Abstract
Objective
Bowman’s capsule rupture (BCR) is a glomerular pathological change, but it is still not well recognized in immunoglobulin A vasculitis nephritis (IgAV-N). The Oxford MEST-C score is a classification for IgA nephropathy; however, its clinical correlation and prognostic value in adult patients with IgAV-N are unclear.
Methods
A retrospective study of 145 adult patients with IgAV-N diagnosed by renal biopsy was conducted. Clinical manifestations, pathological changes and the prognosis of IgAV-N patients were compared depending on the presence or absence of BCR, International Study of Kidney Disease in Children (ISKDC) classification and MEST-C score. The primary endpoint events were end-stage renal disease, renal replacement therapy and all-cause death.
Results
In total, 51 of 145 (35.17%) patients with IgAV-N presented with BCR. Patients with BCR had more proteinuria, lower serum albumin, and more crescents. Compared with IgAV-N patients with crescents only, 51/100 patients with crescents combined with BCR had a higher proportion of crescents in all glomeruli (15.79% vs. 9.09%; p = 0.003). Patients with higher ISKDC grades had more severe clinical presentation, but it did not reflect the prognosis. However, the MEST-C score not only reflected clinical manifestations but also predicted prognosis (p < 0.05). BCR contributed to the effectiveness of the MEST-C score in predicting the prognosis of IgAV-N (C-index: 0.845 to 0.855).
Conclusions
BCR is associated with clinical manifestations and pathological changes in patients with IgAV-N. The ISKDC classification and MEST-C score are related to the patient’s condition, but only the MEST-C score is correlated with the prognosis of patients with IgAV-N, while BCR can improve its predictive ability.
KEY MESSAGES
BCR was associated with clinical manifestations and pathological changes in patients with IgAV-N, particularly crescents.
The ISKDC classification was related to clinical manifestations of patients with IgAV-N, but it wasn’t associated with prognosis.
The Oxford MEST-C score was correlated to clinical presentations and prognosis of patients with IgAV-N, while BCR can improve its predictive ability.
Introduction
Immunoglobulin A vasculitis (IgAV), formerly known as Henoch-Schönlein purpura (HSP), is a small-vessel vasculitis, and its dominant pathological feature is the deposition of IgA immune complexes (IC) in the vessel wall [Citation1]. IgAV is the most common systemic vasculitis in children, with an annual incidence of 3–26.7/100,000 cases, but 0.8–1.8/100,000 cases in adults [Citation2]. IgAV nephritis (IgAV-N), also named Henoch-Schönlein purpura nephritis (HSPN) (ICD-10 code: D69.005+), is an important chronic complication of IgAV and a type of secondary IgA nephropathy (IgAN) [Citation3,Citation4]. In adults, IgAV-N manifests as severe, refractory, prone to recurrence, and commonly presenting as hematuria, with or without proteinuria, and/or nephrotic syndrome [Citation3–5]. The International Study of Kidney Disease in Children (ISKDC) divides IgAV-N into 6 grades, which is of great significance for guiding the clinical diagnosis and treatment of this disease [Citation6–8]. There are correlations between clinical features and ISKDC classifications [Citation9], but previous studies have shown that it cannot accurately reflect the clinical severity and predict the prognosis of patients with IgAV-N, especially adult patients [Citation4,Citation5,Citation8–12]. The Oxford MEST-C classification of IgA nephropathy (IgAN) also focuses on lesions ignored in the ISKDC classification, which can reflect both acute and chronic renal lesions in contrast to the ISKDC classification [Citation12,Citation13]. The IgA Nephropathy Classification Working Group and Kidney Disease: Improving Global Outcomes (KDIGO) suggested that the MEST-C score might be applicable to the assessment of patients with IgAV-N [Citation14,Citation15]. The MEST-C score was not yet sufficient to be used in IgAV-N, particularly in adult patients; thus, its value in IgAV-N remains to be further explored [Citation7–9, Citation11,Citation12,Citation16]. Furthermore, the ISKDC and MEST-C classifications lack the description of glomerular Bowman’s capsule rupture (BCR), a common renal pathological change in heavy immune-mediated glomerulonephritis [Citation17–22]; therefore, the effect of BCR on IgAV-N also needs to be addressed, especially in adult patients with severe renal lesions.
Bowman’s capsule is a cup-like sac around the glomerular capillary network that forms Bowman’s space and serves as a barrier to protect the glomerulus [Citation17,Citation18]. BCR has been identified since the 1970s [Citation23], and recent studies have demonstrated that it is associated with impaired renal function in patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis (ANCA-GN) [Citation24], and can improve the performance of the original prognosis model of ANCA-GN [Citation20]. Importantly, a recent clinical practice guideline issued in 2015 proposed that BCR deserves more attention in renal biopsies [Citation22]. However, research on BCR in adult patients with IgAV-N has not been conducted until now.
This study evaluated adult patients with IgAV-N diagnosed by renal biopsy from June 2015 to November 2020 at the Second Xiangya Hospital of Central South University. For the first time, IgAV-N patients with BCR presented more severe clinical manifestations and renal pathological changes than those without BCR. In addition, the ISKDC classification was related to clinical manifestations, especially serum creatinine, but it was not associated with prognosis. However, the MEST-C score was correlated with the clinical presentations and prognosis of IgAV-N, and BCR improved its predictive ability.
Materials and methods
Patients
A total of 157 patients with IgAV-N (HSPN) and aged older than 18 years were diagnosed by clinical and renal biopsy at the Second Xiangya Hospital of Central South University between 6/9/2015 and 11/17/2020. The diagnosis of adult patients with IgAV-N was based on the EULAR/PRINTO/PReS-endorsed Ankara 2008 criteria [Citation25] and the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases [Citation14]. Patients with concomitant primary kidney diseases or secondary kidney diseases such as diabetes nephropathy, hepatitis B-associated nephritis, hypertensive nephropathy, and gouty nephropathy, (n = 5); poor pathological material that may affect interpretation (n = 3); and an inadequate number of glomeruli in biopsy (<6 glomeruli, n = 4) detected by renal biopsy were excluded. We also excluded autoimmune diseases by collecting the laboratory examination results of antinuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (ANCA), anti-glomerular basement antibody, myeloperoxidase (MPO), proteinase 3 (PR3), and complements. After excluding 12 patients, 145 patients were included in the analysis. The research complied with the Declaration of Helsinki, patient consent was obtained, and the study was approved by the Medical Ethics Committee of the Second Xiangya Hospital.
Clinical and pathologic data collection
Clinical data at biopsy were obtained, including sex, age, clinical symptoms, and laboratory test results, through the medical record system in our hospital. The estimated glomerular filtration rate (eGFR) of all patients was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, and the CKD stage was determined.
Pathological data were obtained from pathological reports, including pathological changes in glomeruli, renal tubules, renal interstitium, and immunofluorescence. BCR was defined as discontinuity of the basement membrane of Bowman’s capsule in renal biopsy sections, stained with periodic acid Schiff (PAS) and Jones methenamine silver (JMS), respectively, and evaluated by an experienced nephropathologist using light microscopy. The pathological slices of patients were reviewed by three pathologists who were unaware of the patient’s specific situation. The ISKDC stage of IgAV-N was determined as discussed previously [Citation6]: grade I (minimal histological alterations), grade II (mesangial proliferation without crescents), grade III (mesangial proliferation with <50% crescents, a. focal, b. diffuse), grade IV (mesangial proliferation with 50 ∼ 75% crescents), grade V (mesangial proliferation with >75% crescents, a. focal, b. diffuse), and grade VI (membranoproliferative-pattern glomerulonephritis). The MEST-C score includes mesangial hypercellularity (M0 < 50%; M1 ≥ 50% of the glomeruli), endocapillary hypercellularity (E0: absent; E1: present), segmental glomerulosclerosis (S0: absent; S1: present), tubular atrophy and interstitial fibrosis (T0: 0 ∼ 24%; T1: 25 ∼ 49%; and T2: ≥50% of the cortical area), and cellular or fibro-cellular crescents (C0: absent; C1: 1 ∼ 24%; and C2: ≥25% of the glomeruli) as in reference [Citation12,Citation15].
Study Outcomes
A total of 145 patients were followed up by telephone and outpatient visits. For patients who were lost to follow-up, their last data were recorded. The median follow-up time of all patients was 23.45 (6.78, 38.20) months. Among them, 28 patients who were followed up for less than 6 months were excluded. The primary endpoint events of this study were defined as end-stage renal disease (ESRD, eGFR < 15 ml/min/1.73m2), need for renal replacement therapy (RRT: dialysis and kidney transplantation), and all-cause death.
Statistical analyses
The Kolmogorov–Smirnov test was used to analyze the normality of the parameter distribution. Continuous variables that conformed to a normal distribution were expressed as means ± standard deviation (sd), while those that conformed to a skewed distribution were expressed as medians and quartile intervals. Categorical variables were expressed as numbers with percentages. Comparisons of continuous variables were based on t-test, the Mann–Whitney U test, the SNK-q test, or the Kruskal–Wallis H test. Comparisons of categorical variables were based on the χ2 test or Fisher’s exact test. Spearman correlation analyses were used to analyze correlations and were shown using a heatmap. Event-free survival for the composite endpoint (all-cause death and ESRD) was derived using the Kaplan–Meier method, and differences between curves were analyzed using the log-rank test. The Cox proportional hazards model was used for univariate and multivariate analyses, statistically significant variables in univariate analysis were included in the model, and the results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Cox models were established, and the C-index was calculated to evaluate the predictive power. Statistical analyses were performed using IBM SPSS for Windows, version 26.0 and R (x64 4.0.5). All tests were two-sided, and a p-value of < 0.05 was considered statistically significant.
Results
Patients’ baseline characteristics
Of these 145 patients with IgAV-N, 78 (53.8%) were female and 67 (46.2%) were male. At the time of biopsy, the median age of the study subjects was 32 (22, 49) years, the median courses of IgAV and IgAV-N were 5 (1, 24) and 2 (0.78, 9) months, respectively, 19 (13.1%) patients had gross hematuria, 6 (4.1%) had no skin purpura, 45 (31.0%) had abdominal purpura and 37 (25.5%) had arthritic purpura. There were 19 (13.1%) patients with a history of hypertension and 5 (3.5%) with diabetes (, Supplementary Table 1). None of the patients had diseases of other systems, such as tumors, cerebrovascular diseases, or central nervous system diseases. The research cohort had a median 24-hour urine protein level of 1085.88 (300.02, 2427.95) mg/day, serum creatinine (Scr) level of 69.20 (55.10, 87.30) µmol/L, serum albumin (ALB) level of 35.60 (29.85, 38.50) g/L, eGFR of 109.39 (82.32, 124.01) ml/min/1.73 m2, and serum IgA level of 2.81 ± 1.16 g/L at baseline (, Supplementary Table 2).
Table 1. Comparison of baseline clinical characteristics, laboratory results and pathological changes in IgAV-N patients with or without BCR.
Clinical features of patients with or without BCR
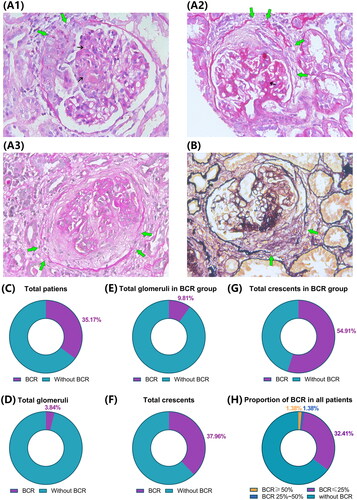
Representative photomicrographs are shown in (). Pathological change in patients with IgAV-N was observed, including obvious discontinuity of the basement membrane of Bowman’s capsule, different stages of crescents, and glomerular mesangial proliferation. Fifty-one (35.17%) patients had BCR, and the glomeruli with BCR accounted for 3.84% of the total glomeruli and 37.96% of the total crescents in all patients. In the BCR group, 9.81% of the total glomeruli and 54.91% of crescents had BCR. There were 2 (1.38%) patients with BCR ≥ 50% and 4 (2.76%) with BCR >25% ().
Figure 1. Typical photomicrograph of Bowman’s capsule rupture (BCR) in patients with IgAV-N by PAS and PASM staining and summary of BCR in renal biopsy. (A, B) Representative periodic acid-Schiff (PAS) and periodic acid-silver metheramine (PASM) photomicrograph of Bowman’s capsule rupture (BCR) in patients with IgAV-N (×400). A1-A3 show cellular, fibro-cellular, and fibrous crescents with BCR, respectively. The green arrows indicate disruption of Bowman’s capsule, and the small black arrows show glomerular mesangial proliferation. (C) Patients with BCR in total cases. (D) Glomeruli with BCR in the total glomeruli. (E) Glomeruli with BCR in the total glomeruli in the BCR group. (F) Glomeruli with BCR in all glomeruli with crescents. (G) Glomeruli with BCR in all glomeruli with crescents in the BCR group. (H) Proportion of glomeruli with BCR in total cases.
To investigate the correlations between BCR and clinical features, patients were divided into 2 groups according to the presence of BCR. Compared with IgAV-N patients without BCR, patients with BCR had a higher frequency of gross hematuria, positive urine leukocytes, severe proteinuria, and higher D-dimer levels, while ALB and IgG were lower (all p < 0.05). However, other indicators, such as the mean arterial pressure, serum creatinine, eGFR, and urinary erythrocytes, were not significantly different between the two groups (all p > 0.05) , Supplementary Table 1, 2).
Distribution of BCR in ISKDC pathological classification
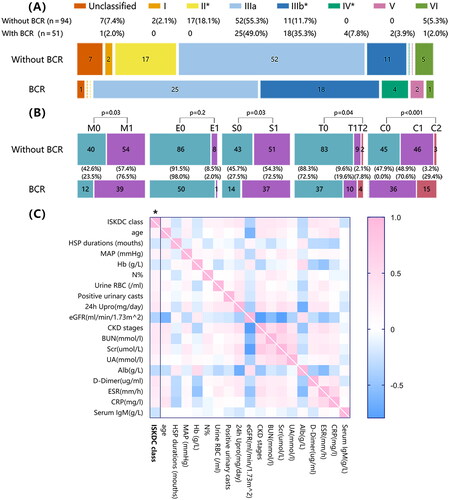
According to ISKDC pathological classification, 2 (1.4%) patients were type I, 17 (11.7%) were type II, 77 (53.1%) were type IIIa, 29 (20.0%) were type IIIb, 4 (2.8%) were type IV, 2 (1.4%) were type V, 6 (4.1%) were type VI, and 8 (5.5%) were unclassified purpura nephritis. The distribution of BCR in each pathological classification indicated more patients had BCR in type IIIb and type IV, while BCR was less common in type II patients ().
Figure 2. Study cohort classified according to the pathological diagnosis, compared based on BCR, and the association between the ISKDC classification and clinical manifestations in patients with IgAV-N. (A) Patients were divided into I∼VI grades according to the ISKDC classification. The ISKDC classification includes grade I (minimal histological alterations), grade II (mesangial proliferation without crescents), grade III (mesangial proliferation with <50% crescents, a. focal, b. diffuse), grade IV (mesangial proliferation with 50 ∼ 75% crescents), grade V (mesangial proliferation with >75% crescents, a. focal, b. diffuse), and grade VI (membranoproliferative-pattern glomerulonephritis). (B) We re-evaluated the patients and reclassified the patients based on the MEST-C score of IgA nephropathy. The MEST-C score includes mesangial hypercellularity (M0 < 50%; M1 ≥ 50% of the glomeruli), endocapillary hypercellularity (E0: absent; E1: present), segmental glomerulosclerosis (S0: absent; S1: present), tubular atrophy and interstitial fibrosis (T0: 0 ∼ 24%; T1: 25 ∼ 49%; and T2: ≥50% of the cortical area), and cellular or fibro-cellular crescents (C0: absent; C1: 1 ∼ 24%; and C2: ≥25% of the glomeruli). *p < 0.05. (C) The comparisons were based on Spearman correlation analyses. The heatmap reflects the mean values of Spearman’s ρ, and the first column with asterisks indicates p < 0.05. MAP, mean arterial pressure, MAP=(SBP + 2 × DBP)/3; Hb, hemoglobin; urine RBC, urine erythrocyte; 24 h Upro, 24 h urine protein; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; Scr, serum creatinine; UA, blood uric acid; Alb, serum albumin; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Association between BCR and crescents, segmental sclerosis, and interstitial inflammation
The BCR group had a higher proportion of crescents, cellular crescents, fibro-cellular crescents (p < 0.01), segmental sclerosis (p < 0.05), and more severe interstitial inflammation (p = 0.01) (). No significant difference was found in immune complex deposition between the two groups (Supplementary Figure 1).
Clinical features and pathological changes in IgA-N patients with BCR combined with crescents
Because all patients with BCR were characterized by at least one crescent, we further analyzed the clinical and pathological features of patients with BCR combined with crescents. Among the patients with crescents, the proportions of crescents, fibro-cellular crescents, and segmental sclerosis were higher in those with BCR (p < 0.05). However, the differences in gross hematuria, proteinuria, CKD stage, and ALB disappeared between the groups with or without BCR (p > 0.05) ().
Table 2. Comparison of the baseline laboratory examination and pathological characteristics of IgAV-N patients with crescents (n = 100) with or without BCR.
Correlation between ISKDC classification and clinical manifestations
Correlation analysis showed that the ISKDC classification correlated with the clinical manifestations of IgAV-N patients. ISKDC grade was positively correlated with age, mean arterial pressure, hematuria, proteinuria, degree of renal function decline, erythrocyte sedimentation rate and C-reactive protein and negatively correlated with hemoglobin (Hb) and ALB ().
Role of BCR and ISKDC classification in predicting prognosis
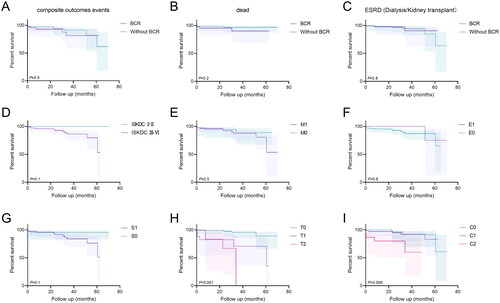
The median follow-up time of the 117 patients involved in the prognosis analysis was 30.5 (15.35, 44.53) months. Among these patients, 12 (10.26%) reached the endpoint, of whom 5 (4.27%) died and 7 (5.98%) developed ESRD (one received kidney transplantation). Kaplan–Meier plots showed no significant difference in event-free survival between patients with or without BCR when all-cause death or ESRD (including dialysis and kidney transplantation) or both were used as endpoint events (all p > 0.05). In addition, we found no correlation between ISKDC classification and the prognosis of patients with IgAV-N (p > 0.05) (), similar to previous studies [Citation4,Citation5,Citation8–12].
Figure 3. Survival in the two groups of IgAV-N patients and survival curves according to MEST-C scores in patients followed up for more than 6 months. (A) Survival without composite endpoint, defined as all-cause death, creatinine doubled, ESRD, dialysis, and kidney transplantation. (B) Survival without all-cause death. (C) Survival without creatinine doubled, ESRD, dialysis, and kidney transplantation. (D) Survival without composite endpoint (all-cause death, creatinine doubled, ESRD, dialysis and kidney transplantation) according to ISKDC pathological classification (class I/II vs. III-VI). (E-I) Survival without composite endpoint according to MEST-C scores, in sequence are M0 vs. M1; E0 vs. E1; S0 vs. S1; T0/T1/T2; C0/C1/C2. p < 0.05, statistically significant.
Clinical and prognostic value of the MEST-C score in IgAV-N
Because IgAV-N and IgAN have similar pathological mechanisms and pathological features [Citation2], previous studies have tested the application of the MEST-C score in IgAV-N [Citation7–9,Citation11,Citation12,Citation16]. In this study, we also observed the role of the MEST-C score in the clinical features, pathological changes, and prognosis of IgAV-N. Patients with BCR had higher M, S, T, and C scores than those without BCR (; all p < 0.05). A more serious clinical manifestation of patients in the M1, T1/T2, and C1/C2 groups was observed compared with that in the M0, T0, and C0 groups, mainly manifested in higher blood pressure, urine RBC and WBC, 24-hour urine protein, lower Hb, eGFR, and ALB, and severe interstitial inflammation (Supplementary Table 3; all p < 0.05). Furthermore, patients with BCR had more severe clinical symptoms than those without BCR in the groups with higher M, S, T, and C scores (p < 0.05) (Supplementary Table 4, 5 and Supplementary Figure 2). However, the compound event-free survival was longer in Groups T0 and C0 than in Groups T1/T2 and C1/C2 (p < 0.001 and p = 0.009, respectively) (). Cox regression analysis showed that interstitial fibrosis/tubular atrophy >50% and Scr were independent risk factors for the compound outcome ().
Table 3. Univariate and multivariate Cox proportional hazard regression analyses for compound endpoint events in patients followed up for more than 6 months.
BCR enhances the value of the MEST-C score in predicting prognosis in IgAV-N
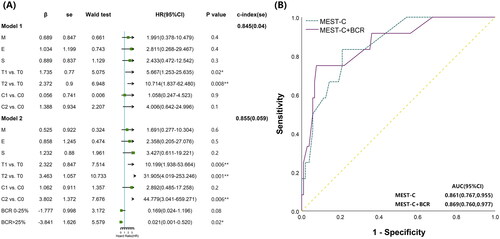
We further evaluated the value of the BCR on the predictive ability of the MEST-C score for prognosis in IgAV-N. Two Cox proportional hazards models of MEST-C (Model 1) and MESTC + BCR (Model 2) were established. The MEST-C score was valuable for predicting prognosis in IgAV-N, and T was an independent risk factor for poor prognosis (p < 0.05). The MESTC + BCR Model showed that T and C were independent risk factors (all p < 0.05), statistically increasing the c-index from 0.845 to 0.855 (). The predictive sensitivity and specificity of the MEST-C Model were 83.3% and 79.0%, respectively, while they were 75.0% and 92.4%, respectively, in the MESTC + BCR Model, associated with the area under the ROC curves (0.869 vs. 0.861) (). BCR may contribute to the effectiveness of the MEST-C score in predicting prognosis in IgAV-N.
Figure 4. Contribution of Bowman’s capsule rupture to the MEST-C score in predicting the prognosis of patients. (A) A Cox proportional hazard model including only MEST-C and MEST-C with BCR was established, and the c-index was calculated. The analysis showed that T, C, and BCR were independent predictors of composite outcomes, and BCR improved the c-index of the model. ROC, receiver operating characteristic; AUC, area under the ROC curve. (B) Comparison of ROC curves between only MEST-C scores and MEST-C with BCR, which were divided into grades 0-2 (grade 0: no BCR, grade 1: ≤25%, grade 2: >25%) for predicting the compound endpoint events. *p < 0.05, **p < 0.01.
Discussion
We found that in adult patients with IgAV-N, BCR was correlated with some clinical manifestations and pathological changes, especially crescents. The ISKDC classification and MEST-C score were both related to clinical manifestations, especially the deterioration of renal function, but only the MEST-C score reflected prognosis. In addition, BCR improved the predictive ability of the MEST-C score in patients with IgAV-N.
Previous studies by our team have found that Bowman’s capsule integrity may prevent the fibrous crescent formation and irreversible damage in experimental crescentic glomerulonephritis [Citation17,Citation18]. An early study on crescentic glomerulonephritis (CGN) and a recent study on ANCA-GN showed that the proportion of fibrous crescents, interstitial inflammation, and myofibroblasts was positively correlated with the degree of BCR [Citation20,Citation26]. In addition, BCR was associated with glomerular CD8+ T-cell infiltration in patients with anti-glomerular basement membrane (GBM) glomerulonephritis and IgAN [Citation17,Citation27]. BCR is also common in ANCA-GN (50–70% of patients) and is associated with severe deterioration of renal function [Citation19–21,Citation24]. Furthermore, in myeloperoxidase-ANCA-associated glomerulonephritis (MPO-ANCA-GN), IgA and IgM deposition were negatively correlated with BCR [Citation28]. These data indicated that although BCR may not be a disease-specific pathological feature, it may be a complication linked to severe renal inflammation and injury in various types of immune-mediated glomerulonephritis. However, the role of BCR in IgAV-N is unknown. In this study, 35.17% of patients with IgAV-N presented BCR, which is less than its incidence in ANCA-GN. In addition, patients with BCR had more serious gross hematuria and proteinuria, suggesting that BCR is correlated with more serious clinical manifestations in patients with IgAV-N.
Crescents, tubulointerstitial inflammation, and glomerular sclerosis are important pathological features of IgAV-N [Citation10]. However, the relationship between BCR and pathological features in patients with IgAV-N is unknown. Here, in IgAV-N, patients with BCR have more serious mesangial hypercellularity, segmental glomerulosclerosis, tubular atrophy, interstitial fibrosis and crescents, indicating that BCR may contribute to renal injury in IgAV-N, a finding that is consistent with that in patients with ANCA-GN [Citation20,Citation26]. Furthermore, because crescents are a symptom of severe pathological changes in patients with IgAN-V (approximately 50% of patients in the data) [Citation8,Citation11,Citation12], we also found that all patients with BCR were characterized by at least one crescent, and patients with BCR had a higher proportion of crescents. The severity of the clinical manifestations is more strongly associated with the presence of crescents, whereas BCR is closely related to crescents, supporting previous studies in CGN [Citation17,Citation18,Citation20,Citation26]. Thus, BCR may be another manifestation of the crescent or a consequence of a severe crescent.
With the increase in the grade of ISKDC classification, patients had more daily urine protein, higher Scr and lower eGFR [Citation9]. Additionally, the mean arterial pressure, hematuria, blood urea nitrogen, blood uric acid, Hb, ALB, D-dimer, ESR, and CRP were related to this classification. Furthermore, previous studies showed that ISKDC classification was not associated with renal outcome [Citation4,Citation5,Citation8–12], a finding that was consistent with ours.
Because ISKDC classification cannot forecast the outcome of patients with IgAV-N and no other recognized prediction tool exists, a new approach must be identified to better evaluate patients with IgAV-N. In addition, the IgA Nephropathy Classification Working Group and KDIGO suggest that the Oxford MEST-C score may be applicable in IgAV-N and should be further validated [Citation14,Citation15]. Recent studies have reported that the MEST-C score is related to clinical features in children with IgAV-N [Citation11,Citation12,Citation16], but its application in adults is limited. A few studies in adults found a better correlation between E/T and the prognosis of IgAV-N [Citation8,Citation12,Citation16], while in pediatric patients, the MEST-C score (especially S and T) is meaningful in predicting prognosis [Citation7,Citation9,Citation11,Citation16]. Thus, we also applied the MEST-C score to evaluate the clinical prognosis of patients with IgAV-N. In IgAV-N, patients in the M1, S0, T1/T2, and C1/C2 groups had more severe clinical manifestations, which were further aggravated in the BCR group, especially in the S0, T1/T2, and C1/C2 groups. Furthermore, the multivariate Cox models showed that the MEST-C score (T and C) was valuable to predict the prognosis of patients with IgAV-N; moreover, it was more valuable when BCR was added to the MEST-C score. This finding was similar to that in previous studies on ANCA, which showed that adding the index of BCR can improve the performance of the original prognostic model [Citation20,Citation24].
Previous studies on CGN have shown that initial damage to the glomerular capillary wall leads to damage to endothelial cells and podocytes. Thereafter, the parietal epithelial cells (PECs) of Bowman’s capsule proliferate to form crescents, and damaged podocytes secrete inflammatory factors and chemokines, causing activation and proliferation of CD4+ and CD8+ T cells, acting together with neutrophils and macrophages to destroy Bowman’s capsule [Citation17,Citation18,Citation26,Citation27,Citation29,Citation30]. After BCR occurs, immunocytes (T cells, macrophages) and interstitial myofibroblasts invade Bowman’s space and glomerular clusters, leading to the formation of fibrous crescents and kidney damage [Citation20,Citation26,Citation27,Citation29–31]. Furthermore, BCR may cause primary urine to diffuse into the interstitium, leading to inflammatory destruction of adjacent nephrons [Citation20]. This finding indicates that as a downstream event of glomerular inflammation and crescent formation, patients with BCR have more severe renal injury [Citation30]. However, whether the mechanism above is involved in the BCR formation of IgAV-N needs warrants further study.
This study has limitations. This was a single-center retrospective study with an insufficient sample size and short follow-up. The effect of treatment on prognosis should be further studied in the future.
Conclusion
The present study demonstrated for the first time that in adult patients with IgAV-N, BCR is associated with more severe clinical manifestations and pathological changes, especially crescents. The ISKDC and MEST-C classifications are related to clinical manifestations, especially renal function, but the ISKDC classification cannot reflect prognosis. The MEST-C score can predict the prognosis of IgAV-N patients, while BCR can enhance the predictive ability of the MEST-C score.
Ethical approval
This study has passed the ethical review of the Second Xiangya Hospital (XY210105).
Author contributions
Conception and design: Sun L, Zhu XJ, Zhao Q, Duan TY, Chen AQ; data acquisition: Zhu XJ, Zhao Q, Duan TY; data analysis/interpretation: Zhao Q, Duan TY, Sun L, Chen AQ, Zhu XJ, Yuan SG; statistical analysis: Zhao Q, Duan TY; drafting of the paper: Zhao Q; revising of the paper: Sun L, Zhu XJ, Zhao Q, Duan TY; supervision or mentorship: Sun L, Zhu XJ, Chen AQ, He LY, Liu H, Yuan SG, Tang CY, Liu YH, Liu Y, Liu FY, Xiao L. All the authors contributed important intellectual content during manuscript drafting and accepted accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of this work.
Supplemental Material
Download PDF (625.8 KB)Acknowledgements
The authors are thankful to the physicians and nurses of the Second Xiangya Hospital, Central South University and patients who made it possible to conduct this study.
Disclosure statement
All the authors reported no conflict of interest.
Data availability statement
The data that underlie the findings of this article are available from the corresponding author (Lin Sun), upon reasonable request.
Additional information
Funding
References
- Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14(7):1–11.
- Calvo-Río V, Loricera J, Martín L, et al. Henoch-Schönlein purpura nephritis and IgA nephropathy: a comparative clinical study. Clin Exp Rheumatol. 2013;31(1 Suppl 75):S45–S51.
- Lu S, Liu D, Xiao J, et al. Comparison between adults and children with Henoch-Schönlein purpura nephritis. Pediatr Nephrol. 2015;30(5):791–796.
- Jelusic M, Sestan M, Cimaz R, et al. Different histological classifications for Henoch-Schönlein purpura nephritis: which one should be used? Pediatr Rheumatol Online J. 2019;17(1):10.
- Inagaki K, Kaihan AB, Hachiya A, et al. Clinical impact of endocapillary proliferation according to the Oxford classification among adults with Henoch-Schönlein purpura nephritis: a multicenter retrospective cohort study. BMC Nephrol. 2018;19(1):208.
- Counahan R, Winterborn MH, White RH, et al. Prognosis of Henoch-Schönlein nephritis in children. Br Med J. 1977;2(6078):11–14.
- Clavé S, Sordet M, Tsimaratos M, et al. Association of kidney biopsy findings with short- and medium-term outcomes in children with moderate-to-severe IgA vasculitis nephritis. Eur J Pediatr. 2021;180(10):3209–3218.
- Kim CH, Lim BJ, Bae YS, et al. Using the Oxford classification of IgA nephropathy to predict long-term outcomes of Henoch-Schönlein purpura nephritis in adults. Mod Pathol. 2014;27(7):972–982.
- Huang X, Ma L, Ren P, et al. Updated Oxford classification and the international study of kidney disease in children classification: application in predicting outcome of Henoch-Schönlein purpura nephritis. Diagn Pathol. 2019;14(1):40.
- Foster BJ, Bernard C, Drummond KN, et al. Effective therapy for severe Henoch-Schonlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J Pediatr. 2000;136(3):370–375.
- Xu K, Zhang L, Ding J, et al. Value of the Oxford classification of IgA nephropathy in children with Henoch-Schönlein purpura nephritis. J Nephrol. 2018;31(2):279–286.
- Wang M, Wang R, He X, et al. Using MEST-C scores and the international study of kidney disease in children classification to predict outcomes of Henoch-Schönlein purpura nephritis in children. Front Pediatr. 2021;9:658845.
- Jimenez A, Chen A, Lin J-J, et al. Does MEST-C score predict outcomes in pediatric Henoch-Schönlein purpura nephritis? Pediatr Nephrol. 2019;34(12):2583–2589.
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276.
- Trimarchi H, Barratt J, Cattran DC, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. 2017;91(5):1014–1021.
- Yun D, Kim DK, Oh K-H, et al. MEST-C pathological score and long-term outcomes of child and adult patients with Henoch-Schönlein purpura nephritis. BMC Nephrol. 2020;21(1):33.
- Chen A, Lee K, D’Agati VD, et al. Bowman’s capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. J Clin Invest. 2018;128(8):3413–3424.
- Chen A, Lee K, Guan T, et al. Role of CD8+ T cells in crescentic glomerulonephritis. Nephrol Dial Transplant. 2020;35(4):564–572.
- Hakroush S, Tampe B. Correspondence on ‘Bowman’s capsule rupture on renal biopsy improves the outcome prediction of ANCA-associated glomerulonephritis classifications. Ann Rheum Dis. 2023;82(5):e125–e125.
- Hakroush S, Tampe D, Korsten P, et al. Bowman’s capsule rupture links glomerular damage to tubulointerstitial inflammation in ANCA-associated glomerulonephritis. Clin Exp Rheumatol. 2021;39 Suppl 129(2):27–31.
- L’Imperio V, Vischini G, Ferraro M, et al. Response to: ‘Correspondence on ‘bowman’s capsule rupture on renal biopsy improves the outcome prediction of ANCA-associated glomerulonephritis classifications’’ by Hakroush and Tampe. Ann Rheum Dis. 2023;82(5):e126–e126.
- Sethi S, Haas M, Markowitz GS, et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol. 2016;27(5):1278–1287.
- Szabó J, Biró I, Tóth T, et al. Granulomatous glomerulonephritis, without systemic disorder. Virchows Arch A Pathol Anat Histol. 1978;381(1):111–119.
- L’Imperio V, Vischini G, Pagni F, et al. Bowman’s capsule rupture on renal biopsy improves the outcome prediction of ANCA-associated glomerulonephritis classifications. Ann Rheum Dis. 2022;81(6):e95–e95.
- Ozen S, Marks SD, Brogan P, et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin a vasculitis-the SHARE initiative. Rheumatology. 2019;58(9):1607–1616.
- Goumenos D, Tsomi K, Iatrou C, et al. Myofibroblasts and the progression of crescentic glomerulonephritis. Nephrol Dial Transplant. 1998;13(7):1652–1661.
- Hu S-Y, Jia X-Y, Li J-N, et al. T cell infiltration is associated with kidney injury in patients with anti-glomerular basement membrane disease. Sci China Life Sci. 2016;59(12):1282–1289.
- Lin W, Shen C, Zhong Y, et al. Glomerular immune deposition in MPO-ANCA associated glomerulonephritis is associated with poor renal survival. Front Immunol. 2021;12:625672.
- Robertson H, Wheeler J, Morley AR. Anti-glomerular basement membrane glomerulonephritis in the mouse: the role of macrophages. Int J Exp Pathol. 1995;76(2):157–162.
- Anguiano L, Kain R, Anders H-J. The glomerular crescent: triggers, evolution, resolution, and implications for therapy. Curr Opin Nephrol Hypertens. 2020;29(3):302–309.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63(3):1164–1177.
