Abstract
Background/Aims
Pancreatic fluid collections (PFC) are debris or fluid of the pancreas that needs to be drained out. This may result from surgery or necrotizing pancreatitis. This meta-analysis compared the outcomes of PFC through endoscopic and percutaneous interventions.
Methods
A medical database was searched up to June 2022, comparing the outcomes of endoscopic drainage (ED) and percutaneous drainage (PD) for the PFC. Eligible studies reporting clinical and technical success and adverse events were selected.
Results
Seventeen studies with 1170 patients were included for meta-analysis, of which 543 patients underwent ED and 627 underwent PD. The odd ratio (OR) of technical success was 0.81 (95% confidence interval (CI) 0.31, 2.1) and clinical success was in the favor of the ED group at OR 2.23 (95% CI 1.45, 3.41). Adverse events OR 0.62 (95% CI 0.27, 1.39) and stent migration OR 0.61 (95% CI 0.10, 3.88) were the same in both groups, but hospital stay pooled mean difference of 15.02 days (95% CI 9.86, 20.18), mortality OR 0.24 (95% CI 0.09, 0.67), and re-interventions OR 0.25 (95% CI 0.16, 0.40) favored ED.
Conclusions
ED is safe and efficient for PFC with higher clinical success, lower mortality rate, hospital stay, and re-interventions compared with PD.
1. Introduction
Pancreatic fluid collections (PFCs) are accumulations of debris or fluid from the pancreas that are encircled by a wall of granulation tissue. They can develop as a result of acute pancreatitis, surgery of the pancreas, abdominal trauma, or chronic obstructive disease of the pancreatic duct [Citation1–3]. According to the recent Atlanta classification [Citation1], there are four unique forms of pancreatic fluid collections, which can be split down as follows: The collections that last for more than four weeks are referred to as pancreatic pseudocysts, while the collections that last for less than four weeks are referred to as acute peripancreatic collections (APC). On the other hand, collections that occur in the context of pancreatic necrosis and last for less than four weeks or more than four weeks are referred to as acute necrotic collections (ANC) and walled-off necrosis (WON), respectively. Patients who have symptomatic, mature PFCs that are transitioning to infectious PFCs or who have maintained a rise in size throughout follow-up should be evaluated for possible intervention therapy. Patients whose PFCs have continued to grow in size throughout the follow-up should also be evaluated. This is because these patients have a higher risk of encountering complications overall [Citation4–6]. Pain, gastrointestinal obstruction, fistulas, and even septic shock might result from some PFCs, whereas others do not cause any symptoms at all. Long-term feeding through the jejunum, total parenteral nutrition, and antibiotics are all possible conservative therapeutic options for patients with PFCs. However, some PFCs may necessitate extra therapies. For example, ERCP with trans-papillary stent insertion, endoscopic ultrasound-guided drainage (EUSD), surgery for cysto-gastrostomy with or without debridement, or percutaneous drainage (PD) is some of the procedures that can help.
Surgery is only performed as a last option when other treatments have failed or when severe issues arise [Citation7,Citation8]. Symptomatic PFCs can be drained percutaneously under local anaesthesia, but an external catheter can decrease quality of life (QOL). Percutaneous catheters require frequent fluid output monitoring, continual flushing to maintain patency, interval catheter changes, skin problems, and visiting nursing services [Citation9–11]. Endoscopic treatment reduces fluid and electrolyte loss and reduces the need for a pancreatic fistula and external drainage catheter.
Endoscopic drainage and percutaneous drainage have not yet been shown to be better for patients with PFCs in terms of how well they work or how safe they are. Studies that compare these two interventions often have small sample sizes, which makes it hard to conclude. Our meta-analysis of the relevant literature was performed to find out whether endoscopic drainage (ED) or percutaneous drainage (PD) was better for PFCs.
2. Materials and methods
This study followed the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [Citation12].
2.1. Search strategy
To find relevant papers, electronic databases were searched such as PubMed, MEDLINE, Web of Science, and the Cochran Library. The publishing period of the search strategy was limited to June 2022, and only available in English. Mesh keywords such as ‘percutaneous’, ‘endoscopic’, ‘endoscopic-ultrasound-guided’, ‘pancreatic drainage’, ‘pancreatic walled-off necrocisis’, ‘pancreatic pseudocysts’, ‘pancreatic fluid collection’, and ‘acute necrotic collection’ and their different combinations were used for the search. All results were inspected, and duplicates were eliminated.
2.2. Studies selection
Studies were included or excluded according to the following criteria.
2.2.1. Studies selection criteria
This analysis included studies that met the following criteria:
All of the patients in the study have pancreatic fluid or debris collections.
All kinds of studies such as; randomized control trials, cohort studies, and retrospective studies (observational studies) were included.
Studies comparing the outcomes of ED and PD modes for PFCs.
Studies only on adult patients (More than 18 years old).
Human studies in English.
2.2.2. Studies exclusion criteria
Studies comparing outcomes other than ED and PD.
Duplicates, case studies, review articles, abstracts, and letters were excluded.
Studies focusing solely on ED or PD outcomes.
Studies that do not provide adequate results or miss our primary outcome.
Animal research or publications in other languages.
2.3. Data extraction
Two authors extracted data from the studies that were selected. Specifically, collected information from each study about the following factors: publication year, country, design of the study, overall number of patients, mean age of the patients, sex proportion of patient populations, number of patients who had undergone ED or PD, clinical success, technical success, 30 days morality, stent migration, type of fluid, re-interventions, hospital stay, and adverse events. Different coefficients were converted into the same units of measurement. Each study was subdivided into two groups: the ED group and the PD group.
2.4. Quality assessment of studies
The technique for assessing the risk of bias published by the Cochrane Collaboration was used in this study [Citation13]. It received a grade of ‘low’ when there was a low risk of bias, a grade of ‘high’ when there was a high risk of bias and a grade of ‘some concerns’ when the information offered was insufficient to make a risk of bias judgment [Citation14]. When analysing research, a variety of aspects were taken into account, including missing outcome data, timeliness of participant identification or recruitment, measurement of outcome, deviations from the intended treatments, and selection of the reported outcomes (Supplementary risk of bias table).
2.5. Outcome and definitions
Our primary outcomes were a clinical success (defined as improvement in the clinical condition of the patients with resolution of sepsis after AP and no need for any kind of surgical intervention being necessary) and technical success (defined as success in stenting and catheterizing PFCs and the capacity to drain them out). Secondary outcomes include adverse events (defined as any type of procedure-related complication occurring during the follow-up period), stent migration (defined as stent occlusion or migration), hospital stay (duration of hospital stay after receiving treatment), and 30-day mortality (number of patients dying within a specific 30-day period). In sub-group analysis, studies were divided into two groups: the POPFC group (post-operative pancreatic fluid collection patients) and the WON group (walled-off necrosis and pancreatic pseudocysts patients).
2.6. Publication bias and study effect
A funnel plot of the results was used for the estimation of the potential for publication bias in this meta-analysis. To figure out how each study affected the overall result, each one was removed one at a time.
2.7. Statistical analysis
This study used the Cochrane Review Manager Software (version 5.4.1) to calculate odds ratios (ORs) for outcomes. By utilizing the Mantel-Haenszel technique of the random effect model the pooled odds ratio (OR) and 95 percent confidence intervals (CI) were calculated for each outcome. To calculate the mean difference, employed the continuous inverse variance approach with a random effect model. Whereas Cochrane x2 and I2 statistics were used to quantify statistical heterogeneity. Heterogeneity is represented by the following criteria; values of 25%–49% indicate less heterogeneity, whereas values of 50%–74% indicate a moderate level of heterogeneity, and values greater than 75% indicate a higher level of heterogeneity [Citation15]. A funnel plot was applied to figure out whether or not there was any sort of publication bias. For the data to be considered statistically significant, the p-value needed to be lower than 0.05.
3. Results
3.1. Search results, study characteristics, and evaluation
318 articles were identified following the elimination of all of the duplicate search results and papers that were not related to our investigation. At the end of the process of assessment, a total of 95 papers were looked into for possible inclusion in the final selection. A total of 17 [Citation16–32] papers were considered for inclusion in the analysis in the end. A detailed PRISMA flow chart on the selection of studies is shown here (). In these studies, a total of 1170 patients participated, 543 of whom received endoscopic pancreatic fluid drainage and 627 of whom underwent percutaneous fluid collection. There were a total of ten studies that reported post-operative pancreatic fluid collections (POPFC), and the remaining seven included either WON fluid collections or a mix of WON and PP collections. The eleven studies that were conducted in Asia (China, Japan, and India), four studies that were conducted in the United States, one study that was conducted in Germany, and one study that was conducted in the United Kingdom were all observational cohort studies. According to the inclusion criteria, the features of the studies that were taken into consideration for the analysis are presented in the tables ( and Citation2). The results of the test of the risk of bias showed that every study had a moderate risk of bias (Supplementary Table 1). He et al. [Citation21] and Jurgenson et al. [Citation24] reported only clinical success. Whereas Kwon et al. [Citation19] and Grobmyer et al. [Citation16] did not report any adverse events.
Table 1. Characteristics of included studies.
Table 2. Characteristics of included studies table 1 continue.
3.2. Primary outcomes
3.2.1. Technical success
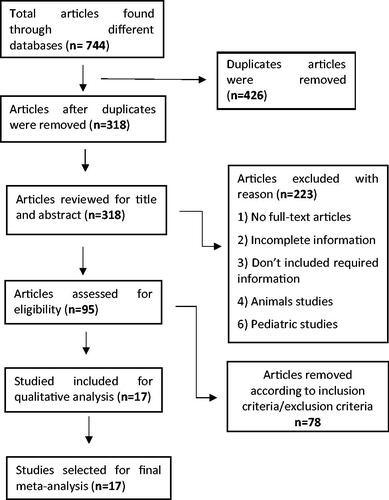
In total, fifteen studies reported technical success. He et al. and Jurgenson et al. did not report technical success. Only six studies were included in the calculation of the odd ratio (OR), while the remaining nine studies achieved 100% technical success for both drainage procedures. There was no significant difference in technical success OR between the two procedures: 0.81 (95% CI 0.31, 2.1), I2 = 0%, p = 0.66 (). Whereas in subgroup analysis POPFC studies had an OR of 1.97 (95% CI 0.38, 10.29) I2=0%, p = 0.42 and WON studies had an OR of 0.49 (95% CI 0.12, 2.05) I2=33%, p = 0.33. Subgroup analysis also revealed no significant differences between the two procedures.
3.2.2. Clinical success
Clinical success was reported in all seventeen studies. Tamura et al. achieved 100% clinical success for both procedures. The OR for the other sixteen studies was 2.23 (95% CI 1.45, 3.41), I2=28%, p = 0.0002. For PFCs, the OR result was significantly in favor of endoscopic intervention. The data from the included studies had low heterogeneity. However, in the subgroup analysis, POPFC studies revealed an OR of 1.38 (95% CI 0.72, 2.63) I2=0%, p = 0.33, indicating that there is no significant difference in clinical success between ED and PD patients (). WON studies revealed an OR of 2.74 (95% CI 1.56, 4.81) I2=50%, p = 0.0005, indicating a significant difference in clinical success in favour of ED with moderate heterogeneity.
3.3. Secondary outcomes
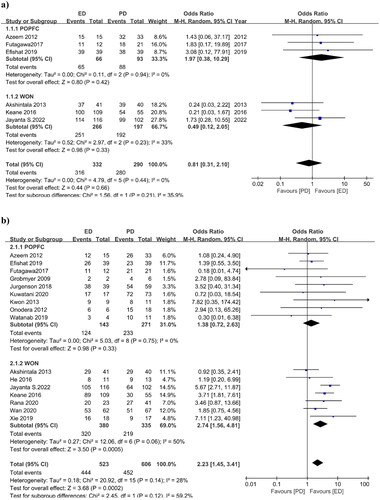
3.3.1. Adverse events
Ten studies reported adverse events for both drainage procedures. The OR for adverse events was 0.62 (95% CI 0.27, 1.39), I2 = 72%, p = 0.25, indicating that there were fewer adverse events in the ED group, but statistically there was no significant difference in adverse events between the two interventions and moderate heterogeneity of the included data (). Only three studies in the POPFC group reported adverse events, with an OR of 2.47 (95% CI 0.92, 6.60) I2=0%, p = 0.25. In the WON group, seven studies produced an OR of 0.37 (95% CI 0.17, 0.81) I2=64%, p = 0.01. This indicates that adverse events for ED procedures are lower in the WON group with moderate heterogeneity, but there is no difference in the POPFC group.
3.3.2. Stent migration
Only six studies have noted stent migration events. There were fewer stent migration events in the ED procedure, but the difference was not statistically significant and showed moderate heterogeneity. The OR for stent migration was 0.61 (95% CI 0.10, 3.88), I2 = 68%, p = 0.60 (). Only two POPFC studies reported stent migration, yielding an OR of 5.99 (95% CI 0.64, 55.97) I2 = 0%, p = 0.12, and four WON studies yielded an OR of 0.23 (95% CI 0.03, 1.59) I2 = 63.9%, p = 0.14. In POPFC studies, stent migration incidents were greater in ED than in PD, but this difference was not statistically significant because only two studies were included, whereas, in WON studies, stent migration events were lower in the ED group, although not statistically significant. The study by Jayanta et al. had a significant impact on the final result, reporting 22 stent migration events in the PD group and only one in the ED group leading to higher heterogeneity.
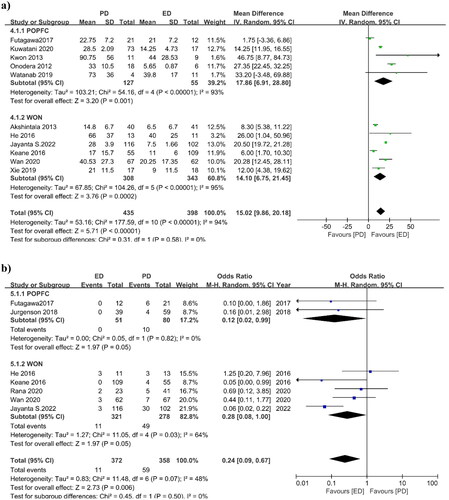
3.3.3. Hospital stay
Eleven studies reported hospital stays after the interventions for both groups. The pooled mean difference in hospital stay was 15.02 days (95%CI 9.86, 20.18), I2=94%, p = 0.00001, indicating that the ED and PD groups had a 15-day difference in hospital stay that is statically significant but presenting higher heterogeneity (). POPFC studies had a pooled mean difference of 17.86 days (95% CI 6.91, 28.80), I2 = 93%, p = 0.001, whereas WON studies had a pooled mean difference of 14.10 days (95% CI 6.75, 21.45), I2 = 95%, p = 0.0002. In terms of length of hospital stay, subgroup analysis also shows that the ED intervention is better than PD but it showed higher heterogeneity.
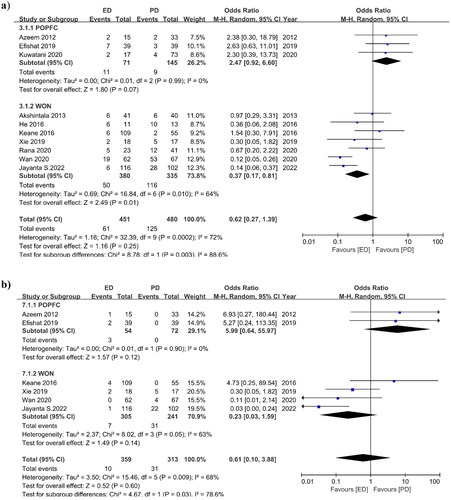
3.3.4. Mortality
The mortality rate for the ED and PD groups was reported in seven studies. The OR for mortality was 0.24 (95% CI 0.09, 0.67) I2=48%, p = 0.006, indicating that the mortality rate is considerably in favor of the ED group (). The number of deaths after the drainage was lower in the ED group than in the PD group. Only two POPFC studies reported an OR of 0.12 (95% CI 0.02, 0.99) I2 = 0%, p = 0.05, and five WON studies produced an OR of 0.28 (95% CI 0.08, 1.00) I2 = 64.0%, p = 0.05. The subgroup analysis was also in favor of the ED technique.
3.3.5. Re-interventions
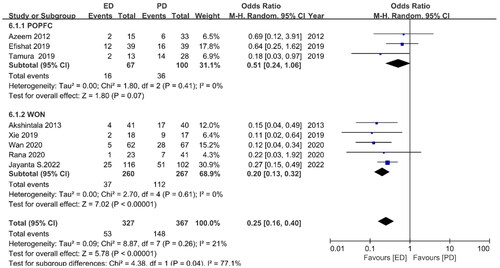
Re-intervention occurrences were observed in eight studies, with an OR of 0.25 (95%CI 0.16, 0.40) I2=21%, p = 0.00001 (). The re-intervention cases of the ED and PD groups varied significantly. POPFC studies had an OR of 0.51 (95% CI 0.24, 1.06) I2 = 64.0%, p = 0.05, while WON studies had an OR of 0.20 (95% CI 0.13, 0.32) I2 = 0%, p = 0.00001. The subgroup analysis also showed that ED was better than PD because the ED group needed fewer re-interventions than the PD group.
3.3.5.1. Publication bias and study effect
The risk of publication bias was found to be negligible in our meta-analysis. On the funnel plot, each outcome data displayed the symmetric distribution of the data. While the effects of each study were evaluated by removing each study one by one, the findings indicated that none of the studies had a significant impact on the conclusion made.
4. Discussion
It is difficult to collect pancreatic fluids (PF) from patients, and this presents a significant obstacle to their care. Surgical drainage for PF is a last choice due to the high risk of complications and the invasive nature of the procedure. In addition to endoscopy and percutaneous drainage, there are other alternatives. Several researchers have discovered an advantage of ED for PFCs. 17 studies reported on the PFC in this meta-analysis. OR was determined independently for POPFC and WON patients in subgroup analysis. According to our findings, the odds were strongly in favor of ED. Subgroup analysis of the POPFC investigations found that in terms of technical success, clinical success, adverse events, recurrence of mortality, and stent migration, both ED and PD had the same results. However, the length of stay in the hospital and the rate of re-intervention in the ED group were much lower than in the PD group. Whereas for WON studies, OR showed that clinical success, hospital stay, recurrence, and mortality were significantly in favor of the ED group while technical success and stent migration were the same for the ED and the PD groups.
A meta-analysis of 13 studies by Mohan et al. [Citation33] reported that ED and PD had similar adverse events and technical success. ED outperformed PD in clinical success (93.2% vs. 79.8%, p = 0.002), disease recurrence, and PF management. This meta-analysis only included single-arm studies involving PFC patients. This meta-analysis did not compare ED and PD. Current meta-analysis solely compared ED and PD for PFCs. Khan et al. [Citation34] reported that the pooled risk ratio (RR) for clinical success was 0.40 (95%CI 0.26, 0.61) in favour of ED in seven trials comparing the outcomes of ED versus PD. Meanwhile, both procedures had a virtually identical RR for adverse events of 0.77 (95%CI 0.46, 1.28). However, only seven papers were included in this analysis, and the results of the comparison between the two methods were different from those of the current study. Cai et al. [Citation35] reported a data of 11 studies meta-analysis with OR for clinical success of 1.39 (95% CI 0.82, 2.37) that is in favour of ED and included studies with missing outcome data. But the current study included 17 studies all reporting our primary outcome clinical success. Studies not reporting clinical success were excluded from the meta-analysis.
Chen et al. [Citation36] reported a meta-analysis of six studies about PFC after pancreatic surgery. According to the reported data, there is no difference in technical success, clinical success, recurrence, and adverse events between the two interventions. ED has comparable safety and efficacy to PD. In the subgroup analysis, the current study also analysed ten studies reporting ED and PD after pancreatic surgery and analysis also showed no significant difference in technical success, clinical success, mortality, recurrence, stent migration, and adverse events but hospital stay and re-intervention rate that were in the favour of ED. Reporting data from 25 studies’ meta-analysis by Ramouz et al. [Citation37] stated that compared to PD, ED produced higher clinical and technical success with fewer procedure-related complications and hospital stay time [Citation38]. In this meta-analysis, single-arm studies and case reports describing the efficacy of only ED were included that did not describe the comparative data between the two interventions. The present study included up to-dated seventeen studies reporting the comparative data of ED and PD.
Endoscopic drainage will accrue an increasing number of benefits as time goes by, particularly when new stents and endoscopic procedures and abilities become available. In the meantime, ED can also perform additional therapies for pancreatic duct strictures and pancreatic duct stones, as well as bile duct strictures and bile duct stones while the PD cannot conduct such additional therapies. ED also demonstrated an improvement in life quality and a reduced risk of infection, whereas PD patients required long-term care, which affected the patient’s life quality and also carried the risk of developing an infection of the skin and an external pancreatic fistula [Citation39]. Albers et al. [Citation40] reported that during the follow-up period of 8.5 ± 5.9 months for the described treatment of IPN (infected pancreatic necrosis), 12 out of 13 patients obtained clinical success by utilizing ED through the utilization of stents and lumen debriment. Glucck et al. [Citation41] reported that compared to only ED a combination of ED and PD can reduce hospital stay significantly (55 days vs. 26 days) for PFC. According to another study, the ED procedure was also used for abdominal abscesses and showed better results compared with the PD procedure [Citation42]. Inamdar et al. [Citation43] suggested ED should be the first line of treatment for malignant biliary strictures (MBS) patients because ED procedures showed fewer adverse events compared to PD. However, the current meta-analysis did not find any significant difference in adverse events between ED and PD. Metal stents can reduce bleeding risk as compared to plastic stents [Citation44–48]. Chen et al. [Citation49] reported that metal stent drainage is more effective with higher clinical success than plastic stents (92% vs. 84%). A recent meta-analysis of fifteen studies found that using metal stents for endoscopic drainage of WON resulted in better clinical outcomes and fewer adverse events compared to using plastic stents [Citation50]. For the patients of necrotizing pancreatic collection, ‘Step-up’ treatment based on minimally invasive procedures is recommended [Citation51,Citation52]. WON patients need special care and multiple drainage secessions due to their aggressive nature. An RCT study of WON patients also demonstrates that there is no difference in technical and clinical success, adverse events between ED and laparoscopic internal drainage, and fewer hospital stays [Citation53]. Hamada et al. reported an analysis of 5 studies of PFC for patients with disconnected pancreatic duct syndrome (DPDS). Results also supported the ED for the PFC [Citation54]. Another meta-analysis of 30 studies of PFC with DPDS by Chong et al. reported that endoscopic transmural drainage is better than transpapillary drainage and endoscopic and surgical drainages interventions showed comparable success rates for the patients of DPDS [Citation55].
ED procedure re-introduce pancreatic fluid (PF) into the GI tract preventing electrolytes and fluid loss compared to the PD procedure where PF is drained out of the body [Citation19,Citation56–58]. ED has only the disadvantage of inconvenient nasocystic drainage and repeated endoscopic procedures due to stent migration and incomplete drainage [Citation59]. So this may be not suitable for all patients. While selecting the appropriate drainage procedure it is very important to take into account the patient’s condition and the risk of the presence of solid debris from the necrotic tissues that cannot be drained out through PD. But ED will be useful in the detection of debris and necrosectomy that is not possible through PD.
Acute pancreatitis can result in different types of fluid collections, including ANCs and PFCs. While PFCs can often be managed with a passive drainage approach, APNCs may require a more aggressive approach and occasionally necessitate necrosectomy. However, the current literature lacks studies that specifically compare these two types of collections. Many studies report on both WON and pancreatic pseudocysts together, while it’s also important to note that WON and some POPFCs may require a more aggressive drainage approach. While some studies have conducted separate meta-analyses for APNCs and POPFCs [Citation37,Citation38], our analysis combines both conditions. This is because there is a lack of comparative studies and it is important to understand the differences in management approaches between these types of collections. However, in our analysis, we conducted a subgroup analysis to compare the management of the two types separately. There are several limitations to this research. The primary concern with our analysis is the lack of randomized controlled trials. Our results show higher heterogeneity for some outcomes; thus, it is essential to keep this in mind when interpreting the results. Additionally, some studies included in our analysis had small sample sizes, and others reported incomplete data with different types of collections. Another limitation is that some studies presented data in different formats, which we standardized to ensure consistency, but this could have potentially influenced the results. Furthermore, the studies we included involved patients with different types, amounts, and locations of pancreatic fluid(PF) disorders. To draw more robust conclusions, future studies should focus on patients with identical PF characteristics and locations.
5. Conclusion
Based on the findings of this meta-analysis, the ED technique for pancreatic fluid(PF) draining is considered both safe and effective. The clinical success rate of this technique is better compared to other interventions, with a reduced rate of adverse events, re-interventions, hospital stays, and mortality. Achieving gratifying results relies on selecting the appropriate patients and utilizing competent medical professionals. However, to fully evaluate the advantages of ED procedures in comparison to other interventions, randomized control trials involving large numbers of patients are necessary.
Ethical Approval and consent to publish
All authors have approved this submission to your esteemed journal. Its publication is also approved tacitly by the responsible authorities where the work was carried out. The authors have no relevant financial or non-financial interests to disclose.
Author contributions
Khizar Hayat: Wrote the original paper; Zhicheng Huang: Edit and correct the paper; Yanhua Wu: Literature search; Chenyu Le: Images Editing; Jianfeng Yang: Provided article ideas and article review.
Supplemental Material
Download MS Word (499.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data related to this study is available, please send mail to Yang Jianfeng for further information.
Additional information
Funding
References
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the atlanta classification and definitions by international consensus. Gut. 2013;62(1):1–13.
- Hookey LC, Debroux S, Delhaye M, et al. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63(4):635–643.
- Varadarajulu S, Bang JY, Phadnis MA, et al. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15(11):2080–2088.
- Andalib I, Dawod E, Kahaleh M. Modern management of pancreatic fluid collections. J Clin Gastroenterol. 2018;52(2):97–104.
- Yokoe M, Takada T, Mayumi T, et al. Japanese guidelines for the management of acute pancreatitis: ochrane guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22(6):405–432.
- Tenner S, Baillie J, DeWitt J, et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–1415; 1416.
- Wroński M, Cebulski W, Witkowski B, et al. Comparison between minimally invasive and open surgical treatment in necrotizing pancreatitis. J Surg Res. 2017;210:22–31.
- Baron TH, DiMaio CJ, Wang AY, et al. American gastroenterological association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158(1):67–75.e1.
- Matthews JB. Prevention, evaluation, and treatment of leaks after pancreatic surgery. J Gastrointest Surg. 2011;15(8):1327–1328.
- Vin Y, Sima CS, Getrajdman GI, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008;207(4):490–498.
- Freeny PC, Hauptmann E, Althaus SJ, et al. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170(4):969–975.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the ochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10: ed000142.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919–i.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- Grobmyer SR, Hunt DL, Forsmark CE, et al. Pancreatic stent placement is associated with resolution of refractory grade C pancreatic fistula after left-sided pancreatectomy. The American Surgeon. 2009;75(8):654–658. Discussion 7–8.
- Onodera M, Kawakami H, Kuwatani M, et al. Endoscopic ultrasound-guided transmural drainage for pancreatic fistula or pancreatic duct dilation after pancreatic surgery. Surg Endosc. 2012;26(6):1710–1717.
- Azeem N, Baron TH, Topazian MD, et al. Outcomes of endoscopic and percutaneous drainage of pancreatic fluid collections arising after pancreatic tail resection. J Am Coll Surg. 2012;215(2):177–185.
- Kwon YM, Gerdes H, Schattner MA, et al. Management of peripancreatic fluid collections following partial pancreatectomy: a comparison of percutaneous versus EUS-guided drainage. Surg Endosc. 2013;27(7):2422–2427.
- Akshintala VS, Saxena P, Zaheer A, et al. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79(6):921–928; quiz 83.e2, 83.e5.
- He WH, Zhu Y, Zhu Y, et al. The outcomes of initial endoscopic transluminal drainage are superior to percutaneous drainage for patients with infected pancreatic necrosis: a prospective cohort study. Surg Endosc. 2017;31(7):3004–3013.
- Keane MG, Sze SF, Cieplik N, et al. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: a 14-year experience from a tertiary hepatobiliary Centre. Surg Endosc. 2016;30(9):3730–3740.
- Futagawa Y, Imazu H, Mori N, et al. The effectiveness and feasibility of endoscopic ultrasound-guided transgastric drainage of postoperative fluid collections early after pancreatic surgery. Surg Laparosc Endosc Percutan Tech. 2017;27(4):267–272.
- Jürgensen C, Distler M, Arlt A, et al. EUS-guided drainage in the management of postoperative pancreatic leaks and fistulas (with video). Gastrointest Endosc. 2019;89(2):311–319.e1.
- Tamura T, Kitano M, Kawai M, et al. Effectiveness of endoscopic ultrasound-guided drainage for noncapsulated postoperative pancreatic collection. Therap Adv Gastroenterol. 2019;12:1756284819884418.
- Xie LT, Zhao QY, Gu JH, et al. Endoscopic Ultrasonography-Guided versus percutaneous drainage for the recurrent pancreatic fluid collections. Med Sci Monit. 2019;25:5785–5794.
- Watanabe Y, Ueda K, Nakamura S, et al. Endoscopic transpapillary pancreatic duct stent placement for symptomatic peripancreatic fluid collection caused by clinically relevant postoperative pancreatic fistula after distal pancreatectomy. Surg Laparosc Endosc Percutan Tech. 2019;29(4):261–266.
- Al Efishat M, Attiyeh MA, Eaton AA, et al. Endoscopic versus percutaneous drainage of post-operative peripancreatic fluid collections following pancreatic resection. HPB (Oxford). 2019;21(4):434–443.
- Kuwatani M, Imamura M, Hayashi T, et al. A drainage strategy for postoperative pancreatic fistula after left-sided pancreatectomy based on the wall status of collected fluid. Langenbecks Arch Surg. 2021;406(3):743–751.
- Rana SS, Shah J, Kang M, et al. Complications of endoscopic ultrasound-guided transmural drainage of pancreatic fluid collections and their management. Ann Gastroenterol. 2019;32(5):441–450.
- Wan J, Wu D, He W, et al. Comparison of percutaneous vs endoscopic drainage in the management of pancreatic fluid collections: a prospective cohort study. J Gastroenterol Hepatol. 2020;35(12):2170–2175.
- Samanta J, Dhar J, Muktesh G, et al. Endoscopic drainage versus percutaneous drainage for the management of infected walled-off necrosis: a comparative analysis. Expert Rev Gastroenterol Hepatol. 2022;16(3):297–305.
- Mohan BP, Shakhatreh M, Dugyala S, et al. EUS versus percutaneous management of postoperative pancreatic fluid collection: a systematic review and meta-analysis. Endosc Ultrasound. 2019;8(5):298–309.
- Khan MA, Hammad T, Khan Z, et al. Endoscopic versus percutaneous management for symptomatic pancreatic fluid collections: a systematic review and meta-analysis. Endosc Int Open. 2018;6(4):E474–e83.
- Cai QC, Zhang YX, Liao Y, et al. Is endoscopic drainage better than percutaneous drainage for patients with pancreatic fluid collections? A comparative meta-analysis. Revista Espanola de Enfermedades Digestivas: organo Oficial de la Sociedad Espanola de Patologia Digestiva. 2021;113(6):454–462.
- Chen L, Li T, Wang B, et al. Endoscopic versus percutaneous drainage for pancreatic fluid collection after pancreatic surgery: an up-to-date meta-analysis and systematic review. Asian Journal of Surgery. 2022;45(8):1519–1524.
- Ramouz A, Shafiei S, Ali-Hasan-Al-Saegh S, et al. Systematic review and meta-analysis of endoscopic ultrasound drainage for the management of fluid collections after pancreas surgery. Surg Endosc. 2022;36(6):3708–3720.
- Chen L, Li T, Wang B, et al. Endoscopic versus percutaneous drainage for pancreatic fluid collection after pancreatic surgery: an up-to-date meta-analysis and systematic review. Asian J Surg. 2022;45(8):1519–1524.
- Tyberg A, Karia K, Gabr M, et al. Management of pancreatic fluid collections: a comprehensive review of the literature. World J Gastroenterol. 2016;22(7):2256–2270.
- Albers D, Toermer T, Charton JP, et al. Endoscopic therapy for infected pancreatic necrosis using fully covered self-expandable metal stents: combination of transluminal necrosectomy, transluminal and percutaneous drainage. Z Gastroenterol. 2016;54(1):26–30.
- Gluck M, Ross A, Irani S, et al. Endoscopic and percutaneous drainage of symptomatic walled-off pancreatic necrosis reduces hospital stay and radiographic resources. Clin Gastroenterol Hepatol. 2010;8(12):1083–1088.
- Carbajo AY, Brunie Vegas FJ, García-Alonso FJ, et al. Retrospective cohort study comparing endoscopic ultrasound-guided and percutaneous drainage of upper abdominal abscesses. Dig Endosc. 2019;31(4):431–438.
- Inamdar S, Slattery E, Bhalla R, et al. Comparison of adverse events for endoscopic vs percutaneous biliary drainage in the treatment of malignant biliary tract obstruction in an inpatient national cohort. JAMA Oncol. 2016;2(1):112–117.
- Abu Dayyeh BK, Mukewar S, Majumder S, et al. Large-caliber metal stents versus plastic stents for the management of pancreatic walled-off necrosis. Gastrointest Endosc. 2018;87(1):141–149.
- Bang JY, Hasan M, Navaneethan U, et al. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. 2017;66(12):2054–2056.
- Khizar H, Hu Y, Wu Y, et al. Efficacy and safety of radiofrequency ablation plus stent versus stent-alone treatments for malignant biliary strictures: a systematic review and meta-analysis. J Clin Gastroenterol. 2023;57(4):335–345.
- Zhang LY, Kunda R, Aerts M, et al. Novel 15-mm-long lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections located ≥10 mm from the luminal wall. Endoscopy. 2022;54(7):706–711.
- Gkolfakis P, Chiara Petrone M, Tadic M, et al. Efficacy and safety of endoscopic drainage of peripancreatic fluid collections: a retrospective multicenter european study. Ann Gastroenterol. 2022;35(6):654–662.
- Chen YI, Barkun AN, Adam V, et al. Cost-effectiveness analysis comparing lumen-apposing metal stents with plastic stents in the management of pancreatic walled-off necrosis. Gastrointest Endosc. 2018;88(2):267–276.e1.
- Khizar H, Yufei H, Yanhua W, et al. Safety and efficacy of lumen-apposing metal stents and double-pigtail plastic stents for endoscopic ultrasound-guided drainage of walled-off necrosis; a systematic review and meta-analysis. Ann Med. 2023;55(1):578–591.
- Hollemans RA, Bakker OJ, Boermeester MA, et al. Superiority of step-up approach vs open necrosectomy in long-term follow-up of patients with necrotizing pancreatitis. Gastroenterology. 2019;156(4):1016–1026.
- Singh AD, Mian A. Role of early endoscopically centered Step-Up interventions in acute necrotizing pancreatitis. Am J Gastroenterol. 2019;114(4):687–688.
- Angadi S, Mahapatra SJ, Sethia R, et al. Endoscopic transmural drainage tailored to quantity of necrotic debris versus laparoscopic transmural internal drainage for walled-off necrosis in acute pancreatitis: a randomized controlled trial. Pancreatology. 2021;21(7):1291–1298.
- Hamada T, Iwashita T, Saito T, et al. Disconnected pancreatic duct syndrome and outcomes of endoscopic ultrasound-guided treatment of pancreatic fluid collections: systematic review and meta-analysis. Dig Endosc. 2022;34(4):676–686.
- Chong E, Ratnayake CB, Saikia S, et al. Endoscopic transmural drainage is associated with improved outcomes in disconnected pancreatic duct syndrome: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21(1):87.
- Tilara A, Gerdes H, Allen P, et al. Endoscopic ultrasound-guided transmural drainage of postoperative pancreatic collections. J Am Coll Surg. 2014;218(1):33–40.
- Téllez-Ávila F, Carmona-Aguilera GJ, Valdovinos-Andraca F, et al. Postoperative abdominal collections drainage: percutaneous versus guided by endoscopic ultrasound. Digest Endoscopy. 2015;27(7):763–767.
- Le Moine O, Matos C, Closset J, et al. Endoscopic management of pancreatic fistula after pancreatic and other abdominal surgery. Best Pract Res Clin Gastroenterol. 2004;18(5):957–975.
- Prachayakul V, Aswakul P. Endoscopic ultrasound-guided biliary drainage as an alternative to percutaneous drainage and surgical bypass. World J Gastrointest Endosc. 2015;7(1):37–44.