Abstract
Background
Little is known about the effectiveness of the newly emerging technology of exergaming in reducing Cancer Related Fatigue (CRF).
Objectives
The study’s primary aim was to examine the effectiveness of exergaming in reducing CRF; the secondary aims were to improve functional capacity/endurance and promote physical activity (PA) among children with acute lymphoblastic leukemia (ALL).
Methods
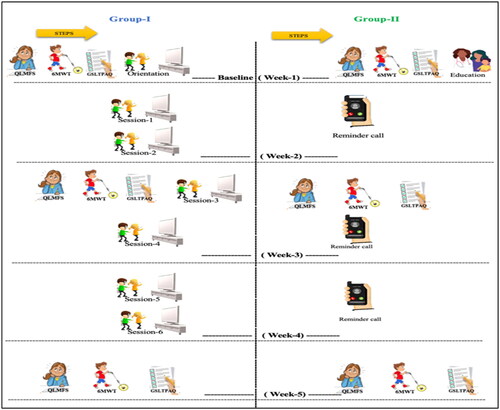
In this Randomized Controlled Trial (RCT), 45 children aged 6–14 years were randomly assigned into group-I, n = 22, and group II, n = 23. Group-I played exergaming of moderate intensity for 60 min, twice a week for three weeks. Group II was given an instructional session regarding the benefits of PA with advice to practice PA for 60 min twice a week. CRF, functional capacity/endurance, and PA were measured using the pediatric quality of life multidimensional fatigue scale (Ped-QLMFS), six-minute walk test (6-MWT), and Godin-Shepard Leisure Time Physical Activity Questionnaire (QSLTPAQ) respectively. All measurements were taken thrice; in the first, third, and fifth weeks of intervention.
Results
Group-I demonstrated a significant reduction of CRF, and a significant increase of functional capacity/endurance compared to group-II over the five weeks study period. The effect of time × intervention interaction was significant. Based on Cohen’s guidelines, CRF and functional capacity/endurance had large effect sizes (η2 = 0.41, p = .00) and (η2 = 0.27, p = .00) respectively.
Conclusion
The protocol of exergaming used in this RCT effectively reduces CRF and promotes functional capacity/endurance and PA in children with ALL undergoing chemotherapy. It may provide an alternative treatment modality to decrease the healthcare load.
Cancer-related fatigue (CRF) is described as physical exhaustion, sleep disturbance, emotional distress, and cognitive dysfunction.
Exergaming reduces CRF and promotes functional capacity/endurance and physical activity in children with acute lymphoblastic leukemia undergoing chemotherapy.
Exergaming may provide an alternative treatment modality to decrease the healthcare load.
Key messages
Introduction
Leukemia is an abnormal cell growth of the bone marrow caused by gene malfunction, leading to the production of abnormal blood cells that circulate in the bloodstream [Citation1]. In Saudi Arabia (SA), leukemia represents 35% of all childhood cancer cases in children below the age of 14 years [Citation2]. Among the different subtypes of leukemia, acute lymphoblastic leukemia (ALL) is the most common among children worldwide. It is treated by a standardized chemotherapy treatment protocol of five phases: Induction, Consolidation, Interim-maintenance, Delayed-intensification, and Maintenance [Citation1,Citation2]. All chemotherapy phases include the use of Methotrexate and/or Vincristine which mainly causes Cancer Related Fatigue (CRF) [Citation3–5].
At different stages of cancer treatment and recovery, pediatric cancer survivors often experience CRF as a devastating symptom [Citation6]. It is described as physical exhaustion, sleep disturbance, emotional distress, and cognitive dysfunction [Citation7]. It also decreases functional capacity/endurance [Citation4]. A wide range of prevalence rates of fatigue has been reported in the literature, ranging from 0.0% to 61.7% [Citation8] and from 10 to 85% [Citation9]. Among cancer survivors in the Arab world, fatigue was the most common symptom (87%–92.5%) [Citation10,Citation11].
CRF influences engagement in everyday activities, mood, sleep, social relations, school attendance, academic achievement, and quality of life [Citation12]. It was significantly associated with low health-related quality of life in pediatric cancer patients including Leukemia [Citation13,Citation14].
Since attention to managing CRF has been drawn, only a few non-pharmacological strategies were tested for their effectiveness in reducing CRF among children [Citation15]. Physical activity (PA) has been proven to be the most effective strategy [Citation16]. Yet adherence to PA remains problematic.
Exergaming, an emerging activity with technology, is a promising intervention to promote physical and psychological health in children suffering from illness [Citation17]. One of the most used exergames is Nintendo Wii. It provides a gaming style that enables interaction through motion sensors providing a unique blend of activity and electronic games [Citation17].
The efficacy of such intervention has not been adequately examined in the literature. Most studies lacked the inclusion of a control group, adequate sample size, Intention-to-Treat analysis, and randomization. Psychological enhancements such as; increased enjoyment, distraction, and socialization were benefits of exergaming in children with cancer [Citation18]. Nonetheless, exergaming has not yet been applied with appropriate intensity and duration among children with ALL to reduce CRF’s debilitating effect. Therefore, the study’s primary aim was to examine the effectiveness of exergaming in reducing CRF; the secondary aims were improving functional capacity/endurance and promoting PA among children with ALL.
Methods
Participants
Forty-five children aged 6–14 years old diagnosed with ALL and receiving chemotherapy regimen that includes Vincristine and/or Methotrexate [Citation11,Citation19] were included. However, the exclusion criteria were: developmental, neurological, or genetic impairments [Citation4,Citation20], chronic lung or heart disease, pulmonary or bone metastasis, neuromuscular disease, children who are receiving radiation therapy [Citation16,Citation21,Citation22] or already receiving physiotherapy [Citation23].
Design and setting
This randomized control trial (RCT) was conducted under the approval of the institutional ethical review board (IRB) of King Fahad Specialist Hospital -Dammam (ONC0352), King Faisal Specialist Hospital and Research Center (KFSHRC) (RAC number: 2191129), King Fahad Medical City (KFMC) (IRB log Number: 19-500E), and King Khaled University Hospital (E-19-3749)- Riyadh, SA. It was registered at clinicaltrials.gov with the ID: NCT04663516. Parents/guardians signed an informed consent form and children were given the chance to give their assent. The study was carried out in KFSHRC and KFMC. The data was collected between November 2019 and November 2020.
Sample size
The sample size was calculated as proposed by Rosner, 2015 [Citation24] considering data from a pilot study conducted with 20 children to calculate the mean score of CRF. The effect size was 0.71, considering a significance level of p < .05 and statistical power of 0.80, with the optimal sample estimated to be 45 children. The participants were randomly assigned into two groups (intervention group; Group-I (n) = 22 and control group; Group-II (n) = 23).
Randomization method
Simple randomization was used to assign the participants to Group I or Group II. All participants were assigned numbers sequentially, those who were assigned odd numbers (one, three, five…) were the participants of Group I, and those who were assigned even numbers (two, four, six….) were included in Group II.
Procedures
Demographic characteristics
The demographic data such as age, sex, weight in kilogram (kg), height in centimeters (cm), nationality, diagnosis, and chemotherapy information were collected at baseline. Body mass index (BMI) percentile (BMI%) was calculated according to the Centers for Disease Control and Prevention (CDC) BMI% calculator [Citation25].
Pediatric quality of life multidimensional fatigue scale (peds QLMFS):
CRF was measured using the valid and reliable Arabic version of peds QLMFS [Citation26]. It is an 18-item questionnaire including ‘general fatigue’, ‘sleep/rest fatigue’, and ‘cognitive fatigue’. The three child self-report versions were used depending on the child’s age: 5–7 years (young child), 8–12 years (child), and 13–18 years (adolescent). Higher scores indicate less intense fatigue symptoms [Citation27]. Before the COVID-19 pandemic, a paper-based appropriate version of the scale was completed by children. During the pandemic, the questionnaires were offered to the participants through a link sent to the parent’s phones to eliminate cross-infection.
The six-minute walk test (6-MWT)
The 6-MWT is a functional test that measures the functional capacity/endurance and submaximal or maximal effort needed to perform activities of daily living [Citation28]. The primary outcome of this test is the distance covered in meters. A lower score reflects less functional capacity conceptualizing the child’s inability to actively participate in functional activities due to CRF [Citation4,Citation29]. The test is valid and reliable for children with cancer [Citation29]. Two small cones were placed to mark the starting and end points for a 25-meter-long corridor. The heart rate (HR) and oxygen saturation (SpO2) were measured 10 min before and one minute after the 6-MWT according to the guidelines of the American Thoracic Society (ATS) [Citation30].
Godin-Shepard leisure time physical activity questionnaire (GSLTPAQ)
Using the GSLTPAQ, PA was estimated by calculating the Leisure time score (LTS) using the equation: LTS = [(times of mild intensity PA/week × three) + (times of moderate-intensity PA/week × five) + (times of vigorous-intensity PA/week × nine). Based on the results, the PA level is determined as active (LTS ≥ 24), sufficiently active (LTS = 18–24), or insufficiently active (LTS ≤ 18) [Citation31,Citation32]. The GSLTPAQ questionnaire is valid for patients with cancer [Citation31].
All measurements were taken thrice: In the first, third, and fifth week respectively.
Monitoring of HR and SpO2
HR and SpO2 were repeatedly measured during the exergaming session (every 15 min) using Polar, Lake Success, NY HR. monitor, and a Portable Beurer pulse Oximeter to monitor the participant’s exertion and intensity of exergaming to be within 50–70% increase of predicted HRmax (pred. HRmax). The pred. HRmax was calculated using the equation 207–0.7 × age [Citation33].
The procedure of the exergaming session
A Wii console was set up in an empty room, where the Wii sensor rested on a table under the screen. Two Wii remotes were available and one Wii balance board (WBB) was fixed on the ground at a two-meter distance from the screen. All equipment was sterilized before and after each use. Extra sport resort accessories were used to enjoy the games and to make it easier for children to understand and practice. The games were introduced and explained to the participants in the first week.
The protocol of exergaming allows the children to choose from the 23 applicable Wii games: 6 Wii Sports Resort (Bowling, Swordplay, canoeing, Archery, Basketball, and Frisbee) and 17 Wii fit plus exergames [12 training plus (Island cycling, Tilt city, Snowball fight, Skateboard Arena, Rhythm Kung Fu, Rhythm parade, Driving range, Segway circuit, Obstacle Course, Bird’s-eye bull’s-eye, Big top juggling, and Perfect Ten); one aerobic (Run) and 4 balance games (Soccer heading, table tilt plus, Penguin slide, and Ski slalom)] [Citation34,Citation35]. The applicability and intensity of these games were previously tested in a preliminary study. The games require full-body engagement to control the avatar and are commensurate with the oncology and CDC guidelines [Citation33,Citation36–38]. There was no limit to how many games the participant plays as long as it was within the 60-min session, moderate intensity (50–70% increase of pred. HRmax), twice a week for three weeks ().
The participants could play with one family member or the researcher to enhance social interaction. The sessions were supervised and managed by trained and licensed researchers. If the participants became tired, they were allowed to rest until they felt ready to continue or decided to stop. The participants were allowed to rest for no more than 10 min in total. Group II was given an instructional session regarding the benefits of PA with advice to practice PA for 60 min twice a week.
Statistical analysis
The statistical package for social sciences (IBM SPSS version 25) was used for data analysis. A confidence interval of 95% was assigned with a p value ≤ .05. The normality of data was checked using Shapiro–Wilks (p > .05). If the data were normally distributed, descriptive statistics are presented as mean, standard deviation, minimum, and maximum for continuous variables and as median, frequency, and percentage for non-normally distributed data and categorical variables. At baseline, the comparison between groups I and II was calculated using the independent sample t-test for normally distributed data and the Mann-Whitney test if the data were skewed. Regarding the nominal data, Chi-square and/or Fisher’s exact test were used to compare the two groups. The significance of Pearson’s Chi-Square value was recorded for items with more than five cells, while Fisher’s exact test value was recorded for items containing less than five cells. A mixed between-/within-subjects ANOVA (split-plot ANOVA) was calculated to assess the overall effect of the exergaming on each outcome variable; CRF (general fatigue, sleep/rest fatigue, cognitive fatigue, and total fatigue), functional capacity/endurance, and PA as well as to assess the intervention × time interaction. According to Cohen’s guidelines [Citation39], partial eta-squared values (η2) of 0.01, 0.06, and 0.14 represent small, medium, and large effect sizes, respectively. Post- hoc analysis using the Bonferroni procedure was performed to examine the difference between groups at three-time points if the ANOVA showed that the exergaming had a significant effect.
Results
Generally, 46 (44.23%) of 104 children were eligible and included in this study. One child withdrew after one session from group I. The reasons for declining participation are co-morbidity (n = 8), off-chemotherapy (n = 24), and could not comply (n = 26). All data were normally distributed and homogenous (p > .05).
The main age of the participants was 9.00 ± 2.35 years. At baseline, according to Fisher’s exact test, there were no significant differences in anthropometric and clinical characteristics between group I and II except for the number of participants in BMI% categories (p = .02). Most of the participants (82.2%) were categorized as healthy weight with a significant difference between groups in favor of group II. About 71.1% were diagnosed with High-Risk ALL (HR-ALL) and 33.3% were in the maintenance phase of chemotherapy ().
Table 1. Demographic and clinical characteristics.
There were non-significant differences between groups regarding CRF, Functional capacity/endurance, and PA level at baseline ().
Table 2. Comparison between groups at baseline.
ANOVA displayed a statistically significant main effect of exergaming on CRF [total fatigue (F (1,43) = 12.41, p = .00)]. There were significant reductions in all dimensions of CRF [general fatigue (F (1,43) =16.57, p = .00) and sleep/rest fatigue (F (1,43) = 2.26, p = .02)] except for cognitive fatigue (p = .21) over the time. Using Cohen’s guidelines, the effect sizes of the exergaming were large on total fatigue (η2 = 0.22) and general fatigue (η2 = 0.27) while it was medium on sleep/rest fatigue (η2 = 0.10). In addition, there was a significant increase of PA (F (1,43) = 10.10, p = .00) in group I compared to group II with a large effect size of exergaming (η2 = 0.19). However, the level of PA did not improve from insufficiently active to active or sufficiently active where Fisher’s exact test was non-significant (p = .34).
Moreover, statistically significant main effects of time on CRF [total fatigue (F (1.63,70.37) = 16.38, p = .00)], sleep/rest fatigue dimension (F (2,68) =7.81, p = .00), and functional capacity/endurance (F (1,43) = 15.10, p = .00). Using Cohen’s guidelines, the effect size of time was large on total fatigue (η2 = 0.27), sleep/rest fatigue (η2 = 0.15), and functional capacity (η2 = 0.26) ().
Table 3. Between – Within subjects’ ANOVA, on CRF, functional capacity/endurance, and PA (n = 45).
The effect of time × intervention interaction was significant for all variables except for PA. The analysis revealed significant main effects on CRF [total fatigue (F (1.63, 70.37) = 30.82, p = .00)] including the three dimensions: general fatigue (F (1.56,66.97) = 6.68, p = .00), sleep/rest fatigue (F (2,86) = 15.07, p = .00), and cognitive fatigue (F (1.64, 70.80) = 4.76, p = .01). In addition, there was a significant main effect on functional capacity/endurance (F (1,43) = 16.06, p = .00). Using Cohen’s guidelines, effect sizes were large for total fatigue (η2 = 0.41), and sleep/rest fatigue (η2 = 0.26). While the effect sizes for general and cognitive dimensions of fatigue were medium (η2= 0.14) and (η2 = 0.10) respectively. Moreover, the effect size on functional capacity/endurance was large (η2= 0.27) ().
Post-hoc analysis using the Bonferroni test revealed that Group I participants conveyed statistically significant attenuation of CRF (total fatigue) (95% CI, −10.47 to −4.69; p = .00) including sleep/rest fatigue dimension (95% CI, −17.59 to −4.27; p = .00) and a higher level of functional capacity/endurance (95% CI, −25.68 to −9.71; p = .00) than group II participants in the 3rd week. In addition, group I participants showed a statistically significant reduction of CRF (total fatigue) (95% CI, −18.64 to −9.76; p = .00) including the three dimensions: general fatigue (95% CI, −25.81 to −4.07; p = .00), sleep/rest fatigue (95% CI, −26.79 to −10.99; p = .00), and cognitive fatigue (95% CI, −16.95 to −0.08; p = .04) compared to group II after 4 weeks. Furthermore, the functional capacity/endurance was significantly higher (95% CI, −51.37 to −9.42; p = .00) in group I participants compared to group II after 4 weeks ().
Table 4. The results of post-hoc comparisons using the Bonferroni test for CRF and functional capacity/endurance over the 3 periods (T1, T2, and T3) within the groups.
All the participants of group-I attended all exergaming sessions. The mean score of SpO2 during sessions was between 95 and 100, and none of them reached less than 92. The increase in HR showed that the mean effort exerted by the participants during the sessions was 56.5% pred. According to the CDC’s guidelines, HRmax ±6.8 commiserating with moderate intensity [Citation38]. None of the participants reached less than 50% of pred. HRmax at any of the six sessions.
Discussion
The study’s primary aim was examining the effectiveness of exergaming in reducing CRF; the secondary aims were improving functional capacity/endurance and promoting PA among children with ALL. The results reported a significant reduction in CRF as well as significant improvement in PA among Group-I participants compared to those in Group II after applying exergaming for 60 min with moderate intensity for six sessions over three weeks.
There is a crucial need to confirm the effectiveness of exergaming using a designed protocol (type, mode of intensity, duration of session, and number of sessions/week). Up to the authors’ knowledge, only one study was partially consistent with the current study’s aims and type of intervention. Yet the protocol of exergaming was inconsistent [Citation19]. Unlike our findings, Hamari et al. could not elicit a significant effect of exergaming in reducing CRF or promoting PA.
In the current study, the exergaming was applied in the hospital under the supervision of a pediatric physiotherapist while in Hamari et al. study, the intervention protocol was done at home. Unfortunately, the home-based program was not followed by the children as instructed. Another major limitation of Hamari et al. study was the inclusion of younger children than the appropriate age for exergaming (three years), as the children have to be at least six years old to be physically and cognitively able to understand the act and concept of imitating and follow the movement of the avatar [Citation17]. Moreover, their study’s protocol stated to apply exergaming daily, and children reported boredom with having to play the same games every day [Citation19]. In the current study, the researchers were keen to solve the previously mentioned issues. Therefore, the protocol offered a variety of games and the freedom to choose the games they like to play within two sessions a week. In addition, the age of the participants was six years and above.
Globally, previous studies that focused on applying moderate to high-intensity exercises (66–90% of pred. HRmax) among children with different types of cancer found no significant reduction in CRF. They recommended using low to moderate-intensity exercises with such a population, especially during chemotherapy treatment [Citation21,Citation40–48]. Therefore, in this study, the protocol of exergaming was applied with moderate intensity (50–70% of pred. HRmax).
Kaushal et al. found a significant effect of aerobic exercises in reducing CRF in children with ALL [Citation23]. Moreover, Yeh et al. reported CRF reduction after applying 30 min of home-based exercise 3 times a week for 6 weeks in children with ALL undergoing the Maintenance phase of chemotherapy. Yet their study had no control group to confirm the significance of the results [Citation43].
Further, traditional exercises were able to elicit a positive impact on CRF during cancer treatment as reported by a few other studies [Citation16,Citation49,Citation50]. In contrast, Van Dijk-Lokkart et al. reported no improvement in CRF in the intervention group which had received 45 min of cardiorespiratory and muscle strength training exercises combined with 60 min of psychoeducation and cognitive behavioral technique over 12 weeks compared to the control group had received usual care [Citation51]. One possible factor for such inconsistency is motivation which can be achieved by making the intervention fun for children.
Three previously carried out interventions included children with an age group similar to ours [Citation22,Citation23,Citation43]. However, they included children with different cancer types which might have been a possible factor for not affecting CRF reduction. Generally, several possible factors might have contributed to the ineffectiveness of other previous interventions to reduce CRF. One possible factor is the adaptation to exercise training [Citation43]. Therefore, in the current study, the variability of the games to be chosen gave the participants the benefit of practicing different activities each session which gave the children the joy of change and avoid the effect of boredom caused by adaptation. Another cause may be the very short duration of intervention [Citation23] using invalid and/or insufficiently sensitive scales to measure CRF and detect change among patients with cancer [Citation16], and the absence of a control group might have limited the possibility of a positive conclusion.
An increase in the distance walked in six- minutes was objectively reported in this study. In contrast to group II, the group I participants showed a borderline significant increase in functional capacity/endurance (p = .07). However, there was a significant improvement in functional capacity/endurance in group I compared to group II over time. The time × intervention interaction effect size was large which leads to a strong acceptance of the proposed hypothesis that exergaming would improve functional capacity/endurance among children with ALL. In other studies, exergaming was used to promote cardiorespiratory fitness for the fact that exergaming causes energy expenditure similar to moderate intensity caused by walking on a treadmill [Citation52,Citation53]. This fact is comparable with what has most recently been proven about the efficiency of exergaming in improving functional capacity.
Several studies reported the effect of an exercise intervention on functional capacity among children with ALL using nine 12-min walk tests. They measured the improvement as the distance covered during nine and 12 min between baseline and after the intervention program [Citation54,Citation55] While other studies revealed improvement of functional capacity after exergaming by the reduction of time needed to complete the Time Up and Go test after exercise intervention compared to baseline [Citation17].
Several biochemical theories for exercise’s impact on cancer patients have been reported [Citation56]. The central nervous system produced micro-stimulation during exercise, which relieves muscle tension, anxiety, and depression. Additionally, exercise enhances metabolism through the clearance of metabolic waste and accumulated adrenaline. Exercise also encourages blood circulation, capillarization, and mitochondrial activity of muscles in the periphery, thereby improving oxygenation and leading to improved organ function and physical well-being which helps reduce fatigue [Citation57,Citation58]. Thus, exercise has beneficial effects on a patient’s functional capacity, CRF, and negative moods [Citation59,Citation60].
In the current study, the exergaming significantly increased the PA. Therefore, the study may provide an intervention recommendation to possibly promote PA among children with ALL. These recommendations might help with other symptoms (sleep disturbance, weakness, and reduction of cognitive function capability) and side effects of chemotherapy that can limit the children’s ability to exercise [Citation41]. Acceptance of what has been hypothesized about playing exergaming during chemotherapy treatment will promote the children’s PA compared to controls is possibly accepted.
Although the exergaming statistically improved the PA in group I compared to group II, the PA level was not improved enough to be changed from insufficiently active to sufficiently active, or from sufficiently active to active based on Fisher’s exact test. This may be attributed to the short duration of the intervention protocol because of conducting the study during the precautions and partial lockdown of the COVID-19 Pandemic.
This study has several limitations. First, the blinding (subject, therapist, or assessor) has not been fulfilled. Second, because of the pandemic, the data could not be collected over an extended period to increase the intervention’s duration and collect data for the follow-up period. The long-term effects of the exergaming program on children with ALL remain uncertain. Finally, PA was not objectively measured, yet the GSLTPAQ questionnaire is valid for patients with cancer [Citation31]. Double-blinded randomized control design studies including a larger sample with a wider age range are recommended. Future studies should consider using objective measures (i.e. pedometers or accelerometers) to record PA. Moreover, it is recommended to investigate the long-term effect of exergaming and include a follow-up assessment. Because home-based programs are less consistently effective, programs should be monitored by an exercise professional [Citation19,Citation43]. Therefore, it is critical to emphasize the difference between hospital-based and home-based exergaming implementation in future research. In addition, the investigation of the risk factors associated with CRF in children is warranted.
Conclusion and clinical implications
The protocol of exergaming used in this RCT effectively reduces CRF and promotes functional capacity/endurance and PA in patients with ALL undergoing chemotherapy compared to the control group. This protocol must be applied under the supervision and management of the physiotherapist in a hospital setting.
Author contributions
Afnan E. Masoud (A.M) and Afaf A. M. Shaheen (A.S) were involved in the conception and design; A.M collected the data and performed the data entry. A.S and A.M analyzed and interpreted the data. A.S and A.M prepared the initial draft of the manuscript. A.S, Maha Fahad Algabbani (M.A), Enas AlEisa (E.A), and Amani AlKofide (A.A) papered the final drafts of the article. Enas AlEisa (E.A), and Amani AlKofide (A.A) provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript. All authors agree to be accountable for all aspects of the work.
Acknowledgments
We are grateful to the Ministry of Education, School members, and all children and their parents for their contributions to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting this study’s findings are available on request from the corresponding author [A.S]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Colby-Graham MF, Chordas C. The childhood leukemias. J Pediatr Nurs. 2003;18(2):1–10. doi: 10.1053/jpdn.2003.9.
- Alghamdi IG, Hussain II, Alghamdi MS. The incidence of leukemia in Saudi Arabia. Descriptive epidemiological analysis of data from the Saudi Cancer Registry 2001–2008. Saudi Med J. 2014;35(7):674–683.
- Oh P-J. Predictors of cognitive decline in people with cancer undergoing chemotherapy. Eur J Oncol Nurs. 2017;27:53–59. doi: 10.1016/j.ejon.2016.12.007.
- Hooke MC, Garwick AW, Gross CR. Fatigue and physical performance in children and adolescents receiving chemotherapy. Oncol Nurs Forum. 2011;38(6):649–657. doi: 10.1188/11.ONF.649-657.
- Hooke MC, McCarthy K, Taylor O, et al. Fatigue and carnitine levels over multiple cycles of chemotherapy in children and adolescents. Eur J Oncol Nurs. 2015;19(1):7–12. doi: 10.1016/j.ejon.2014.07.015.
- Ang SH, Koh SSL, Lee XHHT, et al. Experiences of adolescents living with cancer: a descriptive qualitative study. J Child Health Care. 2018;22(4):532–544. doi: 10.1177/1367493518763109.
- Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(S1):4–10. doi: 10.1634/theoncologist.12-S1-4.
- van Deuren S, Boonstra A, van Dulmen-den Broeder E, et al. Severe fatigue after treatment for childhood cancer. Cochrane Database Syst Rev. 2020;2020(3):CD012681. doi: 10.1002/14651858.CD012681.pub2.
- Christen S, Roser K, Mulder RL, et al. Recommendations for the surveillance of cancer-related fatigue in childhood, adolescent, and young adult cancer survivors: a report from the international late effects of childhood cancer guideline harmonization group. J Cancer Surviv. 2020;14(6):923–938. doi: 10.1007/s11764-020-00904-9.
- Abutaha N, Al-Zharani M, Al-Doaiss AA, et al. Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5. Open Chemistry. 2020;18(1):472–481. doi: 10.1515/chem-2020-0047.
- Al-Gamal E, Long T. Psychometric properties of the Arabic version of the PedsQL family impact scale. J Res Nurs. 2016;21(8):599–608. doi: 10.1177/1744987116670204.
- Berger AM, Abernethy AP, Atkinson A, et al. NCCN clinical practice guidelines cancer-related fatigue. J Natl Compr Canc Netw. 2010;8(8):904–931. doi: 10.6004/jnccn.2010.0067.
- Saito M, Hiramoto I, Yano M, et al. Influence of self-efficacy on cancer-related fatigue and health-related quality of life in young survivors of childhood cancer. IJERPH. 2022;19(3):1467. doi: 10.3390/ijerph19031467.
- Nunes MDR, Jacob E, Bomfim EO, et al. Fatigue and health-related quality of life in children and adolescents with cancer. Eur J Oncol Nurs. 2017;29:39–46. doi: 10.1016/j.ejon.2017.05.001.
- Bhardwaj T, Koffman J. Non-pharmacological interventions for the management of fatigue among children with cancer: a systematic review of existing practices and their effectiveness. BMJ Support Palliat Care. 2017;7(4):404–414. doi: 10.1136/bmjspcare-2016-001132.
- Simioni C, Zauli G, Martelli AM, et al. Physical training interventions for children and teenagers affected by acute lymphoblastic leukemia and related treatment impairments. Oncotarget. 2018;9(24):17199–17209. doi: 10.18632/oncotarget.24762.
- Tripette J, Murakami H, Ryan KR, et al. The contribution of Nintendo Wii Fit series in the field of health: a systematic review and meta-analysis. PeerJ. 2017;5:e3600. doi: 10.7717/peerj.3600.
- Primack BA, Carroll MV, McNamara M, et al. Role of video games in improving health-related outcomes: a systematic review. Am J Prev Med. 2012;42(6):630–638. doi: 10.1016/j.amepre.2012.02.023.
- Hamari L, Järvelä LS, Lähteenmäki PM, et al. The effect of an active video game intervention on physical activity, motor performance, and fatigue in children with cancer: a randomized controlled trial. BMC Res Notes. 2019;12(1):784. doi: 10.1186/s13104-019-4821-z.
- Knowles G, Borthwick D, McNamara S, et al. Survey of nurses’ assessment of cancer-related fatigue. Eur J Cancer Care. 2000;9(2):105–113. doi: 10.1046/j.1365-2354.2000.00197.x.
- Keats MR, Culos-Reed SN. A community-based physical activity program for adolescents with cancer (project TREK): program feasibility and preliminary findings. J Pediatr Hematol Oncol. 2008;30(4):272–280. doi: 10.1097/MPH.0b013e318162c476.
- Hooke MC, Gilchrist L, Tanner L, et al. Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016;63(4):684–689. doi: 10.1002/pbc.25860.
- Kaushal DB, Narendra BD, Smitha D. A comparative study between relaxation technique and aerobic exercise in fatigue during chemotherapy in acute lymphoblastic leukemia in children. Indian J Physiother Occup Ther. 2013;7(3):140–145. doi: 10.5958/j.0973-5674.7.3.081.
- Rosner B. Fundamentals of biostatistics. Boston: Cengage Learning; 2015 [cited 2021 July 20].
- Kuczmarski R, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190.
- Al-Gamal E, Long T. The psychometric properties of an Arabic version of the PedsQL multidimensional fatigue scale tested for children with cancer. Compr Child Adolesc Nurs. 2017;40(3):188–199. doi: 10.1080/24694193.2017.1316791.
- Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL™ in pediatric cancer: reliability and validity of the pediatric quality of life inventory™ generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428.
- Mänttäri A, Suni J, Sievänen H, et al. Six‐minute walk test: a tool for predicting maximal aerobic power (VO 2 max) in healthy adults. Clin Physiol Funct Imaging. 2018;38(6):1038–1045. doi: 10.1111/cpf.12525.
- Bartels B, De Groot JF, Terwee CB. The six-minute walk test in chronic pediatric conditions: a systematic review of measurement properties. Phys Ther. 2013;93(4):529–541. doi: 10.2522/ptj.20120210.
- Laboratories A C. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117.
- Amireault S, Godin G, Lacombe J, et al. The use of the Godin-Shephard Leisure-Time physical activity questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15(1):1–11. doi: 10.1186/s12874-015-0045-7.
- Godin G. The Godin-Shephard leisure-time physical activity questionnaire. HFJC. 2011;4(1):18–22.
- Mahon AD, Marjerrison AD, Lee JD, et al. Evaluating the prediction of maximal heart rate in children and adolescents. Res Q Exerc Sport. 2010;81(4):466–471. doi: 10.1080/02701367.2010.10599707.
- Wii Fit Plus. 2009. Available from: https://nintendo.fandom.com/wiki/Wii_Fit_Plus.
- Wii Sports Resort. 2009. Available from: https://nintendo.fandom.com/wiki/Wii_Sports_Resort.
- Astruc E. Physical activity guidelines for children during and after cancer treatment, in the college at brockport. New York: State University of New York; 2016.
- Perron RM, et al. Do exergames allow children to achieve physical activity intensity commensurate with national guidelines? Int J Exerc Sci. 2011;4(4):257–264.
- Centers for Disease Control and Prevention. How much physical activity do children need? 2014 [cited 2010 June 10]. Available from: https://www.cdc.gov/
- Cohen J. Statistical power analysis for the behavioral sciences. Burlington: Elsevier Science; 2013.
- Hockenberry MJ, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: the child, parent, and staff perspectives. J Pain Symptom Manage. 2003;25(4):319–328. doi: 10.1016/s0885-3924(02)00680-2.
- Hinds PS, Hockenberry M, Rai SN, et al. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. J Pain Symptom Manage. 2007;33(6):686–697. doi: 10.1016/j.jpainsymman.2006.09.025.
- Takken T, van der Torre P, Zwerink M, et al. Development, feasibility and efficacy of a community‐based exercise training program in pediatric cancer survivors. Psychooncology. 2009;18(4):440–448. doi: 10.1002/pon.1484.
- Yeh CH, Man Wai JP, Lin U-S, et al. A pilot study to examine the feasibility and effects of a home-based aerobic program on reducing fatigue in children with acute lymphoblastic leukemia. Cancer Nurs. 2011;34(1):3–12. doi: 10.1097/NCC.0b013e3181e4553c.
- Baky A, Elhakk S. Impact of aerobic exercise on physical fitness and fatigue in children with acute lymphoblastic leukemia. Int J Ther Rehabil Res. 2017;6(2):137. doi: 10.5455/ijtrr.000000255.
- San Juan AF, Chamorro-Viña C, Moral S, et al. Benefits of intrahospital exercise training after pediatric bone marrow transplantation. Int J Sports Med. 2008;29(5):439–446. doi: 10.1055/s-2007-965571.
- San Juan AF, Fleck SJ, Chamorro-Viña C, et al. Effects of an intrahospital exercise program intervention for children with leukemia. Med Sci Sports Exerc. 2007;39(1):13–21. doi: 10.1249/01.mss.0000240326.54147.fc.
- Genc RE, Conk Z. Impact of effective nursing interventions to the fatigue syndrome in children who receive chemotherapy. Cancer Nurs. 2008;31(4):312–317. doi: 10.1097/01.NCC.0000305740.18711.c6.
- Chamorro-Viña C, Ruiz JR, Santana-Sosa E, et al. Exercise during hematopoietic stem cell transplant hospitalization in children. Med Sci Sports Exerc. 2010;42(6):1045–1053. doi: 10.1249/MSS.0b013e3181c4dac1.
- Lam KKW, Li WHC, Chung OK, et al. An integrated experiential training program with coaching to promote physical activity, and reduce fatigue among children with cancer: a randomized controlled trial. Patient Educ Couns. 2018;101(11):1947–1956. doi: 10.1016/j.pec.2018.07.008.
- Diorio C, Celis Ekstrand A, Hesser T, et al. Development of an individualized yoga intervention to address fatigue in hospitalized children undergoing intensive chemotherapy. Integr Cancer Ther. 2016;15(3):279–284. doi: 10.1177/1534735416630806.
- van Dijk-Lokkart EM, Braam KI, van Dulmen-den Broeder E, et al. Effects of a combined physical and psychosocial intervention program for childhood cancer patients on the quality of life and psychosocial functioning: results of the QLIM randomized clinical trial. Psychooncology. 2016;25(7):815–822. doi: 10.1002/pon.4016.
- Hung J-W, Chou C-X, Hsieh Y-W, et al. Randomized comparison trial of balance training by using exergaming and conventional weight-shift therapy in patients with chronic stroke. Arch Phys Med Rehabil. 2014;95(9):1629–1637. doi: 10.1016/j.apmr.2014.04.029.
- Lau PWC, Liang Y, Lau EY, et al. Evaluating physical and perceptual responses to exergames in Chinese children. Int J Environ Res Public Health. 2015;12(4):4018–4030. doi: 10.3390/ijerph120404018.
- Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42(2):127–133. doi: 10.1002/pbc.10481.
- Braam KI, van der Torre P, Takken T, et al. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;2017(3):CD008796. doi: 10.1002/14651858.CD008796.pub3.
- Dimeo F, Schwartz S, Wesel N, et al. Effects of an endurance and resistance exercise program on persistent cancer-related fatigue after treatment. Ann Oncol. 2008;19(8):1495–1499. doi: 10.1093/annonc/mdn068.
- Hockenberry-Eaton M, Hinds PS. Fatigue in children and adolescents with cancer: evolution of a program of study. Semin Oncol Nurs. 2000;16(4):261–272. doi: 10.1053/sonu.2000.16577.
- Zhou X, Edmonson MN, Wilkinson MR, et al. Exploring genomic alteration in pediatric cancer using ProteinPaint. Nat Genet. 2016;48(1):4–6. doi: 10.1038/ng.3466.
- Åstrand P-O. Textbook of work physiology: physiological bases of exercise. Champaign (IL): Human Kinetics; 2003.
- Gilliam MB, Schwebel DC. Physical activity in child and adolescent cancer survivors: a review. Health Psychol Rev. 2013;7(1):92–110. doi: 10.1080/17437199.2011.603641.