic data, which are reported unweighted (). Healthcare professional data were not weighted.
Table 1. Sample characteristics, unadjusted.
Respondent data were collected through informed consent and protected through encryption and access controls to prevent unauthorized disclosure of confidential and sensitive information.
Access to data was tightly controlled with only highly trained and vetted personnel having access to it.
Results
Demographics
A total of 152 patients and 226 healthcare professionals (101 PCPs, 75 gastroenterologists/hepatologists, 50 endocrinologists) participated in the survey. Here we report responses from the patients and 101 PCP respondents. Mean patient age was 40 years with a majority of male respondents (62%) ().
PCPs estimated the proportion of their patients with NASH who suffered from a list of comorbidities and reported on average, obesity (58%), dyslipidemia (54%), hypertension (39%), hypertriglyceridemia (39%), and type 2 diabetes (35%) affected the highest proportion of patients seen by PCPs. This differs greatly from the comorbidities most commonly self-reported by patients which were anxiety (15%), depression (13%), NAFLD (12%) hypertension (11%), and type 2 diabetes (11%).
Pre-diagnosis
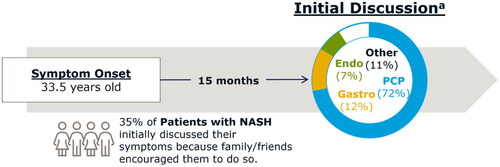
Most patients (83%) reported starting their medical journeys with a discussion of symptoms with a healthcare professional, followed by a diagnosis, and treatment; we refer to this as the most common patient journey when discussing this subset of patients. In the most common patient journey, 72% had their initial discussions with a PCP (). Among all patients, most (88%) reported experiencing non-specific symptoms prior to NASH diagnosis with the most common being fatigue (45%), nausea (43%), loss of appetite (41%), abdominal pain (40%), and swelling in the abdomen (36%). In the most common patient journey, mean age of symptom onset for patients was 33.5 years. Following symptom onset, patients took an average of 15 months to be evaluated by a healthcare professional. In the most common patient journey, 35% of patients reported initially discussing symptoms with a healthcare professional due to encouragement from friends and family. Of all patients surveyed, most were diagnosed at an appointment specifically to discuss their NASH symptoms (38%) or at an appointment made specifically to discuss formal testing for suspected NASH (22%), while 21% reported they were diagnosed at a well-visit or annual exam.
Figure 1. Most common patient journey (comprising 83% of patients). The most common patient journey involved patients discussing symptoms with a healthcare professional, followed by diagnosis and treatment. Most initial discussions (72%) were had with PCPs. Other professionals seen for initial discussions included gastroenterologists, endocrinologists, and others. Patients experienced symptom onset at an average of 33.5 years old, waited an average of 15 months to discuss symptoms with a healthcare professional, and 35% cited encouragement by family/friends as the reason for discussing their symptoms with a healthcare professional. aPercentages do not sum to 100% due to rounding. Endo: endocrinologist; Gastro: gastroenterologist; PCP: primary care physician.
Prior to diagnosis, patients reported seeing an average of 1.7 healthcare professionals for their NASH symptoms. Most saw a PCP (65%), with hepatologists (37%), gastroenterologists (35%) and endocrinologists (14%) seen less frequently prior to diagnosis.
Diagnosis
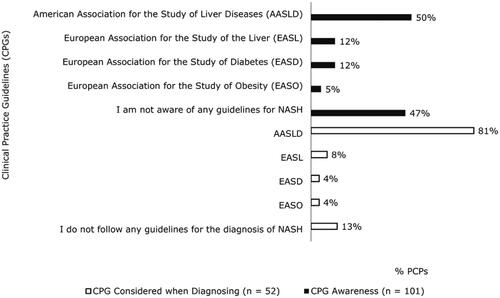
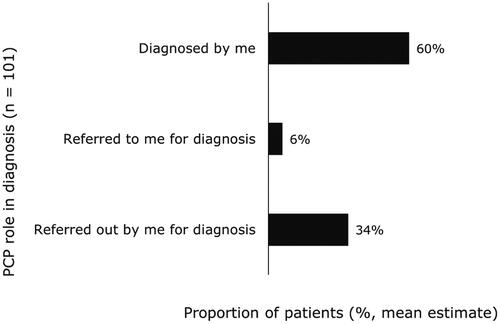
Almost half of PCPs (47%) reported they were not aware of any CPGs for the diagnosis, treatment, or management of NASH (). Half of PCPs (50%) said they were aware of AASLD NASH guidelines. Among the PCPs who reported they were aware of CPGs, 81% reported considering CPGs from AASLD when diagnosing patients with NASH. PCPs estimated they diagnose most patients themselves, while referring about one-third to other healthcare professionals for diagnosis (). However, only 21% of patients reported receiving their official NASH diagnosis from a PCP with most being diagnosed by a specialist, including hepatologists (37%) and gastroenterologists (26%). Of the PCPs who personally diagnose NASH, only 60% distinguish between NASH with or without fibrosis. PCPs were most likely to use liver chemistry tests (87%), ultrasounds (73%), and lipid levels (70%) to confirm a NASH diagnosis.
Management
Of the roughly half (53%) of PCPs who were aware of clinical guidelines for NASH, 81% followed AASLD guidelines for the treatment and management of NASH (). PCPs were asked to rate NASH treatment goals on a scale from 1 to 7 with 1 indicating a goal was ‘not at all important’ and 7 indicating a goal was ‘extremely important.’ The goals that were most frequently rated 6 or 7 by PCPs included delayed progression to cirrhosis (81%), delayed progression of fibrosis stage (80%), prevention of liver transplantation (79%), and weight loss (78%).
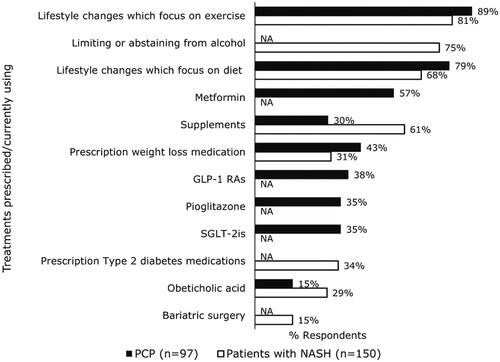
Healthcare professionals rarely referred patients to PCPs for NASH treatment (only 24 of 226 reported doing so), and when they did, the primary reasons for doing so were patients struggling with lifestyle modifications (58%), patients struggling with weight loss (46%), and patients needing treatment of comorbidities (42%). For ongoing management of NASH, PCPs most frequently prescribed lifestyle changes focused on exercise, lifestyle changes focused on diet, and/or metformin (). Patients reported that they were primarily currently using lifestyle changes focused on exercise, limiting alcohol, lifestyle changes focused on diet, and supplements like vitamin E or milk thistle; patients reported lower use of prescription type 2 diabetes medications, prescription weight loss medications, and obeticholic acid ().
Figure 4. PCP-reported treatments recommended for ongoing management of NASH and patient- reported treatments currently using. GLP-1 RAs: glucagon-like peptide-1 receptor agonist; NA: question was not asked of PCPs or patients, respectively; PCP: primary care physician; SGLT-2is: sodium-glucose cotransporter 2 inhibitors.
Most patients (70%) indicated that they had seen a PCP at some point for NASH, more than any other healthcare professional. More than half of patients (60%) selected PCPs as one of the two most influential healthcare professionals in the treatment and management of their NASH (hepatologists being the other). Almost one-third (30%) of patients think of their PCP as the primary coordinator of their NASH care. Patients think that PCPs are influential in their care because they provide trusted advice (68%), ongoing support (59%), and all possible treatment options (53%).
PCPs believed the primary reasons patients discontinued NASH treatment were the asymptomatic nature of the condition (63%), difficulty with lifestyle change adherence (54%), and/or an unwillingness to make lifestyle changes (48%). Almost all patients (89%) agree that their comorbidities have an impact on the severity of their NASH. Patients reported their primary motivators to manage NASH were living longer (55%) and improving their NASH (46%). Patients believed successful NASH management would involve improvement in symptoms (40%), reverting their NASH (38%), and/or resolving their NASH (32%).
Discussion
NASH is underdiagnosed [Citation19], and it is rarely accompanied by specific symptoms until patients develop cirrhosis or hepatic decompensation [Citation20]. We surveyed patients with NASH and healthcare professionals to understand the medical journeys of patients with NASH, the role of PCPs in diagnosis and treatment of NASH, and gaps in diagnosis and treatment of NASH. Despite the non-specific nature of NASH symptoms, our research shows that the path to diagnosis typically begins with patients approaching PCPs to discuss symptoms that they are experiencing. Although non-specific, the symptoms may prompt conversations about NAFLD and NASH, ultimately leading to further diagnostic tests. PCPs are uniquely positioned for early identification and treatment of patients at risk for NASH. PCPs should start conversations about NASH and its associated risks with at-risk patients, regardless of symptoms, as early diagnosis and intervention can impact overall outcomes.
Lifestyle intervention is the cornerstone of NASH treatment across the disease spectrum. NASH is a progressive disease without current FDA approved pharmacological treatments, therefore, halting or delaying progression are the primary goals of NASH management. Early diagnosis and intervention could help halt or delay NASH progression. In our study, almost half of PCPs were not aware of any guidelines for the diagnosis and management of NASH. PCPs may benefit from increased awareness of guidelines on identifying patients at risk for NASH in the primary care setting, like those with obesity and adiposity-based chronic diseases. Recently published clinical practice guidelines from AACE and AASLD on the evaluation and treatment of NAFLD offer guidance on the use of NITs in NASH, which includes a new algorithm to evaluate liver disease severity and prognosis [Citation10]. The EASL recommends the use of conventional ultrasound as a first-line tool for diagnosing steatosis because it is widely available, safe, and inexpensive. However, conventional ultrasound has reduced accuracy in patients with obesity [Citation21] and can only detect steatosis >12.5–20% [Citation22]. Recently updated (AGA) guidance has also emphasized the use of NITs in a risk stratification algorithm for patients suspected of having NAFLD/NASH [Citation23].
PCPs in the US face many challenges to improving the diagnosis and treatment of patients with NASH. Access to screening technologies limit PCPs in diagnosing patients with NASH. Evaluating the extent of liver fibrosis is important because it is strongly associated with risk of patient morbidity and mortality [Citation24,Citation25]. The current gold-standard for evaluation of liver fibrosis is a liver biopsy, but biopsies carry risk of complications and have limitations (e.g. only a small portion of the liver is sampled) [Citation1,Citation26]. Current and emerging guidance is better informing PCPs on the use of NITs to better identify which of their patients are low risk and can be managed in primary care, and which should be referred for more specialized testing and intervention [Citation1,Citation27–29]. Non-invasive methods for evaluating liver fibrosis and steatosis are improving and will reduce the reliance on biopsies for the identification of patients with more progressive disease. It is hoped that broad access to NITs and following improved guidance from AGA and EASL will rule out advanced fibrosis in primary care settings and improve diagnostic outcomes for patients with NASH.
Until there are FDA approved pharmacologic therapies for NASH, patient care needs to focus on a multidisciplinary approach to managing comorbidities and lifestyle changes [Citation30]. Our research shows that PCPs are viewed as helpful in managing lifestyle changes related to diet and exercise by patients and by the few healthcare professionals that reported referring patients with NASH to PCPs. Perceptions of the level of difficulty or unwillingness of patients to adhere to lifestyle changes to manage NASH were previously discussed in a parallel manuscript describing the medical journey of patients with NASH from the perspectives of hepatologists and gastroenterologists [Citation17].
Studies show that lifestyle-, drug-, or surgically-induced weight loss can improve NASH [Citation11,Citation31–33]. Similarly, effectively managing T2DM can lead to liver fibrosis improvement. A study involving serial liver biopsies of Japanese patients with NAFLD found that improvement of liver fibrosis was significantly associated with use of insulin and decrease in HbA1C levels [Citation34]. Many patients with NASH also have hypertension. Several studies found that patients with both NASH and hypertension saw improvement in steatosis, inflammation, and fibrosis when treated with angiotensin II receptor antagonists, but further work is needed [Citation35–37]. Glucagon-like peptide-1 (GLP-1) analogs are indicated for T2DM and obesity have been shown to lead to high rates of NASH resolution compared to placebo in Phase 2 trials [Citation38,Citation39]. These results suggest that control of comorbidities can improve NASH and PCPs are best suited to help patients with management of their comorbidities.
This research relies on self-reported PCP and patient experiences. Some results may reflect inaccurate recall on the part of survey participants [Citation40]. The groups of healthcare professionals and patients are independent from one another, the patients surveyed were not matched with the healthcare professionals surveyed. Additionally, the survey was not tested or validated. Some disagreement between answers provided by healthcare professionals and patients (e.g. who officially diagnosed a patient with NASH) may reflect real differences rather than differences in perception. NASH is underdiagnosed, and most PCPs do not perform the type of invasive testing needed to confirm the diagnosis of NASH. Further, NASH is also largely asymptomatic with many patients being unaware they have it, but all patients in our survey were aware of their symptoms and able to recall details of their NASH diagnosis. Patient respondents may therefore represent a particularly well-informed and engaged subset of patients with NASH; thus, we must be cautious in generalizing the experiences of these surveyed patients to the wider population of patients with NASH.
Conclusions
This paper identifies PCPs as the initial medical touchpoint for patients with NASH and emphasizes the importance of increasing disease awareness and management among PCPs. Current treatments for NASH involve managing comorbidities which benefit from a good healthcare professional-patient relationship and frequent follow-up appointments. PCPs are well positioned to manage the comorbidities of patients and can work to achieve improvements in management of obesity and adiposity-based chronic diseases including T2DM, hypertension and dyslipidemia. However, more work needs to be done in disseminating best practice recommendations to PCPs and providing the necessary infrastructure to make the wholistic care of patients with NAFLD more manageable.
Previous presentation and publication
Portions of this data were presented as a poster at the North American Primary Care Research Group (NAPCRG) annual meeting from November 19-23, 2021. The abstract of this presentation was published in The Annals of Family Medicine as a supplement from the conference, [April 2022, 20 (Supplement 1) 2649].
A parallel manuscript entitled ‘Nonalcoholic steatohepatitis medical patient journey from the perspective of hepatologists, gastroenterologists and patients: a cross-sectional survey’ was published in BMC Gastroenterology (2022)22:335. The article focused on how hepatologists and gastroenterologists can leverage their roles as coordinators of care for patients with NASH.
Ethical approval and consent to participate
The study was reviewed by the Western Institutional Review Board and was determined to qualify for exempt status due to the anonymous nature of the survey. The study was conducted in accordance with all relevant guidelines and regulations. Respondents reviewed information about the purpose and nature of the survey. Respondents selected a yes/no option indicating informed consent to participate in the study prior to entering the screening portion of the survey. If they consented, they continued to the survey questions. Respondents could discontinue the survey at any time.
Consent for publication
Not applicable.
Authors’ contributions
DRC, KN, and MR interpreted data, critically reviewed and edited the manuscript for important intellectual content and approved the final version to be published.
AA, JS, and TF contributed to the conception and design of the work, analysis and interpretation of data, critical revisions of manuscript drafts, and approved the final version of the manuscript.
Dissclosure statement
AA, TF, and JS are employees and shareholders of Novo Nordisk, Inc., which funded this research. DRC is employed by the Global Liver Institute, which has received grants and sponsorships from several companies in the NASH therapeutic space (990 publicly available). KN has no relevant financial conflicts of interest. MR has received consulting fees from 89Bio, Alnylam, Amgen, AMRA, BMS, Boehringer Ingelheim, Centara, Coherus, Cymabay, Fractyl, Galecto, Gelesis, Genfit, Gilead, Lipocine, Madrigal, Metacrine, Novartis, Novo Nordisk, Pfizer, Rivus, Sagimet, Siemens, Terns, Thetis, and Viking. MR has served on advisory committees for Enanta, Intercept, and NGM Bio.
Supplemental Material
Download PDF (399 KB)Supplemental Material
Download PDF (350.9 KB)Acknowledgements
The authors thank John Newman, PhD and Elizabeth Tanner, PhD of KJT Group, Inc., Rochester, NY for providing medical writing support, which was funded by Novo Nordisk, Inc., Plainsboro, NJ in accordance with Good Publication Practice (GPP3) guidelines.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the corresponding author, KN.
The datasets analyzed during the current study are not publicly available due to the presence of proprietary information, but data are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Sheka AC, Adeyi O, Thompson J, et al. Nonalcoholic steatohepatitis: a review. Jama. 2020;323(12):1–10.
- Perumpail BJ, Khan MA, Yoo ER, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–8276.
- Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13(12):2062–2070.
- Younossi ZM, Paik JM, Al Shabeeb R, et al. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology. 2022;76(5):1423–1437.
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133.
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
- Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113(11):1649–1659.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–357.
- Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(2):475–485 (2219-2840 (Electronic)).
- Berzigotti A, Tsochatzis E, Boursier J, et al. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75(3):659–689.
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. e5; quiz e14–5.
- Wong VW-S, Chan RS-M, Wong GL-H, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59(3):536–542.
- Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155–1163.
- Younossi ZM, Stepanova M, Lawitz EJ, et al. Patients with nonalcoholic steatohepatitis experience severe impairment of health-related quality of life. Am J Gastroenterol. 2019;114(10):1636–1641.
- McSweeney L, Breckons M, Fattakhova G, et al. Health-related quality of life and patient-reported outcome measures in NASH-related cirrhosis. JHEP Rep. 2020;2(3):100099.
- Porayko MK, Articolo A, Cerenzia W, et al. Differences in NAFLD/NASH management by provider specialty: opportunities for optimizing multidisciplinary care. J Multidiscip Healthc. 2022;15:1533–1545.
- Rinella M, Cryer DR, Articolo A, et al. Nonalcoholic steatohepatitis medical patient journey from the perspective of hepatologists, gastroenterologists and patients: a cross-sectional survey. BMC Gastroenterol. 2022;22(1):335.
- Rich NE, Oji S, Mufti AR, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198–210.e2.
- Rinella ME, Lominadze Z, Loomba R, et al. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4–12.
- Povsic M, Wong OY, Perry R, et al. A structured literature review of the epidemiology and disease burden of non-alcoholic steatohepatitis (NASH). Adv Ther. 2019;36(7):1574–1594.
- de Moura Almeida A, Cotrim HP, Barbosa DBV, et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008;14(9):1415–1418.
- Bril F, Ortiz-Lopez C, Lomonaco R, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35(9):2139–2146.
- Kanwal F, Shubrook JH, Younossi Z, et al. Preparing for the NASH epidemic: a call to action. Gastroenterology. 2021;161(3):1030–1042.e8.
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565.
- Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443–457.e17.
- Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73(6):1322–1332.
- Pandyarajan V, Gish RG, Alkhouri N, et al. Screening for nonalcoholic fatty liver disease in the primary care clinic. Gastroenterol Hepatol . 2019;15(7):357–365.
- Blonde L, Umpierrez GE, Reddy SS, et al. American association of clinical endocrinology clinical practice guideline: developing a diabetes mellitus comprehensive care plan—2022 update. Endocr Pract. 2022;28(10):923–1049.
- Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161(5):1657–1669.
- Hardy T, Anstee QM, Day CP. Nonalcoholic fatty liver disease: new treatments. Curr Opin Gastroenterol. 2015;31(3):175–183.
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129.
- Glass LM, Dickson RC, Anderson JC, et al. Total body weight loss of ≥ 10% is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60(4):1024–1030.
- Lassailly G, Caiazzo R, Ntandja-Wandji LC, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290–1301.e5.
- Hamaguchi E, Takamura T, Sakurai M, et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care. 2010;33(2):284–286.
- Georgescu EF, Ionescu R, Niculescu M, et al. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15(8):942–954.
- Yokohama S, Yoneda M, Haneda M, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40(5):1222–1225.
- Alam S, Kabir J, Mustafa G, et al. Effect of telmisartan on histological activity and fibrosis of non-alcoholic steatohepatitis: a 1-year randomized control trial. Saudi J Gastroenterol. 2016;22(1):69–76.
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690.
- Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124.
- Short ME, Goetzel RZ, Pei X, et al. How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51(7):786–796.