Abstract
Background
More than half of the metastatic breast cancer patients with brain metastases (BCBM) are HER-2 negative, and the prognosis of HER2-negative BCBM is dismal. But few clinical trials have investigated systemic therapies for this subgroup of patients.
Methods
This real-world study included 58 HER2-negative BCBMs who received low-dose apatinib (250 mg daily) in combination with chemotherapy between 18 March 2017 and 31 December 2021. The objective response rate (ORR) of the central nervous system, clinical benefit rate (CBR), progression-free survival of central nervous system (CNS-PFS) and overall survival (OS) were analyzed. Univariate and multivariate Cox regression model was used to estimate the prognostic factors for CNS-PFS and OS.
Results
At the cut-off date, the median follow-up time was 28.2 months. Of the 58 patients, 36 patients were HR+/HER2-, and 22 patients were TNBC. The CNS-ORR was 17.2% (95%CI 9.6% to 28.9%) and the CBR was 53.4% (95%CI 40.8% to 65.7%). The median duration of CNS-PFS for the entire cohort was 6.4 months, and the median OS was 10.7 months. The median CNS-PFS and OS were not affected by hormone receptor status, disease-free survival, the number of prior lines of therapy and local treatment. The most common grade 2–3 adverse events associated with low-dose apatinib were hypertension (20.6%), elevated bilirubin (10.4%), hypothyroidism (10.3%), and hand-foot skin reaction (10.3%).
Conclusion
Apatinib-based chemotherapy demonstrates potential feasibility with acceptable tolerance for HER2-negative BCBM. Its clinical application in BCBM still needs further verification.
KEY MESSAGE
HER2-negative breast cancer patients with brain metastases (BCBM) face an extremely poor prognosis, and a lack of effective treatment options. Apatinib, as a small molecule anti-angiogenic TKI, might have special central nervous system activity. Apatinib-based chemotherapy exhibits good tolerance and gains a favorable survival for HER2-negative BCBM.
Introduction
Brain metastases (BMs) will develop in 15–30% of breast cancer during the metastatic course of this disease [Citation1–3], and the frequency is going on increasing in the last few years due to the improvement of potent systemic treatment as well as the early detection imaging techniques. BMs are associated with an extremely dismal prognosis despite the addition of neurosurgery and radioation, patients could seldom live longer than 1 year [Citation1–4].
The blood-brain barrier (BBB), which is comprised of endothelial cells with tight junctions and finite membrane vesicle transporters, encases the brain. This structure poses a significant challenge to the development of systemic therapies for BMs as almost all large-molecule agents, and more than 98% of small-molecule agents, are not capable to penetrate the intact BBB [Citation4,Citation5]. For HER2-positive metastatic breast cancer (MBC) patients, anti-HER2 treatment has successfully changed the developing process of patients with BM (BCBM) [Citation6,Citation7]. Anti-HER2 antibody agents, such as trastuzumab and pertuzumab, are assumed to be limited in their ability to cross an intact BBB, but they could delay the onset of central nervous system (CNS) lesions [Citation6]. Moreover, small HER2-targeting tyrosine kinase inhibitors (TKIs), like tucatinib, pyrotinib, lapatinib and neratinib, are all supposed to be able to penetrate BBB, and therefore become promising therapeutic options [Citation7–9]. More encouragingly, the novel antibody-drug conjugate trastuzumab deruxtecan has even bright a median progression-free survival (PFS) as long as 15 months for patients with stable BM at enrollment in DESTINY-Breast 03 study [Citation10].
Conversely, more than half of the patients with BCBM were HER2-negative, but few clinical trials exclusively investigated systemic therapies for CNS involvement in this subtype of patients [Citation11]. In a retrospective study with 4118 patients diagnosed with BMs from the French Epidemiological Strategy and Medical Economics research programme [Citation3], 70.7% tumors were HER2-negative, and their overall survival (OS) was significantly shorter than HER2–positive subtypes: median OS was 18.9 months for hormone-receptor-positive and HER2-positive (HR+/HER2+), 13.1 months for HR–/HER2+, 7.1 months for HR+/HER2–, and 4.4 months for triple-negative breast cancer (TNBC) patients (p < 0.0001). Similar results were also reported in several other retrospective studies [Citation12].
The poorer prognosis observed in HR+/HER2-disease is most likely attributed to the fact that BM is typically a late event as the metastatic disease progression persists, when tumors are almost resistant to endocrine therapy and chemotherapy [Citation13]. In the case of TNBC, the aggressiveness and lack of systemic control of the metastatic disease possibly contribute to the high incidence of CNS involvement and dismal prognosis [Citation14]. There is a clear need for effective treatment for HER2-nagetive BCBM in clinic.
Brain metastases from breast cancer are characterized by highly vascular and morphologically malformed vessels. Anti-angiogenesis therapy could normalize intratumor blood vessels and facilitate the delivery of antitumor drugs, thus its combination with chemotherapy is assumed to reverse multidrug resistance. Several studies suggest that chemotherapy combined with bevacizumab, a monoclonal antibody directed against VEGF, is effective for BCBM regardless of HR or HER2 status [Citation15,Citation16], and the risk of CNS hemorrhage is relatively low (0.8–3.3%). In a phase II clinical trial, the conjunction of bevacizumab and carboplatin led to a high rate and durable response (CNS-ORR 63%) in BCBM [Citation16].
Apatinib is an anti-angiogenic TKI targeting VEGFR-2 through selective competition for ATP binding sites [Citation17]. Clinical research on MBC have shown that apatinib monotherapy was effective against both heavily pretreated triple-negative and other subtypes of breast cancer with manageable toxicities [Citation18,Citation19]. Apatinib alone has shown specific CNS activity in some case reports [Citation20,Citation21]. The combination of apatinib and chemotherapy could obtain synergistic effects in cell lines of breast cancer [Citation22]. Herein, we hypothesize that apatinib, as a small-molecule oral TKI, is potential to penetrate across BBB and could be a promising choice for HER2-negative BCBM. In this study, we retrospectively investigated the effectiveness and safety of low-dose apatinib combined with chemotherapy in pretreated HER2-negative BCBM based on real-world data and tried to identify patients who may benefit from this regimen.
Patients and methods
Study design
This is a retrospective, real-world study (RWS) conducted by the Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. HER2-negative breast cancer patients with CNS disease between 18 March 2017 and 31 December 2021 were enrolled. The follow-up was conducted through telephone or data tracking of hospital medical records. The cut-off date for data collection was 15 April 2022. All the patients who were still alive by the cut-off time provided signed informed consent during April, 2022 and December 2022 on their following hospital visits. The study adhered to the ethical principles of the Declaration of Helsinki and was approved by the institutional review board of the National Cancer Hospital & Shenzhen Hospital with approval no. YW2022-31-2.
Inclusion and exclusion criteria
Patients were included in the study if they received at least one month of low-dose apatinib (250 mg daily) combined with chemotherapeutic drugs as suggested by the treating physician. Dose modification and treatment discontinuation were decided by the physicians according to the efficacy, tolerance, and willingness of the patient. Other eligibility criteria included: (1) HER2-negative breast cancer for primary tumor specimen (IHC category 0, 1+ or IHC 2+, negative results of FISH), if re-biopsy of the metastatic lesion was feasible, hormone receptor status was identified by the latest metastatic tumor specimen; (2) CNS metastases were confirmed by pathologic diagnosis or dynamic contrast-enhanced brain images; (3) any prior systemic treatment of breast cancer including apatinib, bevacizumab or other VEGFR inhibitors were allowed, (4) previous whole brain radiation therapy, stereotactic radiosurgery and surgical resection were allowed; (5) Eastern Cooperative Oncology Group (ECOG) scored 0-2. Patients were excluded for the following reasons: (1) undefined HER2 status; (2) apatinib monotherapy; (3) other primary malignancy. In total, ninety-four consecutive HER2-negative BCBM were reviewed and 58 eligible patients were finally included in the study.
Data collection and assessment
Demographic and medical data were collected, including age, ECOG score, metastasis site, and previous treatment. Intracranial progression was defined as 20% or greater increase in the change of the sum of diameters of all measurable brain lesions, occurrence of new brain lesions and worsening of neurological symptoms. CNS response was assessed in isolation from extra-CNS metastatic diseases based on investigator’s determination. The objective response rate (ORR) was defined as the proportion of participants who got a complete response (CR) and partial response (PR). Clinical benefit rate (CBR) was defined as the proportion of participants who got CR, PR, or stable disease (SD) for at least 24 weeks. CNS-PFS was defined as the time from initiating apatinib to intracranial progression or death from any cause. Since fairly high proportions of progressive extra-CNS disease occurred earlier than intracranial progression, total PFS (tPFS) was also calculated for both CNS and extra-CNS disease, which was defined as the time from the beginning of apatinib to tumor progression. Overall survival (OS) was defined as the time from the beginning of apatinib to deathfrom any cause. Survival after brain metastases (SBM) was defined as the time from the initial diagnosis of brain metastasis to death from any cause. Tumor response was estimated by Response Evaluation Criteria In Solid Tumors (RECIST) criteria version 1.1.
Statistical analysis
The survival data were evaluated by the Kaplan–Meier method. Patients were censored at the date of their last visit to the hospital. The Cox proportional hazards model was used for the univariate and multivariate analyses to identify the factors significantly related to PFS and OS. All reported P values and 95% confidence interval (CI) were two-sided. The significant cutoff of P-value was 0.05. All data analyses were performed utilizing SPSS 22.0 software (IBM SPSS, Armonk, NY, USA).
Results
Patient characteristics
In total, 94 consecutive HER2-negative BCBM patients were reviewed and 58 patients were eligible and ultimately included in the apatinib efficacy cohort. Among them, thirty-six patients were HR+/HER2-, and twenty-two patients were TNBC. Demographics and disease features for the subgroups of HR+/HER2- and TNBC were presented in .
Table 1. Demographic characteristics of patients with brain metastases.
The median time from confirmation of metastatic disease to the development of BMs was 13.6 months (range 0 to 98 months) and 6.5 months (range 0 to 68 months) for HR+/HER2- and TNBC patients, respectively. And the median time from initial confirmation of BMs to apatinib-containing treatment was 2.0 months (range 0 to 59 months) and 1.0 month (0 to 18 months) for HR+/HER2- and TNBC, respectively. Eleven patients had symptoms at baseline of apatinib-based chemotherapy. The most common symptoms were headache (n=4), weakness in the limbs (n=4), dizziness (n=3), convulsion (n=2), dysarthria (n=2), and paraesthesia (n=2). All patients with BMs also had extracranial metastasis. The median number of previous lines of systematic treatment (including hormonal therapy and chemotherapy) in the metastatic setting was 3 (range 0 to 10) in HR+/HER2- subgroup and 1(range 0 to 8) in TNBC subgroup. The majority of patients (65.5%, 38/58) received at least two lines of prior treatment for MBC before apatinib-containing treatment, representing a heavily pretreated population. Moreover, 32.7% (19/58) of the whole group had received anti-angiogenisis drugs ever before, including ten of them treated by anti-VEGFR TKIs. In the HR+/HER2- subset, eight of them had received CDK4/6 inhibitors (palbociclib or abemaciclib).
Assessment of tumor response
At the time of the data cut-off, the median follow-up period was 28.2 months. Only one was lost to follow up. Responses were observed in both HR+/HER2- and TNBC patients (). The CNS-ORR was 17.2% (95%CI 9.6% to 28.9%). An additional 20 patients maintained SD lasting 24 weeks, resulting in a CBR of 53.4% (95%CI 40.8% to 65.7%). It seemed that TNBC patients got a higher CNS-ORR (27.3% vs. 11.1%), but there was no significant difference (p = 0.11). CBR was similar in both groups (54.5% vs.52.8%, p = 0.90). The majority (35/58, 60.3%) of the patients got progressive disease (PD) because of simultaneous CNS and non-CNS progression. However, stable disease or tumor regression of brain lesions was observed in 24.1% of patients whose extracranial disease was evaluated as PD per RECIST version 1.1.
Table 2. Response to apatinib-based chemotherapy for HER2-negative breast cancer patients with brain metastases.
Survival outcomes
Among the 58 patients, the median tPFS was 5.0 months, but the median duration of CNS-PFS was 6.4 months (seen in ). The estimated 6-month CNS-PFS rate and 1-year CNS-PFS rate for the entire cohort was 53.4% (95%CI 40.3% to 66.5%) and 25.4% (95%CI 13.1% to 37.7%). The median CNS-PFS did not seem to be affected by DFS, lines of previous treatment and prior local CNS-directed treatments (data not shown). There were no significant discrepancies in CNS-PFS between HR+/HER2- and TNBC subsets (6.8 vs 4.5 months, HR 1.04, p = 0.91, seen in ), either. However, patients who had previously received anti-VEGFR TKIs experienced a much shorter CNS-PFS than those who had not (3.0 vs. 6.8 months, HR 2.80, p = 0.03).
Figure 1. Survival curve in HER2-negative breast cancer patients with brain metastases (BMs) at baseline. (A) CNS-PFS curves of patients with BMs; (B) OS curves of patients with BMs.
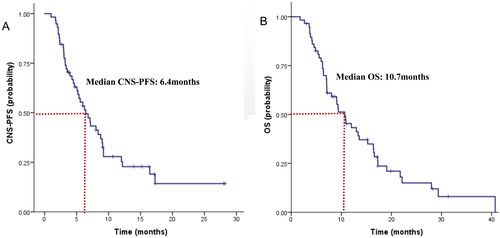
Figure 2. Survival curve in HER2-negative breast cancer patients with brain metastases at baseline. (A) CNS-PFS curves of patients with BMs for HR+/HER2- and TNBC subgroups; (B) OS curves of patients with BMs for HR+/HER2- and TNBC subgroups.
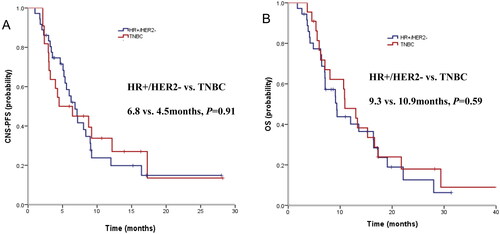
The median duration of OS was 10.7 months (seen in ). The estimated 1-year OS rate was 43.3% (95%CI 30.0% to 56.6%), and 18-month OS rate was 23.6% (95%CI 11.6% to 35.6%). Interestingly, the median OS for HR+/HER2- with BMs was similar to that of TNBC subsets (9.3 m vs. 10.9 m, HR 1.18, p = 0.59, seen in ). Potential prognostic factors of OS were evaluated by exploratory Cox proportional hazards model. ECOG score might be an independent predictive factor of OS (HR 0.51, p = 0.04). But the median OS was not affected by DFS, a number of prior lines of therapy, or local treatment (data not shown). Previous use of anti-VEGFR TKIs seemed to be a negative prognostic factor of OS, but it did not gain statistical significance.
Furthermore, we estimated the median OS after the initial detection of brain metastases (SBM). The SBM was 14.8 months for the total population, and it was 14.8 months and 13.1 months for HR+/HER2- and TNBC patients, respectively (p = 0.85; HR 0.95, 95%CI 0.52–1.71).
Safety
All included patients had accepted at least one-month of apatinib-containing treatment and were assessed for safety. As the information was collected retrospectively from medical records, the omission of some mild nonhematologic adverse events (AEs) was inevitable. We collected the most common grade 2 or more severe AEs that had been previously reported in the trials of single-agent of apatinib. No grade 4 or treatment-associated deaths were observed. The grades 2–3 AEs were presented in . The most frequent grade nonhematologic 2–3 AEs with low-dose apatinib were hypertension (20.6%), elevated bilirubin (10.3%), hypothyroidism (10.3%), and hand-foot skin reaction (10.3%). Two patients experienced grade 2 bleeding, including one case of encephalorrhagia. Three patients (5.2%) got serious oral ulcers. Ten patients discontinued the treatment due to AEs when combined with chemotherapy, and eight of them successfully rechallenged apatinib after adjusting the dose of chemotherapy.
Table 3. Grade 2 and 3 toxicities were reported to be possibly, probably, or definitely attributed to low-dose apatinib.
Discussion
Although local treatment has been the mainstay for many years for HER2-negative breast cancer, the occurrence of BMs is often concomitant to extracranial disease progression. Patients could get survival benefits especially when systemic treatment options are available. In asymptomatic patients, a potent systemic therapy option might be preferred given the short- and long-term adverse effects on neurological cognition following the application of local surgery and radiation [Citation23]. However, at the current time, no breast-cancer-specific treatments are approved for BCBM.
The impassability of the BBB impedes the widespread use of many effective drugs. Small molecules such as TKIs, with favorable properties that allow them for a more efficient penetration through the BBB, are attractive candidates for BCBM. The most remarkable representative is that the addition of anti-HER2 TKI tucatinib to trastuzumab and chemotherapy in the HER2CLIMB trial doubled the intracranial ORR to 47.3% and obtained a median OS of 18.1 months [Citation8]. For HER2-negative breast cancer, some small molecule compounds also exhibit special CNS activity. Talazoparib is a poly ADP-ribose polymerase (PARP) inhibitor that prevents double-stranded DNA repair in BRCA 1/2-mutated cancer. In phase III clinical study (EMBRACA), among 63 patients with BCBM and germline BRCA1/2 mutations [Citation24], talazoparib significantly prolonged the median PFS compared with chemotherapy (HR = 0.32, 95% CI: 0.15 to 0.68), suggesting that talazoparib can effectively penetrate the BBB. The cyclin-dependent kinase (CDK) 4/6 inhibitor abemaciclib, is another small-molecule chemical which could achieve therapeutic concentrations in brain metastases tissues approximating that in plasma [Citation25]. In a phase II trial focused on BCBM, abemaciclib obtained an intracranial CBR of 24% in heavily pretreated MBC patients [Citation26].
As reported before, single-agent angiogenesis inhibitor apatinib only showed passable efficacy in clinical trials for MBC. In two multicenter phase II studies for MBC, a higher dose of apatinib (500 and 750 mg/day) alone got an ORR of 10.7% to 16.7%, and a median OS of 10.3–10.6 months [Citation18,Citation19]. A series of studies have revealed that incorperating angiogenic inhibitor therapy enables to improvement in the efficacy and PFS, and is one of the most promising choices for HER2-negative MBC. In one report of the 2022 American Society of Clinical Oncology (ASCO) annual meeting, the combination of low-dose apatinib with chemotherapy in HER-2 negative advanced breast cancer significantly extended the PFS in apatinib-based chemotherapy group compared to chemotherapy alone (182 days vs 63 days; p = 0.043) [Citation27]. In another phase II trail, the addition of apatinib to neoadujvant chemotherapy (AC-T) exhibited excellent efficacy and good tolerance, with 54.8% (95%CI: 36.0–72.7) of patients reaching pCR in both breast and lymph nodes [Citation28]. Nevertheless, it is important to note that none of these trials focused on patients with active brain metastases. Our study could be an important complement to current clinical practice.
To our knowledge, this is the first study to exclusively assess anti-angiogenic TKIs combined with chemotherapy in patients with brain metastases from HER2-negative breast cancer. We found that low-dose apatinib-based chemotherapy was generally well tolerated and gained a median CNS-PFS of 6.4 months and the median OS was 10.7 months. The 1-year OS was 43.3%, and 18-month OS was 23.6%. Moreover, the TNBC subset achieved a favourable SBM for as long as 13.1 months. Our survival results did seem to exceed those of historical data with chemotherapy alone, especially for TNBC. Although direct comparisons across studies may not be proper due to patient heterogeneity, there is an obvious difference in OS between apatinib-based chemotherapy and chemotherapy alone, justifying further exploration in future trials.
Furthermore, we observed durable intracranial responses in a considerable proportion (24.1%) of patients even after extracranial disease progression, indicating a favorable disease control for BCBM. We did not identify significant prognostic factors for clinical outcomes. Previous use of anti-VEGFR TKIs before the occurrence of BMs perhaps impedes the reuse of it in BCBMs. But all these above results should be interpreted with caution, given the quite small sample-size and the nature of the retrospective study.
Nowadays, a number of promising drugs are in development for HER2-negative BCBM. Novel antibody-drug conjugate, Sacituzumab Govitecan, is an anti-trop-2 monoclonal antibody conjugated to SN-38. Further analysis of patients with BMs from the ASCENT study demonstrated that SG was numerically more effective than the chemotharapy of physician’s choice [Citation29]. Nanoparticle delivery systems are another promising strategy to deliver cytotoxic chemicals across the BBB. Nanoparticle formulations of doxorubicin and etirinotecan have demonstrated special efficacy in BCBM [Citation30,Citation31]. All in all, the ultimate goal of treatment modalities is to reduce the incidence of BMs and extend the survival time for them. A better understanding of the biology of brain metastases and the inherent CNS microenvironment will open up an increased range of opportunities to find new ways for these patients [Citation32].
Conclusion
Aapatinib-based chemotherapy demonstrates potential feasibility with acceptable tolerance for HER2-negative BCBM. We believe our results support further investigation on the application of apatinib in the systemic treatment of HER2-negative breast cancer patients with brain metastases.
Author contributions
Caiwen Du: conception and design of the manuscript, giving the final approval of the manuscript version to be published; Xue Bai, Xiaofeng Xie, Jiayi Huang, Liping Chen, Lin Song, Xiaofeng Lan, Qiuyi Zhang, Jinfeng Guo: a collection of the data; Xuelian Chen: analysis and interpretation of the data, drafting the manuscript. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (46 KB)Acknowledgements
We would like to thank all the patients and investigators for their kind contribution.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bartsch R, Berghoff AS, Preusser M. Optimal management of brain metastases from breast cancer. Issues and considerations. CNS Drugs. 2013;27(2):1–8.
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617.
- Darlix A, Louvel G, Fraisse J, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of Central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000.
- Fidler IJ. The biology of brain metastasis: challenges for therapy. Cancer J. 2015;21(4):284–293.
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14.
- Swain SM, Baselga J, Miles D, et al. Incidence of Central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121.
- Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial Central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117(9):1837–1846.
- Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619.
- Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353–361.
- Hurvitz S, Kim S-B, Chung W-P, et al. Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Presented at the 2021 san antonio breast. Cancer Symposium. 2022;82(4_Supplement):GS3-01–GS3-01.
- Rostami R, Mittal S, Rostami P, et al. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol. 2016;127(3):407–414.
- Niikura N, Hayashi N, Masuda N, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147(1):103–112.
- Liu MC, Cortés J, O’Shaughnessy J. Challenges in the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer with brain metastases. Cancer Metastasis Rev. 2016;35(2):323–332.
- Zimmer AS. Triple-negative breast cancer Central nervous system metastases from the laboratory to the clinic. Cancer J. 2021;27(1):76–82.
- Labidi SI, Bachelot T, Ray-Coquard I, et al. Bevacizumab and paclitaxel for breast cancer patients with Central nervous system metastases: a case series. Clin Breast Cancer. 2009;9(2):118–121.
- Leone JP, Emblem KE, Weitz M, et al. Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res. 2020;22(1):131.
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380.
- Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135(8):1961–1969.
- Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14:820.
- Song Y, Liu B, Guan M, et al. Successful treatment using apatinib in intractable brain edema: a case report and literatures review. Cancer Biol Ther. 2018;19(12):1093–1096.
- Wang J, Chen Y, Chen R, et al. Application of apatinib after multifaceted therapies for metastatic breast cancer. Transl Cancer Res. 2020;9(8):4488–4497.
- Chen J, Deng S, Zhang Y, et al. Apatinib enhances the anti-tumor effect of paclitaxel via the PI3K/p65/bcl-xl pathway in triple-negative breast cancer. Ann Transl Med. 2021;9(12):1001.
- Tanguturi S, Warren LEG. The current and evolving role of radiation therapy for central nervous system metastases from breast cancer. Curr Oncol Rep. 2019;21(6):50.
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763.
- Sahebjam S, Le Rhun E, Kulanthaivel P, et al. Assessment of concentrations of abemaciclib and its major active metabolites in plasma, CSF, and brain tumor tissue in patients with brain metastases secondary to hormone receptor positive (HR+) breast cancer. J Clin Oncol. 2016;34(15_suppl):526–526.
- Tolaney SM, Sahebjam S, Le Rhun E, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res. 2020;26(20):5310–5319.
- Yang CQ, Zhang JS, Wang K. Low-dose apatinib combined with neoadjuvant chemotherapy in the treatment of early-stage triple-negative breast cancer (LANCET): a single-center, single-arm, phase II trial. J Clin Oncol. 2022;40(16_suppl):e12613–e12613.
- Chen ZH, Wang XJ, Huang J, et al. Apatinib combined with chemotherapy versus single chemotherapy in HER-2 negative advanced breast cancer: a randomized, controlled, open-label phase II study. J Clin Oncol. 2022;40(16_suppl):1072–1072.
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541.
- Linot B, Campone M, Augereau P, et al. Use of liposomal doxorubicin-cyclophosphamide combination in breast cancer patients with brain metastases: a monocentric retrospective study. J Neurooncol. 2014;117(2):253–259.
- Perez EA, Awada A, O’Shaughnessy J, et al. Etirinotecan pegol (NKTR-102) versus treatment of physician’s choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16(15):1556–1568.
- Brosnan EM, Anders CK. Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med. 2018;6(9):163.
