Abstract
Objective
The aim of this study was to investigate diagnosis of lipoprotein-associated phospholipase A2 (Lp-PLA2) in early diabetic nephropathy (DN).
Methods
A total of 342 type 2 diabetes mellitus (T2DM) patients hospitalized in department of metabolism and nephrology in our hospital from January 2019 to December 2019 were randomly selected. Patients were divided into three groups via urine albumin level: diabetes mellitus (DM) group, simple diabetes group (114 patients, urinary albumin creatinine ratio (UACR) < 30 mg/g); DN1 group, early DN group (114 patients, UACR: 30–300 mg/g); DN2 group: clinical DN group (114 patients, UACR > 300mg/g). Eighty healthy adults were examined at the same time. Lp-PLA2, fasting blood glucose (FBG), creatinine (Cr), triglyceride (TG), total cholesterol (TCHOL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), haemoglobin A1c (HbA1c), blood urea nitrogen/creatinine (BUN/Cr), estimated glomerular filtration rate (eGFR), 24-h urine protein, albumin and creatinine of all subjects were detected and compared. Pearson’s correlation analysis and multiple ordered logistic regression were used to investigate the correlation between serum Lp-PLA2 level and DN. The possibility of Lp-PLA2 in the diagnosis of early DN was studied by using the subject working curve.
Results
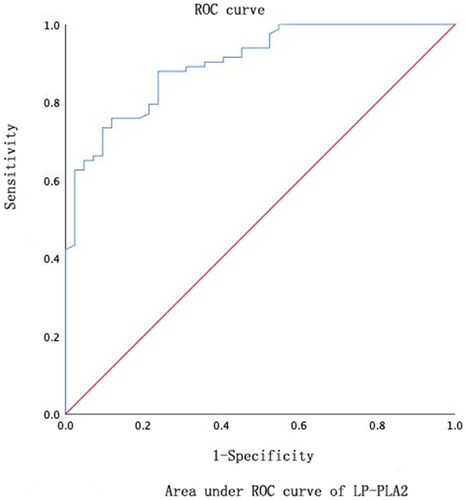
Lp-PLA2 level in DN1 and DN2 groups was significantly higher than that in DM group, with statistical difference (p < .05). With the progression of DN, the level of Lp-PLA2 gradually increased p < .05. Lp-PLA2 was positively correlated with FBG, TG, LDL and HbA1c (R = 0.637, p < .01; R = 0.314, p = .01; R = 0.213, p = .01; R = 0.661, p ≤ .01), was negatively correlated with HDL (r = –0.230, p < .01). The results showed that Lp-PLA2 was an independent factor in the evaluation of early DN. The area under the curve for the evaluation of serum Lp-PLA2 level in early DN was 0.841, the optimal critical value was 155.9 ng/mL, the sensitivity was 88% and the specificity was 76.2%.
Conclusions
Lp-PLA2 is an independent factor for the evaluation of early DN, and can be used as an important potential specific indicator for the diagnosis of early DN, meanwhile monitoring the progression of DN.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease that causes the metabolic disorder of glucose, fat and protein due to the absolute or relative deficiency of insulin in the body, and the incidence has increased rapidly in recent years [Citation1]. Diabetic nephropathy (DN) is one of its most common microvascular complications and one of the main causes of death in diabetic patients. Clinical symptoms of early DN are not typical, mostly showing chronic progressive development and irreversible, eventually progressing to renal failure. Renal puncture biopsy is the gold standard for the diagnosis of DN, but this invasive examination does not meet practical clinical practice. At present, continuous albuminuria is still used as the main marker of DN diagnosis [Citation1].
Lipoprotein-associated phospholipase A2 (Lp-PLA2), belonging to the phospholipase superfamily, can hydrolyse the phospholipid components of low-density lipoprotein (LDL) to produce lipid pro-inflammatory substances such as lyso-phosphatidylcholine (LPC) and free fatty acids (FFA) participating in the inflammatory reaction in the body [Citation2]. Currently, a large number of studies have identified Lp-PLA2 as a strong predictive marker of cardiovascular events. Lp-PLA2 produces damaging vasoactive and inflammatory molecules, such as LPC and oxidized FFA, which promote vascular wall damage and lesions [Citation3]. Although Lp-PLA2 is strongly associated with oxidative stress and cardiovascular complications, its role in diabetes is uncertain and its ability to act as a cardiovascular risk marker in diabetes remains controversial [Citation4].
The importance of Lp-PLA2 as a marker of large vessel disease is undisputed, but its association with microvascular disease is poorly studied. Although Lp-PLA2 was proven to be associated with an increased risk of diabetic retinopathy in humans, and Lp-PLA2 inhibition was successfully used to prevent retinal vascular permeability in animal models [Citation2]. To date, however, reliable data on the potential relevance of Lp-PLA2 as a biomarker of early microvascular renal alteration remain lacking.
Based on the above, this study mainly explored the application value and sensitivity of Lp-PLA2 for early DN diagnosis, thus to provide a potential diagnostic marker with high sensitivity.
2. Study subjects and methods
2.1. Study subjects
According to the diagnostic criteria for T2DM established by WHO in 1999 [Citation5], 342 T2DM patients who were admitted in the Department of Nephrology and Metabolism of our hospital from January 2019 to December 2019 were selected as the study subjects. The reasons for admission of the patient were diabetic-associated poor glycaemic control and severe diabetic complications. At the same time, 80 healthy people who underwent physical examination in the physical examination centre in the same period were randomly selected as the control group.
Morning urinary albumin creatinine ratio (UACR) was tested using the DCA200R instrument (Bayer, Leverkusen, Germany). According to the test results, T2DM patients were divided into three groups: the DM group with pure diabetes (114 patients, UACR <30 mg/g), the DN1 group with early DN (114 patients, UACR: 30–300 mg/g), the DN2 group with clinical DN (114 patients, UACR >300 mg/g). Inclusion criteria: T2DM; aged 18–75 years. Exclusion criteria: type 1 DM; other primary or secondary renal diseases; cancer, severe cardiovascular and cerebrovascular diseases, infectious disease, acute coronary syndrome, autoimmune disease, malignancy, severe liver insufficiency within 3 months; dialysis treatment and drugs affecting renal function within 3 months; women during pregnancy and lactation.
G-Power software was used to calculate sample size. Select the statistical method (F tests) in G-Power software, and then select the classification (fixed effects, omnibus one-way). Set the parameter as 0.05, the inspection efficiency as 0.8 and the number of groups as 3. Input the data of our team’s pre experiment, including the number of groups, the variance within the group, the average number of each group, and the sample size of each group. The calculated effect size f is 0.26, and the total sample size is 342 cases, so there are 114 cases in each group.
2.2. Collection of clinical data
At admission or physical examination, the gender, age and medical history were collected. Additionally, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP) and body mass index (BMI) were calculated. The blood pressure was measured twice while the patient was seated, after at least 15 min of rest, using a standard mercury sphygmomanometer and an appropriately sized cuff. The mean value was calculated for each pair of measurements.
2.3. Test of blood and urine specimens
Venous blood samples were taken on fasting (abrosia over 12 hours) for fasting blood glucose (FBG), total cholesterol (TCHOL), triglycerides (TGs), LDL, high-density lipoprotein (HDL), blood urea nitrogen (BUN), glycosylated haemoglobin A1C (HbA1c) and Lp-PLA2. Morning urine and 24 h urine samples from all participants were collected to determine levels of albumin, creatinine and the UACR and estimated glomerular filtration rate (eGFR). The 24-h urine protein levels were measured via the trichloroacetic acid method using a photometer [Citation6]. The serum creatinine concentrations were determined by the Jaffe reaction [Citation7]. The modification of diet in renal disease (MDRD) formula was used to determine the eGFR [Citation8]. FBG, TCHOL, TG, LDL, HDL, Cr and BUN were detected by the Roche fully automatic biochemical analyser (Cobas600, Roche, Basel, Switzerland). HbA1c was tested by a high-pressure liquid chromatograph (LC-2030C 3D, Shimadzu Corporation, Kyoto, Japan). Lp-PLA2 was determined by using an enzyme-linked immunosorbent kit (EH304RB, Thermo Fisher, Waltham, MA).
2.4. Statistical methods
All data were processed and analysed by SPSS 23.0 software (SPSS Inc., Chicago, IL). After normal distribution test, measurement data are expressed by the mean ± standard deviation (x ± s). Group comparison for measurement data used one-way analysis of variance, with least significant difference (LSD) test for homogeneity of variance and Tamhane’s T2 test for heterogeneity of variance. Categorical data were analysed by the Chi-square test. The association of Lp-PLA2 and various factors was analysed by Pearson’s correlation or Spearman’s rank correlation test. Binary logistic regression analysis was established by using UACR as the diagnostic criterion for DN. Subject working curve was established by using UACR greater than 30 mg/g to establish the diagnostic level and cut-off value of Lp-PLA2. p < .05 was considered to be statistically significant.
3. Results
3.1. General clinical data and laboratory test indicators
A total of 342 patients with T2DM met the inclusion criteria, aged between 32 and 88 years old (mean age 63.9 ± 10.9 years), including 188 males (54.97%). There was no significant difference in sex, age and BMI (p > .05). However, the differences of course of diabetes, comorbidity between group were statistically significant (p < .01). The severity of diabetes increased with the course of the disease, and patients with DN had the longest duration of diabetes. Patients’ comorbidity also increased with the severity of diabetes (all p < .01, ).
Table 1. Demographic data of the four groups.
The four groups of patients showed no difference comparing SBP, DBP, BMI, BUN, serum Cr and TCHOL levels (p > .05). Compared with the DM group, HbA1c, eGFR, Lp-PLA2, UACR, 24-h urine protein and 24-h urine albumin were significantly higher than in the DN1 group, with significant difference (p < .05). Meanwhile, HbA1c, Lp-PLA2 and UACR in the DN2 group were also significantly higher than that in the DN1 group, and the difference was significant (p < .05). In four groups, 24-hour urinary creatinine decreased with the severity of diabetes (p < .05) ().
Table 2. Clinical and biochemistry characteristics of the four groups.
3.2. Correlation analysis of Lp-PLA2 and each factor
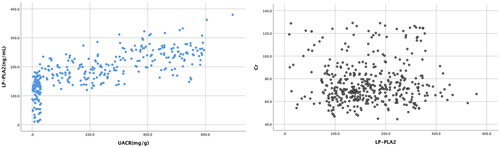
Lp-PLA2 was positively correlated with TG (r = 0.314, p < .01), LDL (r = 0.213, p < .01), FBG (r = 0.637, p < .01), HbA1c (r = 0.661, p < .01), UACR (r = 0.657, p < .01) and negatively with HDL-C (r = −0.230, p < .01). It was not considered that Lp-PLA2 was correlated to SBP, DBP, BMI, BUN, Cr and TCHOL (p > .05) (). The correlation between UACR and Lp-PLA2, Cr and Lp-PLA2 is shown in .
Table 3. Correlation between Lp-PLA2 and each indicator.
3.3. Binary logistic regression analysis with DN as dependent variable
Lp-PLA2, FGB, FIN, HbA1c and HOMA-IR were used as independent variables for binary logistic regression analysis. The results showed that Lp-PLA2 (OR = 1.071, p < .01, 95% CI = 1.053–1.089) and HbA1c (OR = 0.351, p < .001, 95% CI = 0.236–0.524) were independent risk factors ().
Table 4. Binary logistic regression analysis.
4. Discussion
In recent years, with the change of people’s lifestyle, the global prevalence of DM is increasing, and China has become the country with the largest number of DM patients in the world, especially with T2DM patients. DN is one of the most common complications of DM and is a major cause of the development of chronic renal disease and end-stage renal disease, with about 20–40% of T2DM patients complicated with DN. Pathological changes in DN include increased glomerular filtration, progressive proteinuria, decreased GFR and final progression to end-stage renal disease and renal failure [Citation9], a process that is often irreversible. As kidney injury progresses, patient life expectancy and life quality will be severely affected. Therefore, early diagnosis and timely treatment are the key to delay the course of DN and improve the long-term outcomes of patients. Although renal biopsy is considered the gold standard for confirmed kidney injury in DM patients, it is less used in clinical practice. Regular testing of UACR and eGFR are still used clinically as indirect indicators of DN diagnosis.
The traditional view has been that abnormal glucose and lipid metabolism caused by hyperglycaemia and renal hemodynamic disorders caused by insulin resistance are the main causes of DN. In the long-term high filtration, high pressure state, glomerular basement membrane thickening and matrix deposition trigger glomerulosclerosis [Citation10]. However, in recent years, in vitro tests and renal pathology showed that inflammatory response is the important mechanism for the development of DN in DM patients [Citation11,Citation12]. The large accumulation of insulin in DM patients interferes with the metabolic process of lipids and aggravates the oxidative stress response of the body [Citation13]. The environment of hyperglycaemia dramatically increases the glycosylation products in the body. They activate the cellular inflammatory signalling cascade together [Citation14]. Inflammatory response predates the appearance of urinary microalbumin. Under multifactorial conditions such as endothelial dysfunction and microinflammatory status, monocyte macrophage infiltration and glomerular and matrix injury progress eventually lead to end-stage renal failure [Citation15]. There is an independent relationship between various types of inflammatory mediators including cytokines, chemokines and adhesion factors and urinary albumin excretion. It indicates that they play an indispensable role in the pathogenesis of DN [Citation16]. Based on these studies, inflammatory factors for early diagnosis have become a new direction and goal to explore the clinical diagnosis and treatment of DN.
Lp-PLA2, an inflammatory marker, is a platelet-activated factor acetylhydrolase mainly secreted by monocyte macrophages, T lymphocytes and mast cells, which can degrade platelet-activated factors. About 70–80% of Lp-PLA2 binds to LDL and acts on oxidized phospholipids, hydrolysing glycerol phosphate to produce FFA and LPC [Citation17]. FFA and LPC, acting as proinflammatory mediators, can lead to increased vascular endothelial permeability and stimulate the production of cytokines and adhesion molecules. Lp-PLA2 can also enhance the cellular oxidative stress response and promote the apoptosis of endothelial cells [Citation18]. Disruption of endothelial function is considered an early event in renal microangiopathy, laying the foundation for the subsequent clinical manifestations of nephropathy [Citation19].
Lp-PLA2, a highly specific marker of vascular inflammation, has a low biological variability and is more stable than hypersensitive CRP (hs-CRP). Numerous clinical studies have shown that Lp-PLA2 levels are strongly associated with the risk of cardiovascular events. A meta-analysis involving 15 studies and more than 30,000 subjects confirmed that higher Lp-PLA2 activity or quality was independently associated with cardiovascular events in patients with stable coronary atherosclerotic heart disease who were not treated with Lp-PLA2 inhibition [Citation2]. Meanwhile, Lp-PLA2 is also closely related to the occurrence and development of DM microvascular complications [Citation20]. Preclinical studies have shown that Lp-PLA2 inhibitors have good therapeutic effects on diabetic retinal macular oedema [Citation21]. DN and DR often occur concomitantly and promote each other with a similar pathophysiological basis [Citation22]. However, compared with other diabetic complications, the correlation between DN and Lp-PLA2 was the most significant [Citation23]. However, most previous studies used Lp-PLA2 as a non-independent DN detection index, and suggested that Lp-PLA2 could detect early DN in combination with hs-CRP or cystatin C [Citation24,Citation25]. Our study showed that compared with other laboratory indicators, Lp-PLA2 was highly sensitive to both early DN and progressive DN, and can be used as a potential indicator for diagnosing early DN and judging the occurrence and development of DN.
This study found that Lp-PLA2 level was significantly higher in both the DM group and the DN1 and DN2 groups than in the health control group. Meanwhile, the Lp-PLA2 levels were significantly increased in the DN1 group when compared with the DM group. The Lp-PLA2 levels were also significantly higher in the DN2 group than in the DN1 group. This suggests that Lp-PLA2 can not only be used as a highly sensitive indicator for the diagnosis of early DN, but also can dynamically monitor the DN progression. There was evidence that pointed out a positive relation between Lp-PLA2 activity and fasting glucose in diabetes, which suggested that hyperglycaemia may also affect the activity and the biological function of this enzyme [Citation26]. Pearson’s correlation analysis showed that Lp-PLA2 levels in diabetic patients were positively associated with FBG and HbA1c, suggesting that Lp-PLA2 levels can respond to insulin resistance in type 2 diabetes. In addition, Lp-PLA2 level was positively correlated with TG, LDL-c and negatively with HDL-C, which is consistent with the findings of Noto et al. [Citation27]. Research showed that the expression level of Lp-PLA2 in blood and kidney tissue of septic mice was increased and correlated with prognosis [Citation28], and Lp-PLA2 is closely related to lipid metabolism [Citation29]. In this study, it was not considered that Lp-PLA2 was correlated to SBP, DBP, BMI, BUN and Cr. This may be because Lp-PLA2 is more sensitive to glucose metabolism and lipid metabolism disorders than kidney damage, which needs further research to confirm.
Using an ordered multivariate logistic regression analysis with different levels of UACR as covariates and each index as a covariate, this study further found that Lp-PLA2 was an independent influencing factor and a potential indicator for the assessment of early DN. The study showed that Lp-PLA2 was positively correlated with UACR. With the development of DN, the level of Lp-PLA2 gradually increased, which indicated that Lp-PLA2 was involved in the occurrence and development of DN. In this study, Lp-PLA2 was positively correlated with UACR level [Citation30], which further supports our conclusion. In addition, ROC showed that the area under the curve of serum Lp-PLA2 for DN diagnosis was 0.841, sensitivity is 88% and specificity is 76.2%, suggesting that the detection of serum Lp-PLA2 can effectively screen out T2DM patients with possible early renal damage, and can dynamically monitor the progression of DN, which is conducive to early treatment.
Increased plasma level of Lp-PLA2 is associated with incidence and development of DN in patients with T2DM [Citation22]. An elevated level of Lp-PLA2 in patients with T2DM may precede the incidence of albuminuria, but whether it can be used as an indicator ahead of the onset of DN needs further exploration. Lp-PLA2 should be considered as a biomarker for early detection and follow-up of DN [Citation28], which means the use of an Lp-PLA2 inhibitor may inhibit the progression of DN. This is an interesting conjecture that needs further verification.
There are some limitations. First, this study does not have sufficient evidence to show that Lp-PLA2 can be used as an independent diagnostic factor of DN, which requires further research. Although we excluded patients with acute and chronic inflammation and severe cardiovascular and cerebrovascular diseases, Lp-PLA2, as a recognized indicator of acute inflammation, cannot be ruled out as a potential impact of acute inflammatory response on this indicator in this study. Second, in order to protect the participant, the participants in this study did not exclude primary kidney disease through pathological examination, which may cause bias in the results.
5. Conclusions
This study found that Lp-PLA2 can serve as a potential detection indicator of early DN and can dynamically monitor the disease progression of DN. Early kidney injury is reversible before the appearance of persistent proteinuria and has important practical implications for the early diagnosis of DN, but it is not enough to confirm early renal damage by monitoring the UACR alone. Early Lp-PLA2 testing is of great clinical significance to predict and diagnose early DN and further monitor DN progression.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Tianjin Medical University.
Consent form
Informed consent was signed by all participants in this study.
Author contributions
Conception and design of the work: ZY; data collection: CXD, LSY and SYN; supervision: ZY; analysis and interpretation of the data: CXD, LSY and SYN; statistical analysis: ZY and SYN; drafting the manuscript: ZY; critical revision of the manuscript: all authors; approval of the final manuscript: all authors.
Disclosure statement
All of the authors had no personal, financial, commercial or academic conflicts of interest separately.
Data availability statement
All data generated or analysed during this study are included in this published article.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Diabetes Branch of Chinese Medical Association. Guidelines for the prevention and control of type 2 diabetes in China (2017 edition). Chin J Diabetes. 2018;10(1):1–8.
- Siddiqui MK, Kennedy G, Carr F, et al. Lp-PLA2 activity is associated with increased risk of diabetic retinopathy: a longitudinal disease progression study. Diabetologia. 2018;61(6):1344–1353. doi: 10.1007/s00125-018-4601-7.
- Moschos MM, Pantazis P, Gatzioufas Z, et al. Association between platelet activating factor acetylhydrolase and diabetic retinopathy: does inflammation affect the retinal status? Prostaglandins Other Lipid Mediat. 2016;122:69–72. doi: 10.1016/j.prostaglandins.2016.01.001.
- Fortunato J, Bláha V, Bis J, et al. Lipoprotein-associated phospholipase A(2) mass level is increased in elderly subjects with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:278063. doi: 10.1155/2014/278063.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S.
- Momeni A, Shahidi S, Seirafian S, et al. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128–132.
- Bartels H, Böhmer M, Heierli C. Serum creatinine determination without protein precipitation. Clin Chim Acta. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9.
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116.
- Tuttle KR. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes. 2017;66(1):14–16. doi: 10.2337/dbi16-0056.
- Elsherbiny NM, Galil KHAE, Gabr MM, et al. Reno-protective effect of NECA in diabetic nephropathy: implication of IL-18 and ICAM-1. Eur Cytokine Netw. 2012;23(3):78–86. doi: 10.1684/ecn.2012.0309.
- Alkhalaf A, Kleefstra N, Groenier KH, et al. Effect of benfotiamine on advanced glycation endproducts and markers of endothelial dysfunction and inflammation in diabetic nephropathy. PLOS One. 2012;7(7):e40427. doi: 10.1371/journal.pone.0040427.
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482.
- Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854.
- Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–340. doi: 10.1038/nrneph.2011.51.
- Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, et al. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015;2015:948417. doi: 10.1155/2015/948417.
- MacPhee CH, Moores KE, Boyd HF, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338(2):479–487. doi: 10.1042/bj3380479.
- Li D, Zhao L, Yu J, et al. Lipoprotein-associated phospholipase A2 in coronary heart disease: review and meta-analysis. Clin Chim Acta. 2017;465:22–29. doi: 10.1016/j.cca.2016.12.006.
- Leung WK, Gao L, Siu PM, et al. Diabetic nephropathy and endothelial dysfunction: current and future therapies, and emerging of vascular imaging for preclinical renal-kinetic study. Life Sci. 2016;166:121–130. doi: 10.1016/j.lfs.2016.10.015.
- Acharya NK, Qi X, Goldwaser EL, et al. Retinal pathology is associated with increased blood–retina barrier permeability in a diabetic and hypercholesterolaemic pig model: beneficial effects of the Lp-PLA2 inhibitor darapladib. Diab Vasc Dis Res. 2017;14(3):200–213. doi: 10.1177/1479164116683149.
- Faselis C, Katsimardou A, Imprialos K, et al. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):117–124. doi: 10.2174/1570161117666190502103733.
- Hu Y, Li TT, Zhou W, et al. Lipoprotein-associated phospholipase A2 is a risk factor for diabetic kidney disease. Diabetes Res Clin Pract. 2019;150:194–201. doi: 10.1016/j.diabres.2019.03.026.
- Xu RX, Zhang Y, Li XL, et al. Relationship between plasma phospholipase A2 concentrations and lipoprotein subfractions in patients with stable coronary artery disease. Clin Chim Acta. 2015;446:195–200. doi: 10.1016/j.cca.2015.04.032.
- Wang YZ, Ren LJ, Ni WJ. Correlation of the combined detection of Lp-PLA2, hs-CRP and early kidney disease in type 2 diabetes. Chin J Pract Med. 2017;44(2):27–29.
- Zhang WY, Lu Y, Zhang QQ, et al. The value of lipoprotein-associated phospholipase A2 and cystatin C in early diagnosis of diabetic kidney disease. Chin J Diabetes. 2021;29(8):601–605.
- Garg S, Madhu SV, Suneja S. Lipoprotein associated phospholipase A2 activity & its correlation with oxidized LDL & glycaemic status in early stages of type-2 diabetes mellitus. Indian J Med Res. 2015;141(1):107–114. doi: 10.4103/0971-5916.154512.
- Noto H, Chitkara P, Raskin P. The role of lipoprotein-associated phospholipase A(2) in the metabolic syndrome and diabetes. J Diabetes Complications. 2006;20(6):343–348. doi: 10.1016/j.jdiacomp.2006.07.004.
- Liang G, Wu R, Jiang L, et al. The role of lipoprotein-associated phospholipase A2 in acute kidney injury of septic mice. Transl Androl Urol. 2020;9(5):2192–2199. doi: 10.21037/tau-20-1173.
- Xu L, Wang R, Liu H, et al. Resveratrol treatment is associated with lipid regulation and inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) in rabbits fed a high-fat diet. Evid Based Complement Alternat Med. 2020;2020:9641582. doi: 10.1155/2020/9641582.
- Jin W. Correlation analysis between the level of lipoprotein associated phospholipase A2 and the stage of nephropathy in patients with type 2 diabetes. China: Zhengzhou University of China. 2019.