 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and aims
We aimed to assess the potential socio-demographic, clinical, and lifestyle-related risk factors for kidney function decline (KFD), defined as ≥30% estimated glomerular filtration rate (eGFR) decline, in an Iranian cohort study.
Methods
7190 participants (4049 women) aged 20–90 years with 2–5 eGFR data from examinations (2001–2005 to 2015–2018) were included. Cox proportional hazard models were used to examine the association between potential risk factors and eGFR decline.
Results
During 11.5 years of follow-up, 1471 (889 women) participants had incident KFD with a crude incidence rate of 192.1 (182.6–202.2) per 10,000 person-year. Among the total population, older age, female gender, prehypertension, hypertension, diabetes, widowed/divorced states, higher triglycerides (TG), prevalent cardiovascular diseases (CVD), and higher baseline eGFR were significantly associated with higher, while moderate physical activity and a positive family history of diabetes were associated with lower risk of KFD (all p values <.05). Prevalent CVD in women but not men, diabetes, and hypertension among postmenopausal than premenopausal women were significant risk factors of KFD. According to the presence of chronic kidney disease (CKD) at baseline, higher eGFR decreased the risk of KFD in patients with CKD and increased KFD risk in those without CKD (all p for interactions <.05).
Conclusion
KFD is associated with multiple modifiable risk factors among the Iranian urban population that is affected by gender, menopausal status, and initial kidney function. Interventions targeting these factors might potentially help reduce the burden of KFD.
Menopausal status may influence the relationship between cardiometabolic risk factors and KFD;
The impact of higher baseline eGFR on the risk of KFD differed between subjects with preserved kidney function and CKD patients.
The interaction between gender, menopausal status, and baseline kidney function with different risk factors on KFD may help to make renal risk prediction scores to identify those in the general population at risk who may benefit from early prevention.
Key messages:
Introduction
The Middle East and North Africa (MENA) region has a high and increasing burden of CKD, with more than 48 million subjects suffering from CKD in 2017 [Citation1]; also, the age-standardized prevalence rate of CKD has increased by 37.6% for both genders during the past 3 decades [Citation2]. The prevalence and incidence of CKD also appear to be high among Iranians [Citation3,Citation4], with more than 2% of the population developing CKD annually [Citation4]. While current evidence and clinical practice guidelines regarding primary and secondary prevention of cardiovascular morbidity and mortality focus on the progression of CKD toward end-stage kidney diseases (ESKD), several reports have shown an increased rate of poor health outcomes among people with a moderate deterioration in kidney function [Citation5–10]. Considering the high CVD burden attributable to impaired kidney function in the MENA region [Citation1], it is important to identify risk factors for eGFR decline as a surrogate endpoint for kidney disease incidence and progression.
Multiple studies have reported gender-specific differences in the risk factors for eGFR decline [Citation11–15]. Among CKD patients, the rate of decline in eGFR has been reported to be faster in men, with a higher risk of progression to kidney replacement therapy among men compared to women [Citation16], while women of postmenopausal age are shown to have a faster rate of kidney disease progression [Citation17]. However, results about gender disparities in KFD are still controversial; therefore, the role of its sex-specific predictors remains to be further elucidated.
Most studies on the predictors of KFD have used incident CKD or ESKD, and the remaining studies on longitudinal changes of eGFR are limited by relatively small numbers [Citation18–22] and lack of sequential measurements in kidney function [Citation14,Citation15,Citation18–20,Citation22]. More importantly, to our knowledge, the effect modification of the presence or absence of CKD and menopausal status on potential risk factors for KFD was not addressed in previous studies using appropriate statistical methods. Moreover, current studies have been conducted mainly on American, European, and East Asian populations [Citation14,Citation15,Citation18–25] with a lower age-standardized rate of CVD disability-adjusted life-years (DALYs) attributable to impaired kidney function than MENA region [Citation1].
Hence, this study aimed to investigate the association of potential socio-demographic, clinical, and lifestyle-related risk factors with the risk of KFD, defined as eGFR decline ≥30% [Citation7,Citation26,Citation27], and also the potential effect modification of gender, menopausal status, and baseline kidney function status on KFD risk in the large-scale, long-term, and population-based cohort study known as Tehran Lipid and Glucose Study (TLGS).
Materials and methods
Study design and participants
The TLGS is a large-scale, population-based, prospective cohort study first established to determine the epidemiology of non-communicable diseases (NCDs) and their subsequent risk factors. TLGS enrollment was carried out in two phases: the first enrolment phase was from January 31, 1999, to July 03, 2001, and the second one was from October 20, 2001, to September 22, 2005. Follow-up examinations occurred at approximately 3-year intervals: 2005–2008 (phase 3), 2008–2011 (phase 4), 2011–2014 (phase 5), and 2015–2018 (phase 6). The design, registration, data collection protocols, and survey instruments of the TLGS have been described previously [Citation28].
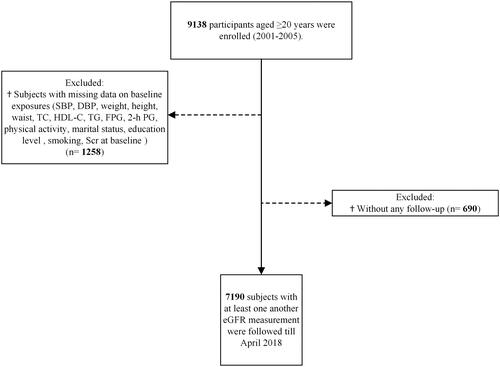
In the current study, 9138 individuals who were 20 years or older and entered the second phase (2001–2005) were considered for inclusion. As shown in , after excluding those with missing values for any of the analyzed variables (N = 1258, considering overlapping features) and subjects without any follow-up measurements after baseline recruitment (N = 690), 7190 individuals (women = 4049) remained for the analysis.
Figure 1. Flow chart of inclusions and exclusions of the study participants. SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglycerides; FPG: fasting plasma glucose; 2-h PG: 2-hour post-challenge plasma glucose; Scr: serum creatinine; eGFR: estimated glomerular filtration rate.
The proposal of this study was approved by the institutional review board of the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences, and each participant gave written informed consent.
Clinical and laboratory measurements
A standard questionnaire was used to collect demographic information, smoking status, past medical history of CVD, family history of diabetes (FH-DM), drug history, education status, physical activity, and marital status from the study participants. Body measurements of the study population [weight (kg) and height (cm)] were recorded while participants were minimally clothed and removed their shoes. Waist circumference (WC) was measured at the level of the umbilicus while wearing light clothing. After resting for 15 min sitting, the systolic and diastolic blood pressures (SBP and DBP) were measured two times by trained personnel on the right arm with a standard mercury sphygmomanometer. We used the Modifiable Activity Questionnaire (MAQ) to collect data on physical activity [Citation29]; the psychometric properties of the Iranian version of MAQ have been examined among the population from TLGS, with high reliability and acceptable validity [Citation30]. After a 12–14 h overnight fasting, blood samples for biochemical analysis were collected between 7:00 and 9:00 am. For the 2-h post-challenge plasma glucose (2-h PG), subjects not using glucose-lowering medication took 82.5-gram glucose monohydrate solution, and a blood sample was taken 2 h later. Details for laboratory measurements including plasma glucose and lipid profile (i.e. TG, total cholesterol [TC], and high-density lipoprotein cholesterol [HDL-C]) are reported elsewhere [Citation28]. Kidney function was measured by serum creatinine levels (Scr), assayed by kinetic colorimetric Jaffe using commercial kits (Pars Azmoon Inc., Tehran, Iran) and a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, The Netherlands). The sensitivity of the assay was 0.2 mg/dL, and reference intervals according to the recommendations of the manufacturer were 80–115 μmol/L in men and 53–97 μmol/L in women. Both intra- and inter-assay coefficient of variations were <3.1% in all phases. After every 25 tests, an assay performance monitoring was conducted using lyophilized serum controls in normal and abnormal ranges. Analysis of each sample was done only if the internal quality control met acceptable criteria and it was performed on the same day of blood collection at the TLGS research laboratory.
Outcome
The study endpoint was KFD, defined with ≥30% decline in eGFR during follow-up from baseline. This endpoint was recently recommended as a surrogate marker for CKD progression [Citation7,Citation26,Citation27].
Definition of terms
Potential exposures included age (20–34 years (reference), 35–49 years, 50–64 years, and ≥65 years for both genders), gender (male as reference), general obesity status defined as BMI <25 kg/m2 [reference], 25–30 kg/m2 (overweight), and ≥30 kg/m2 (obese). Other exposures included education (<6 years, 6–12 years, and >12 years [reference]), marital status (single, married [reference], divorced/widowed), and smoking (current, past, and never [reference]). Based on the recommendations of JNC 7 Guidelines [Citation31], we categorized the baseline blood pressure of the study participants into 3 groups; optimal, normal: SBP <120 mmHg and DBP <80 mmHg (reference), prehypertension: SBP 120–139 mmHg and/or DBP 80–90 mmHg, and hypertension: SBP ≥140 mmHg or DBP ≥90 mmHg or taking antihypertensive medications. FH-DM was defined as a positive report of diabetes in a first-degree relative. History of CVD was any history of percutaneous coronary intervention, acute coronary syndrome leading to CCU admission, coronary artery bypass graft, angiographic proven coronary artery disease, or history of cerebrovascular accidents.
Diabetes mellitus was defined as having FPG ≥7 mmol/L (≥126 mg/dL) and/or 2-h PG ≥11.1 mmol/L (≥ 200 mg/dL) and/or the use of glucose-lowering medications. Additionally, prediabetes status was defined with 5.6≤ FPG <7 mmol/L (100–125 mg/dL) or 7.8 ≤ 2-h PG <11.1 mmol/L (140–199 mg/dL). Having FPG <5.6 mmol/L and 2-h PG <7.8 was considered normal glucose status. Physical activity levels were categorized based on the average metabolic equivalent tasks score (METs); sufficiently active: physical activity of ≥1500 MET mins/wk, moderately active: 600–1500 MET mins/wk, and inactive: <600 MET mins/wk. CKD at baseline was defined as eGFR <60 mL/min/1.73 m2 [Citation32]. eGFR was estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [Citation33]:
In this equation, eGFR is expressed as mL/min per 1.73 m2, Scr is expressed as mg/dL, κ is 0.7 and 0.9 for women and men, respectively, α is −0.329 and −0.411 for women and men, respectively. The Scr levels of study participants were standardized by reducing their levels by five percent as the CKD-EPI equation requires standardized creatinine values [Citation34].
Statistical analysis
Baseline characteristics of the study population were shown as mean (standard deviation: SD) values and frequencies (%) for continuous and categorical variables, respectively. For covariates with a skewed distribution (e.g. TG), the median (interquartile range [IQR]) was reported. We compared characteristics of participants according to gender as well as between respondents and non-respondents (those with missing data at baseline or without any follow-up after baseline measurement) using the Student’s t-test, Kruskal–Wallis test, and the Pearson’s χ2 test, as appropriate. The person-year method was used to obtain incidence rates of KFD, which was reported as the number of cases per 10,000 person-years. The association of potential risk factors with incident KFD was analyzed using multivariable Cox regression models to calculate the hazard ratio (HR) and 95% confidence interval (CI). We checked the proportionality assumptions to be appropriate (using Schoenfeld’s global test of residuals). No evidence of lack of proportionality in the HRs was found. We also performed a competing risk analysis using the Fine and Grey method [Citation35] to account for the competing risk of death before the incident KFD. We chose the potential risk factors using a priori approach that might be considered potential confounders from important studies in this field [Citation4,Citation15,Citation19]. As needed, variables with a skewed distribution were log-transformed before analysis (e.g. TG). The event date was calculated as the time of incident eGFR decline of ≥30%. The event date for subjects with incident KFD was defined as the mid-time point between the date of the follow-up visit at which the event was detected for the first time and the last attended follow-up visit before the diagnosis; survival time was defined as the difference between the date of the event and the date of baseline visit. For the censored participants, survival time was calculated as the interval between the baseline visit and the time of death, loss to follow-up, or end of the study (April 2018), whichever occurred first. Several subgroup analyses were carried out; the analyses stratified by CKD at baseline, gender, and menopausal status (considering the age of 48.2 years as menopausal age among Iranian women [Citation36]) were further performed. Additionally, as a sensitivity analysis, multiple imputations (20 datasets corresponding to the ∼20% missingness in data, with a rule of thumb of at least 1 imputation per % of incomplete cases [Citation37,Citation38]) of missing data of exposure variables using two-fold fully conditional specification (FCS) algorithm [Citation39] and Rubin’s rules [Citation40] was carried out. We did not impute the outcome variable, as we analyzed only participants for whom incident outcome could be ascertained. For conducting statistical analysis, STATA version 14 SE (StataCorp LP, TX, USA) was used. All p-values were two-tailed. p-value <.05 was considered significant for all tests.
Results
The study population included 7190 participants (4049 women) with a mean age (SD) of 43.54 (14.6) years. As shown in Table S1, study respondents had higher BMI, lower HDL-C levels, and were less physically active, while marriage was found to be less prevalent among the non-respondents. Non-respondents were also more likely to be current smokers, take glucose-lowering and antihypertensive medications, and have higher FPG and CVD prevalence than study respondents. No significant differences regarding age, 2-h PG, WC, SBP, DBP, TC, TG, eGFR at baseline, gender proportion, education level, family history of diabetes, and taking lipid-lowering medications existed between respondents and non-respondents.
The baseline characteristics of the subjects in both genders are shown in ; accordingly, men were older, had lower HDL-C levels, higher WC, SBP, DBP, and TG values, the prevalence of CVD, and FH-DM, were more likely to be current smokers, and physically inactive, while women had lower eGFR values, higher BMI, 2-h PG, TC levels, consumption of medications, were less likely to be highly educated and married.
Table 1. Baseline characteristics of the study participants stratified by gender: Tehran Lipid and Glucose Study.
During the median follow-up (IQR) of 11.48 (9-39-12.85) years, 1471 participants had incident KFD, and 326 died before the outcome occurrence.
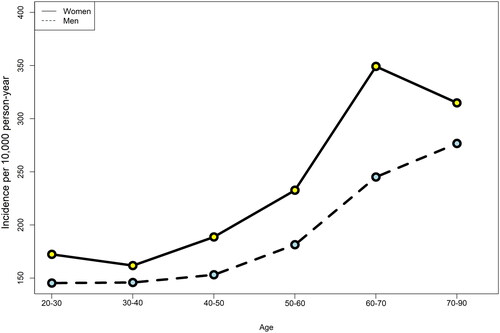
presents the sex-specific crude incidence rates per 10,000 person-year among men and women by 10-year age group categories. In men and women, the incidence of KFD increased at ages 30–40 years and above. The crude incidence rate of KFD was 192.1 (182.6–202.2) per 10,000 person-year in the total population.
Figure 2. The sex-specific crude incidence rate of kidney function decline per 10,000 person-year, stratified by 10-year age group categories. Legend: Due to the small numbers of those aged ≥80 years, all subjects aged ≥70 years were categorized into one age group.
shows the crude and adjusted HR and 95% CI of incident KFD associated with potential risk factors. Accordingly, in the univariable model (model 1), age groups of 50-64 and ≥65 years, BMI ≥30 kg/m2, higher WC, female gender, prehypertension, hypertension (43% on antihypertensive medications), prediabetes, diabetes (45% on glucose-lowering medications), being widowed/divorced, education level <6 years, increasing TG, using lipid-lowering medications, and prevalent CVD significantly increased the risk of KFD (all p values < .05). Additionally, among the potential predictors, family history of diabetes and moderate physical activity were associated with lower risks of KFD [HR: 95% CI: 0.82 (0.71–0.94) and 0.86 (0.75–0.98), respectively]. In the multivariable-adjusted analysis including the baseline eGFR (model 3), each 1 mL/min/1.73 m2 greater eGFR increased the risk of the incident outcome by 6% [HR: 95% CI: 1.06 (1.05–1.06)]. Additionally, the association of the age groups of 35–49, 50–64, and ≥65 years, female gender [1.61 (1.40–1.85)], prehypertension [1.21 (1.06–1.38)], hypertension [1.73 (1.48–2.01)], diabetes [1.30 (1.10–1.53)], moderate physical activity [0.84 (0.73–0.96)], being widowed/divorced [1.23 (1.01–1.49)], increasing TG [1.09 (1.01–1.17)], prevalent CVD [1.36 (1.09–1.68)], and FH-DM [0.87 (0.76–1.00)] with the KFD were significant in model 3. In the competing risk analysis, results remained unchanged after considering the impact of all-cause mortality events as a competing risk ().
Table 2. Association of risk factors with decline in kidney function in the Tehran Lipid and Glucose Study.
Subgroup analysis
Results of the subgroup analysis by CKD status, gender, and menopausal status are shown in . As shown in , among those without prevalent CKD, increasing age, baseline eGFR (p for interaction .037 and <.001, respectively), increasing TG, female gender, prehypertension, hypertension, diabetes, being unmarried, and prevalent CVD increased the risk of KFD. In contrast, moderate physical activity and FH-DM were associated with lower risk. However, for those with CKD, only hypertension was associated with a higher risk of incident KFD, while lower education levels (p for interaction <.05) and higher baseline eGFR decreased the risk.
Table 3. Sub-distribution Hazard ratios and 95% CI of risk factors for the decline in kidney function, stratified by baseline eGFR, in the Tehran Lipid and Glucose StudyTable Footnotea.
Table 4. Sub-distribution Hazard ratios and 95% CI of risk factors for the decline in kidney function, stratified by gender, in the Tehran Lipid and Glucose StudyTable Footnotea.
Table 5. Sub-distribution Hazard ratios and 95% CI of risk factors for the decline in kidney function, stratified by menopausal status among women, in the Tehran Lipid and Glucose StudyTable Footnotea.
When stratifying the results by gender, as shown in , all age groups, hypertension, and increasing baseline eGFR among both genders were associated with a higher incidence of KFD. Moreover, prehypertension [HR: 95% CI: 1.34 (1.13–1.59)] and prevalent CVD [1.60 (1.20–2.13), p for interaction <.05], being widowed/divorced [1.27 (1.02–1.59)] increased the risk of incident KFD among women. Among men, the lowest education levels (p for interaction <.05) decreased the risk of KFD by 26%. Compared to normal glucose levels, diabetes and increasing TG in men were also associated with a significantly higher risk of incident outcome [1.45 (1.11–1.90) and 1.17 (1.04–1.31), respectively].
As shown in , when we categorized female participants according to menopausal status, hypertension [HR: 95% CI: 1.84 (1.37–2.46)] and diabetes [1.32 (1.01–1.73)] were associated with a greater risk of incident KFD only among postmenopausal women (p for interactions <.05). Moreover, increasing eGFR and age were associated with higher KFD risk in both subgroups; however, the association of the former with the KFD risk was significantly higher among premenopausal women (p for interaction <.05).
The pattern of HRs and their 95% CIs in the sensitivity analysis with multiple imputations in the Cox regression analysis were generally in line with the main analysis (Table S2).
Discussion
We found that among the total population, older age, female gender, prehypertension, hypertension, diabetes, widowed/divorced states, higher TG, prevalent CVD, and higher initial eGFR were associated with greater but moderate physical activity and having a positive family history of diabetes with lower KFD risk.
We also found some significant effect modifications of gender, menopausal status, and having CKD on the impact of different risk factors on incident KFD. The unfavorable effect of prevalent CVD was more pronounced in women than men. Among postmenopausal women, the unfavorable impact of major CVD risk factors was more prominent, while higher initial eGFR was associated with greater risk in premenopausal women. Considering kidney function status, higher baseline eGFR was protective only among the CKD population.
Among traditional risk factors, in line with previous studies [Citation19,Citation20,Citation22,Citation25], we found that older age is associated with a higher risk of KFD. However, Baba et al. [Citation41] found that the higher the baseline eGFR, the higher the rate of decline in kidney function, and that the decline rate became slower with aging since the baseline eGFR was lower in older subjects. Higher age was not a risk factor among those with initial CKD in our study; several reports exist on the link between aging and kidney function decline among the CKD population [Citation42,Citation43]; in fact, several reports exist that young CKD patients are more predisposed to progress to ESKD rather than elderly ones [Citation42,Citation44,Citation45]. These differences might also be due to the selection of the participants; Baba et al. [Citation41] excluded subjects with underlying comorbidities. Another possible explanation might be because of the greater competing risk for death, which we accounted for and observed that age was not associated with incident KFD among those with CKD.
Female gender, per se, was a risk factor for KFD. Similarly, in some [Citation15,Citation21] but not all previous studies [Citation18,Citation22], women had a higher rate of eGFR decline than men. Furthermore, findings of a study of more than 5 million individuals from 34 multinational cohorts reported the female gender as a risk factor for CKD [Citation46]. It is important to note that the progression of CKD might reflect more than biological differences between the sexes, such as differences in lifestyle, cultural, socio-economic, and psychological factors that are affected by gender; however, the precise mechanisms are not fully understood [Citation47]. Several studies, including large meta-analyses, have shown a higher prevalence of CKD among women [Citation48–50]; in contrast, incident ESKD is more common in men [Citation51–53], which might be indicative of gender-specific trajectories in eGFR decline with aging.
High blood pressure is another well-known risk factor for CKD progression [Citation14,Citation15,Citation19,Citation21,Citation22]. The current study showed a higher risk of incident KFD associated with hypertension in both men and women, with a higher impact among postmenopausal women. Encouraging lifestyle changes, including reducing salt intake, which is more than two times greater than the World Health Organization recommended levels among Iranians [Citation54], and introducing diets that are low in animal protein [Citation55–57] as well as antihypertensive medication adherence [Citation58] according to international guidelines might be effective interventions in slowing the progression of renal disease through improving blood pressure control. As shown in a systematic review and meta-analysis, the prevalence of medication adherence among patients with hypertension was 33% in Iran [Citation59]; the corresponding value globally was reported at 45% [Citation60]. A study of 3305 adults with CKD showed that low medication adherence increased the risk for CKD progression by 27% [Citation58]. In the current study, we did not have data regarding medication adherence.
Similar to the results of previous studies [Citation19,Citation21], we found that diabetes was a risk factor for KFD. Our study did not find an interaction between gender and diabetes; however, men had a higher risk for incident KFD than premenopausal women (data not shown). Also, diabetes was associated with greater risk in postmenopausal women than premenopausal ones. Among patients with diabetes, the male gender was associated with faster eGFR decline in previous studies [Citation61,Citation62]. We also extended the previous research by showing the differential impact of diabetes on incident KFD across gender and menopausal status.
Regarding social status and modifiable risk factors, being unmarried and living alone has been associated with a greater risk of rapid eGFR decline among middle-aged and elderly subjects with preserved kidney function [Citation63]; our study found similar findings. In the current study, lower education levels among men and CKD patients were associated with lower risk. Qin et al. [Citation18] followed 2518 Chinese adults without CKD and found no association between higher education levels and incident KFD, similar to our population without CKD. We believe that dietary choices, occupational status, and psychosocial factors might justify the impact of lower education on KFD incidence. We also found inconsistent findings regarding physical activity across subgroups, showing a possible protective effect of moderate physical activity among those without CKD; high physical activity has been linked with a lower risk of developing CKD [Citation64].
In our study, the presence of a family history of diabetes resulted in about 13% lower risk for incident KFD, an issue that was reported previously in our population [Citation4]. It is speculated that subjects with a family history of diabetes may be more aware of the risks associated with CKD and more proactive in maintaining a healthy lifestyle, monitoring their blood glucose levels, and seeking medical attention, when necessary, from a young age. Moreover, previous studies conducted among American and Chinese populations found no risk of CKD for those with a positive family history of diabetes [Citation65,Citation66].
We also found that high baseline eGFR increased the risk of KFD in both men and women of the general population, which was in line with previous reports [Citation22,Citation25,Citation41]. Melsom et al. [Citation67] showed that among those without CKD, higher measured GFR at baseline was associated with faster GFR decline regardless of the presence or absence of diabetes; for every 10-mL/min higher baseline GFR, the annual rate of GFR decline was 0.31 mL/min faster in the Norwegian cohort and 0.46 mL/min faster in the Pima cohort. The reason why the incidence of KFD was higher with a higher baseline eGFR among the non-CKD population is unclear. Salt and sodium intake, which are higher among the Iranian population [Citation54], are reported to be associated with higher filtration fraction, especially among hypertensive subjects [Citation41,Citation68]. Moreover, we also noted that eGFR ≥82.23 mL/min/1.73 m2 was associated with a more than 20% increase in the risk of future prehypertension [Citation69], a potential risk factor for eGFR decline [Citation18]. In this line, a previous study also showed that the risk of doubling the serum creatinine increased at higher and lower levels of eGFR compared to intermediate levels, but the risk of chronic dialysis treatment was inversely associated with eGFR [Citation70]. In contrast, we also found that higher eGFR was a protective factor among CKD patients. Previous work shows that the lower eGFR, the higher the risk of CKD progression and incident ESKD among those with CKD [Citation71,Citation72].
In the current analysis, cardiometabolic risk factors, including diabetes, hypertension, and obesity, had stronger effects in developing KFD among postmenopausal women compared to premenopausal ones; many studies addressed the protective role of women’s sex steroids (i.e. estradiol) and duration of exposure to these hormones (i.e. reproductive life span) in the development of CKD [Citation73,Citation74]. Experimental studies have demonstrated that estrogen exerts its biological actions through estrogen receptors [Citation75], which are present in the kidney’s mesangial, endothelial, and vascular smooth muscle cells [Citation76]. Although the precise underlying mechanisms are not clear, studies suggest that after menopause, the decline in serum estrogen levels and its renoprotective effects can accelerate the progression of glomerulosclerosis and kidney dysfunction [Citation77,Citation78]; the finding is based on the fact that studies including postmenopausal women did not show a greater risk of progressive CKD in men [Citation17,Citation44]. In our study, the impact of diabetes on KFD in men and postmenopausal women but not in premenopausal women adds to the importance of hormonal and biological consequences of menopause.
The key strength of our study is being the first to investigate the risk factors of KFD in the MENA region with a high burden of CKD, using the well-known and large TLGS cohort. Second, using subsequent measurements and a relatively long follow-up for assessing renal function to identify most potential risk factors. Third, data were obtained using direct administrative measures, and standard questionnaires were used for the minimal self-reported data.
There were some limitations in our study. First, we did not have data on albuminuria in subjects included in the study. However, in an individual meta-analysis of 1.7 million participants of 35 cohorts in the CKD Prognosis Consortium (including TLGS), data on albuminuria were available in 679,322 and 431,532 for the mortality and ESRD outcomes, respectively [Citation7]; the authors found that the strong association between eGFR decline and ESRD and mortality events did not change after further adjustment for albuminuria, suggesting eGFR decline as a robust determinant. Second, although GFR was not directly measured in our study, using eGFR with Scr is accurate and valid against gold standards [Citation79,Citation80]. Finally, there was also the risk of unmeasured confounders such as serum electrolyte levels (e.g. sodium, potassium, calcium, and phosphorus), which we might have failed to take into account.
Conclusions
Taken together, important predictors for KFD identified among the Iranian population were older age, female gender, high blood pressure, diabetes, widowed/divorced states, higher TG, prevalent CVD, and higher initial eGFR. Moderate physical activity and having a positive family history of diabetes were protective factors. Higher initial eGFR was a risk factor in those without CKD, while it was protective in CKD patients. Importantly, hypertension and diabetes were risk factors in postmenopausal women. Our findings shed more light on the interaction between gender, menopausal status, and baseline kidney function with different risk factors on KFD. The issue should be considered in personalized preventive medicine.
Author contributions
S.M. and F.H. raised the presented idea and designed the study; S.M. analyzed and interpreted the data; S.M. and D.A. drafted the initial manuscript; M.A reviewed the manuscript; F.H. and F.A. critically reviewed the project and approved the final version of the manuscript; all authors read and approved the final manuscript.
Supplemental Material
Download MS Word (21.5 KB)Supplemental Material
Download MS Word (17.7 KB)Acknowledgments
We express our appreciation to the participants in the Tehran Lipid and Glucose Study for their enthusiastic support as well as the staff of the Tehran Lipid and Glucose Study Unit of the Research Institute for Endocrine Sciences for their valuable help.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting this study’s findings are available from the corresponding author, F.H., upon reasonable request.
Additional information
Funding
References
- Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):1–14.
- Tabatabaei-Malazy O, Saeedi SM, Khashayar P, et al. Regional burden of chronic kidney disease in North Africa and Middle East during 1990–2019; results from global burden of disease study 2019. Front Public Health. 2022;10:1015902.
- Hosseinpanah F, Kasraei F, Nassiri AA, et al. High prevalence of chronic kidney disease in Iran: a large population-based study. BMC Public Health. 2009;9(1):1–8.
- Tohidi M, Hasheminia M, Mohebi R, et al. Incidence of chronic kidney disease and its risk factors, results of over 10 year follow up in an Iranian cohort. PLOS One. 2012;7(9):e45304.
- Barzilay JI, Davis BR, Pressel SL, et al. The effects of eGFR change on CVD, renal, and mortality outcomes in a hypertensive cohort treated with 3 different antihypertensive medications. Am J Hypertens. 2018;31(5):609–614.
- Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504–2511.
- Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531.
- Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009;20(12):2625–2630.
- Guo Y, Cui L, Ye P, et al. Change of kidney function is associated with all‐cause mortality and cardiovascular diseases: results from the Kailuan study. J Am Heart Assoc. 2018;7(21):e010596.
- Turin TC, James MT, Jun M, et al. Short‐term change in eGFR and risk of cardiovascular events. J Am Heart Assoc. 2014;3(5):e000997.
- Carrero JJ, Hecking M, Chesnaye NC, et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–164.
- Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69(2):375–382.
- Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11(2):319–329.
- Halbesma N, Brantsma AH, Bakker SJL, et al. Gender differences in predictors of the decline of renal function in the general population. Kidney Int. 2008;74(4):505–512.
- Kronborg J, Solbu M, Njølstad I, et al. Predictors of change in estimated GFR: a population-based 7-year follow-up from the Tromsø study. Nephrol Dial Transplant. 2008;23(9):2818–2826.
- Minutolo R, Gabbai FB, Chiodini P, et al. Sex differences in the progression of CKD among older patients: pooled analysis of 4 cohort studies. Am J Kidney Dis. 2020;75(1):30–38.
- Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant. 2003;18(10):2047–2053.
- Qin X, Wang Y, Li Y, et al. Risk factors for renal function decline in adults with normal kidney function: a 7-year cohort study. J Epidemiol Community Health. 2015;69(8):782–788.
- Young BA, Katz R, Boulware LE, et al. Risk factors for rapid kidney function decline among African Americans: the Jackson Heart Study (JHS). Am J Kidney Dis. 2016;68(2):229–239.
- Wang F, Zhang L, Zuo L, et al. Mortality and renal function decline among a community-based Chinese population with normal or mildly impaired renal function. Nephrol Dial Transplant. 2011;26(9):2847–2852.
- Yokoyama H, Kanno S, Takahashi S, et al. Determinants of decline in glomerular filtration rate in nonproteinuric subjects with or without diabetes and hypertension. Clin J Am Soc Nephrol. 2009;4(9):1432–1440.
- Jiang S, Sun X, Gu H, et al. Age-related change in kidney function, its influencing factors, and association with asymptomatic carotid atherosclerosis in healthy individuals—a 5-year follow-up study. Maturitas. 2012;73(3):230–238.
- Halbesma N, Jansen DF, Stolk RP, et al. Changes in renal risk factors versus renal function outcome during follow-up in a population-based cohort study. Nephrol Dial Transplant. 2010;25(6):1846–1853.
- Toyama T, Kitagawa K, Oshima M, et al. Age differences in the relationships between risk factors and loss of kidney function: a general population cohort study. BMC Nephrol. 2020;21(1):1–9.
- Koraishy FM, Hooks-Anderson D, Salas J, et al. Fast GFR decline and progression to CKD among primary care patients with preserved GFR. Int Urol Nephrol. 2018;50(3):501–508.
- Steubl D, Buzkova P, Garimella PS, et al. Association of serum uromodulin with ESKD and kidney function decline in the elderly: the cardiovascular health study. Am J Kidney Dis. 2019;74(4):501–509.
- Nishimoto M, Murashima M, Yoshida H, et al. Impact of self-reported walking habit on slower decline in renal function among the general population in a longitudinal study: the Japan specific health checkups (J-SHC) study. J Nephrol. 2021;34(6):1845–1853.
- Azizi F, Ghanbarian A, Momenan AA, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II. Trials. 2009;10(1):5.
- Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in pima indians. Diabetes Care. 1990;13(4):401–411.
- Momenan AA, Delshad M, Sarbazi N, et al. Reliability and validity of the modifiable activity questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15(5):279–282.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
- Levey AS, Eckardt K-U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Earley A, Miskulin D, Lamb EJ, et al. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156(11):785–795.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
- Rajaeefard A, Mohammad-Beigi A, Mohammad-Salehi N. Estimation of natural age of menopause in iranian women: a meta-analysis study. Koomesh. 2011;13(1):1–7.
- Bodner TE. What improves with increased missing data imputations? Struct Equ Modeling. 2008;15(4):651–675.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399.
- Welch C, Bartlett J, Petersen I. Application of multiple imputation using the two-fold fully conditional specification algorithm in longitudinal clinical data. Stata J. 2014;14(2):418–431.
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 2004.
- Baba M, Shimbo T, Horio M, et al. Longitudinal study of the decline in renal function in healthy subjects. PLOS One. 2015;10(6):e0129036.
- O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765.
- Chang W-X, Arai S, Tamura Y, et al. Time-dependent risk factors associated with the decline of estimated GFR in CKD patients. Clin Exp Nephrol. 2016;20(1):58–70.
- Van Pottelbergh G, Bartholomeeusen S, Buntinx F, et al. The evolution of renal function and the incidence of end-stage renal disease in patients aged≥ 50 years. Nephrol Dial Transplant. 2012;27(6):2297–2303.
- Kaewput W, Thongprayoon C, Chewcharat A, et al. Rate of kidney function decline and factors predicting progression of kidney disease in type 2 diabetes mellitus patients with reduced kidney function: a nationwide retrospective cohort study. Ther Apher Dial. 2020;24(6):677–687.
- Nelson RG, Grams ME, Ballew SH, et al. Development of risk prediction equations for incident chronic kidney disease. JAMA. 2019;322(21):2104–2114.
- Bairey Merz CN, Dember LM, Ingelfinger JR, et al. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol. 2019;15(12):776–783.
- Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481.
- Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLOS One. 2016;11(7):e0158765.
- Mills KT, Xu Y, Zhang W, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88(5):950–957.
- Iseki K, Nakai S, Shinzato T, et al. Patient registration committee of the Japanese society for dialysis T. Increasing gender difference in the incidence of chronic dialysis therapy in Japan. Ther Apher Dial. 2005;9(5):407–411.
- Hecking M, Bieber BA, Ethier J, et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the dialysis outcomes and practice patterns study (DOPPS). PLOS Med. 2014;11(10):e1001750.
- Gilg J, Castledine C, Fogarty D. UK RRT incidence in 2010: national and Centre-Specific analyses. Nephron Clin Pract. 2012;120(s1):c1–c27.
- Rezaei S, Mahmoudi Z, Sheidaei A, et al. Salt intake among iranian population: the first national report on salt intake in Iran. J Hypertens. 2018;36(12):2380–2389.
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330(13):877–884.
- Chen TK, Sperati CJ, Thavarajah S, et al. Reducing kidney function decline in patients with CKD: core curriculum 2021. Am J Kidney Dis. 2021;77(6):969–983.
- Bellizzi V, Di Iorio BR, De Nicola L, et al. Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int. 2007;71(3):245–251.
- Cedillo-Couvert EA, Ricardo AC, Chen J, et al. Self-reported medication adherence and CKD progression. Kidney Int Rep. 2018;3(3):645–651.
- Oori MJ, Mohammadi F, Norouzi-Tabrizi K, et al. Prevalence of medication adherence in patients with hypertension in Iran: a systematic review and meta-analysis of studies published in 2000–2018. Arya Atherosclerosis. 2019;15(2):82.
- Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine. 2017;96(4):e5641.
- De Hauteclocque A, Ragot S, Slaoui Y, et al. The influence of sex on renal function decline in people with type 2 diabetes. Diabet Med. 2014;31(9):1121–1128.
- Meguro S, Tomita M, Kabeya Y, et al. Factors associated with the decline of kidney function differ among eGFR strata in subjects with type 2 diabetes mellitus. Int J Endocrinol. 2012;2012:1–6.
- Zhou W, Li Y, Ning Y, et al. Social isolation is associated with rapid kidney function decline and the development of chronic kidney diseases in middle-aged and elderly adults: findings from the China health and retirement longitudinal study (CHARLS). Front Med. 2021;8:782624.
- Parvathaneni K, Surapaneni A, Ballew SH, et al. Association between midlife physical activity and incident kidney disease: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2021;77(1):74–81.
- Collins AJ, Vassalotti JA, Wang C, et al. Who should be targeted for CKD screening? Impact of diabetes, hypertension, and cardiovascular disease. Am J Kidney Dis. 2009;53(3 Suppl 3):S71–S7.
- Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008;51(3):373–384.
- Melsom T, Nair V, Schei J, et al. Correlation between baseline GFR and subsequent change in GFR in Norwegian adults without diabetes and in Pima Indians. Am J Kidney Dis. 2019;73(6):777–785.
- Smyth A, O’Donnell MJ, Yusuf S, et al. Sodium intake and renal outcomes: a systematic review. Am J Hypertens. 2014;27(10):1277–1284.
- Hadaegh F, Hasheminia M, Abdi H, et al. Prehypertension tsunami: a decade follow-up of an Iranian adult population. PLOS One. 2015;10(10):e0139412.
- Tonelli M, Klarenbach SW, Lloyd AM, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011;80(12):1306–1314.
- Ali I, Chinnadurai R, Ibrahim ST, et al. Adverse outcomes associated with rapid linear and non-linear patterns of chronic kidney disease progression. BMC Nephrol. 2021;22(1):1–10.
- Levey AS, De Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80(1):17–28.
- Kang SC, Jhee JH, Joo YS, et al. Association of reproductive lifespan duration and chronic kidney disease in postmenopausal women. Mayo Clin Proc. 2020;95(12):2621–2632.
- Qian D, Wang Z-F, Cheng Y-C, et al. Early menopause may associate with a higher risk of CKD and all-cause mortality in postmenopausal women: an analysis of NHANES, 1999–2014. Front Med. 2022;9:823835.
- Rogers JL, Mitchell AR, Maric C, et al. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R794–R799.
- Potier M, Elliot SJ, Tack I, et al. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol. 2001;12(2):241–251.
- Elliot SJ, Karl M, Berho M, et al. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol. 2003;162(5):1441–1448.
- Goldberg I, Krause I. The role of gender in chronic kidney disease. EMJ. 2016;1(2):58–64.
- Levey AS, Coresh J, Tighiouart H, et al. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16(1):51–64.
- Kwong Y-TD, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56(1):39–49.
