?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Severe renal impairment is a common complication of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and is associated with poor prognosis and shorter survival. It is urgent to find effective treatments to improve the prognosis of AAV patients. This study was designed to assess the efficacy and safety of protein A immunoadsorption (PAIA) and therapeutic plasma exchange (TPE) for AAV with severe renal involvement.
Methods
A total of 48 AAV patients with renal involvement admitted to the Second Xiangya Hospital from January 2018 to February 2021 were selected. Clinical data, myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), remission at 6 months, and outcomes were evaluated. The primary outcomes of interest were death and renal survival as defined by the occurrence of end-stage renal disease (ESRD).
Results
PAIA was effective in the removal of MPO-ANCA and IgG, and showed superior over TPE in the clearance of MPO-ANCA within 1 month after treatment. After a median follow-up of 14.5 months, PAIA therapy showed an advantage in reducing mortality over TPE. There was no difference in the development of ESRD between the two groups. Multivariate Cox regression analysis indicated that higher serum creatinine (SCr) and lower haemoglobin level were independent risks of ESRD. Age > 60, lower serum albumin (ALB), and failure to achieve remission at 6 months were independent risks of death.
Conclusions
PAIA treatment reduces MPO-ANCA and IgG as well as mortality in AAV patients, and may be beneficial for severe AAV in clinical practice. Higher SCr, lower serum ALB or haemoglobin levels, age > 60, and failure to achieve remission at 6 months independently predict the ESRD or death of AAV patients with severe renal involvement.
Compared with therapeutic plasma exchange, protein A immunoadsorption treatment eliminates myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) and IgG better and reduces mortality in ANCA-associated vasculitis (AAV) patients with severe renal involvement.
Higher serum creatinine, lower serum albumin or haemoglobin levels, age > 60, and failure to achieve remission at 6 months independently predict the end-stage renal disease (ESRD) or death of AAV patients with severe renal involvement.
KEY MESSAGES
1. Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is an autoimmune systemic disease characterized by the presence of circulating ANCA and necrotizing inflammation of small- and medium-sized vessels [Citation1]. AAV can cause damage and dysfunction of multi-organs and tissues, among which the lung and kidney are the most common [Citation2]. Renal involvement manifests as rapidly progressive glomerulonephritis with decreased renal function with proteinuria, microscopic haematuria and/or hypertension [Citation3], and is a major risk factor for AAV mortality [Citation4]. Treatment strategies for AAV are designed to attenuate disease progression, usually with immunosuppressant maintenance therapy [Citation5,Citation6]. Despite timely immunosuppressive therapy, about 20–35% of patients with AAV progress to end-stage renal disease (ESRD) within 5 years, placing a heavy financial burden on individuals and society [Citation7]. As European League Against Rheumatism (EULAR) recommended, adjuvant therapeutic plasma exchange (TPE) is decent for severe AAV patients [Citation8]. The MEPEX study found that in AAV patients with SCr > 500 μmol/L, the use of TPE in addition to conventional oral glucocorticoids (GCs) and cyclophosphamide (CYC) was associated with a 24% lower risk of ESRD or death at 12 months than intravenous methylprednisolone [Citation9]. A meta-analysis showed that TPE concomitantly used with GC and CYC was superior to GC pulse therapy in the critical outcome of ESRD at 3 and 12 months [Citation10]. However, a recent multicentre randomized controlled trial (PEXIVAS study) that evaluated TPE and two oral GC regimens in patients with severe AAV concluded that TPE did not reduce the incidence of death or ESRD, but increased the risk of severe infection and may do more harm than good in the long term [Citation11]. Further attention and research should be given to these subjects.
Protein A immunoadsorption (PAIA) is a blood purification technology for selective removal of antibody and antigen–antibody complexes from plasma [Citation12]. PAIA was reported to be superior to TPE in removing pathogenic antibodies and reducing allergic reactions, infectious diseases, or bleeding [Citation13]. One session of PAIA efficiently reduced IgG1, IgG2 and IgG4 by more than 60%, and reduced IgG3, IgM and IgA to varying degrees (20–40%) [Citation14]. Researchers are continuously expanding the application of PAIA in autoimmune conditions such as myasthenia gravis and systemic lupus erythematosus [Citation15–17], but its efficacy on AAV remains unclear. A few cases of AAV with PAIA treatment indicated that PAIA could efficiently remove autoantibodies and control disease progression [Citation18,Citation19]. A recent retrospective study suggested that compared to GC combined with CYC/rituximab (RTX) therapy, additional use of PAIA as adjuvant treatment significantly improved remission rate, ESRD or death from AAV with severe kidney involvement [Citation20]. However, study comparing TPE with PAIA in severe AAV treatment is still lacking.
The current retrospective cohort study was performed to evaluate the efficacy of PAIA or TPE for AAV with severe kidney involvement, and meanwhile, explore the predictive indicator of ESRD/death outcomes in these subjects.
2. Methods
2.1. Patients
Eighty-three adults with AAV who underwent PAIA or TPE at the Second Xiangya Hospital from 1 January 2018 to 28 February 2021 were screened in the study. The exclusion criteria were as follows: (1) without severe renal involvement (severe renal involvement was defined by estimated glomerular filtration rate (eGFR), 50 mL/min per 1.73 m2 at diagnosis of renal disease secondary to AAV [Citation11]); (2) disease course >12 months; (3) treated with PAIA with concurrent TPE; (4) combined with other primary or secondary glomerular diseases, or other systemic diseases; (6) lost to follow-up. Eventually, 48 patients, including 18 patients treated with additional PAIA and 30 patients treated with TPE were included. A flow diagram of the selection of the participants is presented in . Clinical and follow-up data, as well as laboratory parameters (i.e. myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), IgG), were collected. The study was performed with the informed consent of all patients, and the procedure was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University, and is following the principles of the Declaration of Helsinki.
2.2. Treatment procedure
All patients received GC combined with an immunosuppressive agent (CYC or RTX) for induction of remission according to disease conditions. The initial dose (prednisone-equivalent dose of 0.8–1.0 mg/kg per day) and reducing regimen of corticosteroids were made at the discretion of the attending physician. Some of the patients also received intravenous methylprednisolone (500 mg/day for 3–5 days). CYC was intravenously administered at a dose of 400–1000 mg, monthly. RTX was administered according to the number of B cells in peripheral blood.
All patients in the PAIA group were treated with protein A immunosorbent column (KONPIA, KONCEN, Guangzhou, China) under the production instructions, and internal jugular vein indwelling double-lumen catheter was used for vascular access. The immunoadsorption column was reused no more than 10 times, while the plasma separator and piping system were used once. The PAIA treatment frequency was administered based on the ANCA titres, kidney function and the general conditions of patients and decided by the attending physicians. Anticoagulants are used according to the patient’s clotting function, closely monitored and adjusted as needed.
TPE was performed using a KM-9000 (Kawasumi, Tokyo, Japan) Plasauto iQ automatic blood purification system and a PE-08 membrane plasma separator. Fresh-frozen plasma was used as a replacement solution. Femoral vein or internal jugular vein was selected for vascular access. The whole blood was discharged from the vascular pathway at a rate of 150 mL/min and separated into plasma by a plasma separator at a rate of 1 L/h. 1–1.5 times of plasma volume was exchanged each time according to the 8th edition of the therapeutic apheresis guidelines [Citation21]. The choice of TPE or PAIA was a mutual decision after the doctor informed the patient of the characteristics of the two methods.
2.3. Clinical assessment
The primary outcomes of interest were all-cause mortality and the occurrence of ESRD defined by permanent renal-replacement therapy. Disease activity was evaluated by Birmingham Vasculitis Activity Score (BVAS) and remission (BVAS = 0) at 6 months was observed [Citation22]. Adverse events were asked and counted during and in between the treatments.
2.4. Statistical analysis
Statistics for continuous variables with normal distribution used means and SD and medians with IQR for non-normally distributed continuous variables. Categorical variables are expressed as percentages (%). Student’s t-test, Mann–Whitney’s U-test, Fisher’s exact test or a Chi-square test was appropriately used for comparison between groups. Cumulative incidence was estimated by the Kaplan–Meier method, and differences were assessed with the log-rank test. Univariate and multivariate analyses were performed using Cox proportional hazards regression to identify prognostic factors. p Value <.05 was considered statistically significant. The analyses were performed using SPSS statistics 28.0 software (SPSS Inc., Chicago, IL) and Graphed Prism 6 (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. Baseline characteristics
A total of 83 patients meeting AAV diagnostic criteria were initially screened from 1 January 2018 to 28 February 2021 in the study. According to the inclusion and exclusion criteria, 48 patients including 18 patients with PAIA treatment and 30 patients with TPE treatment were enrolled in the retrospective study. The baseline characteristics are shown in , and the extrarenal manifestations of these patients are listed in . Overall, the median age was 61 years old, and 34 patients (70.8%) were males. The median disease duration was 1.5 months. Forty-six patients (95.8%) were positive for MPO-ANCA, and the other two (4.2%) were positive for PR3-ANCA. The median baseline serum creatinine (SCr) and eGFR were 497.6 μmol/L and 9.8 mL/min/1.73 m2, respectively. Thirty-two patients (66.7%) required haemodialysis with SCr ≥ 500 μmol/L. All patients had active disease at baseline, with a median baseline BVAS of 15.9. Forty-seven patients (97.9%) received GC in combination with CYC, and one patient (2.1%) received GC in combination with RTX as induction of remission. There were no significant differences between the baseline of PAIA and TPE groups. The median follow-up time was 14.5 months.
Table 1. Baseline characteristics of patients with AAV.
Table 2. Baseline extrarenal organ involvement.
3.2. MPO-ANCA and Ig levels
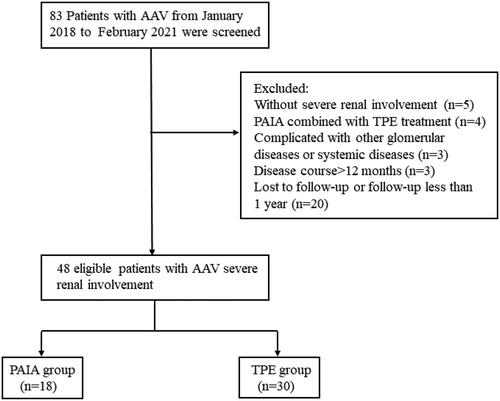
An average of 5.4 ± 1.9 sessions of PAIA were performed in 18 patients compared with 5.1 ± 2.1 sessions of TPE in 30 patients. MPO-ANCA titres were measured before and after therapy from 17 patients in the PAIA group and 17 patients in the TPE group. Before treatment, MPO-ANCA levels in the PAIA group and TPE group were 122.5 (68.6–616.8) RU/mL and 117.1 (91.5–238.8) RU/mL, respectively. Within 1 month after induction treatment, MPO-ANCA decreased significantly in both groups (28.5 (9.1–77.0) vs. 67.5 (28.2–90.6)) (). The reduction rate of MPO-ANCA in the PAIA group versus the TPE group was 80.9% (54.2–90.5%) versus 52.7% (30.2–78.4%) (p = .029) ().
Figure 2. Changes of (A) MPO-ANCA titres and (B) decline rate in PAIA group and TPE group before treatment and within 1 month of treatment (n = 17 in PAIA group, n = 17 in TPE group). (C) Trends of MPO-ANCA titres in two patients during PAIA treatment. (D) Changes in MPO-ANCA titres within 6 months after treatment in PAIA group (n = 13). (E) Changes in serum IgG, IgA and IgM levels before and after PAIA or TPE treatment (n = 13 in PAIA group, n = 6 in TPE group). *p < .05, **p < .01. (1-b) Before the first PAIA treatment; (1-a) after the first PAIA treatment; (2-b) before the second PAIA treatment; (2-a) after the second PAIA treatment; (3-b) after the second PAIA treatment before the third PAIA treatment; (3-a) after the third PAIA treatment; (4-b) before the fourth PAIA treatment; (4-a) after the fourth PAIA treatment; (5-b) before the fifth PAIA treatment; (5-a) after the fifth treatment; (6-b) before the sixth PAIA treatment; (6-a) after the sixth PAIA treatment. PAIA: protein A immunoadsorption; TPE: therapeutic plasma exchange; pre-PAIA: before PAIA treatment; post-PAIA: after PAIA treatment.
Trends of MPO-ANCA in two patients were recorded during PAIA treatment: one patient received six sessions of PAIA and the other received five sessions. As shown in , the titre of MPO-ANCA decreased to varying degrees after each adsorption and increased before the next treatment of PAIA. In addition, changes in MPO-ANCA over time were collected from 13 patients in the PAIA group within 6 months. MPO-ANCA levels decreased significantly 1 month after treatment (p < .05) and then maintained at a low level ().
Serum IgG, IgA and IgM levels were collected from 13 patients in the PAIA group and six patients in the TPE group before and after treatment. Compared to baseline, IgG, IgA and IgM were significantly decreased (79.4% (70.8–89.8%), 43.7% (35.7–51.1%) and 50.7% (30.4–65.5%) after PAIA treatment, respectively. In the TPE group, IgG, IgA and IgM were reduced by 30.4% (20.9–49.1%), 38.7% (4.4–52.8%) and 37.7% (0.3–59.1%) after treatment. PAIA showed a significant advantage in IgG clearance compared with that of IgA and IgM than TPE therapy (p < .01) ().
3.3. Outcomes
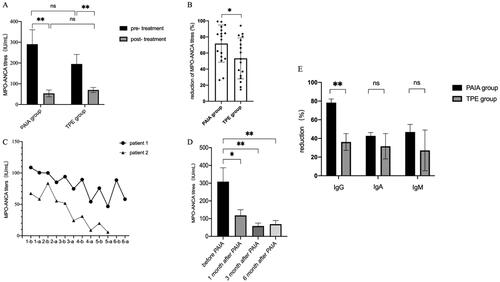
In the PAIA group, 12 patients (66.7%) achieved remission at 6 months, among which three patients progressed to ESRD and one patient died during follow-up. By the end of the follow-up, there were two patients died and eight patients developed ESRD in the PAIA group. In the TPE group, 17 patients (56.7%) achieved remission at 6 months, and 11 of them developed ESRD during follow-up. There were 12 patients died (two ESRD patients) and 13 patients developed ESRD in the TPE group at the end of follow-up. Kaplan–Meier’s survival analysis was performed to examine ESRD and death between groups (). The all-cause mortality was significantly lower in the PAIA group than in the TPE group (HR = 0.24, 95%CI: 0.08–0.71, p = .039), but there was no difference in the cumulative incidence of ESRD (HR = 0.63, 95%CI: 0.27–1.46, p = .283). When ESRD or death was identified as a composite endpoint, additional PAIA treatment was found to induce a better outcome than the TPE group (HR = 0.46, 95%CI: 0.24–0.90, p = .021).
Figure 3. Kaplan–Meier’s curves for (A) all-cause mortality, (B) ESRD and (C) all-cause mortality or ESRD. There were 18 patients in the PAIA group and 30 patients in the TPE group. Significance was determined by the log-rank test. ESRD: end-stage renal disease; PAIA: protein A immunoadsorption; TPE: therapeutic plasma exchange.
3.4. Risk factors for ESRD and death
Cox regression analysis identified risk factors associated with all-cause mortality () and ESRD () with severe renal involvement in AAV patients. Multivariate cox regression analysis was adjusted for the significant factors in univariate analysis and indicated that age > 60 (HR = 10.67, 95%CI: 2.16–52.59), lower serum albumin (ALB) (HR = 0.85, 95%CI: 0.75–0.96) and failure to achieve remission at 6 months (HR = 0.16, 95%CI: 0.04–0.70) were independent risk factors for death. Higher SCr (HR = 1.002, 95%CI: 1.000–1.004) and lower haemoglobin (HR = 0.97, 95%CI: 0.94–0.99) at baseline were independent risk factors of renal outcome.
Table 3. Cox regression analysis of risk factors predicting death due to AAV with severe kidney involvement.
Table 4. Cox regression analysis of risk factors predicting ESRD of AAV with severe kidney involvement.
3.5. Adverse events
Adverse events were mild and none were severe. Two patients (11.1%) in the PAIA group developed transient hypotension during the procedure, which was corrected by supplementing ALB or saline and generally did not affect the treatment process. Two patients (6.7%) in the TPE group developed allergic reactions and improved after anti-allergic treatment.
4. Discussion
AAV is a systemic autoimmune disease with an untreated 1-year mortality rate of 80%. Although immunosuppression with CYC and corticosteroids is effective in improving renal recovery, relapse and low-grade persistent disease result in poor quality survival [Citation23,Citation24]. Over the past few decades, the mortality rate of AAV has decreased significantly with the improvement of diagnostic techniques and treatments for disease awareness. However, compared with the general population, there was still a mortality ratio of 2.6 in AAV patients due to complications of the disease, such as renal failure or serious infection [Citation25]. International guidelines recommend TPE as adjuvant therapy for patients with AAV and severe organ involvement, while existing research recommendations vary regarding the use of TPE in AAV and its long-term benefits remain controversial [Citation26]. The largest randomized trial to date the TPE and GC in severe AAV (PEXIVAS) study failed to show added benefit for TPE in the prevention of death or ESRD for the management of patients with severe AAV [Citation11]. Therefore, it is important to identify AAV patients who may benefit from TPE. A guideline panel classified AAV patients into four tiers based on risk for ESRD, as assessed by baseline SCr level and baseline risk of developing ESRD at 1 year. Plasma exchange is recommended for patients with moderate-high (SCr > 300–500 μmol/L, risk of developing ESRD at 1 year > 7.5–25%) or high risk (SCr > 500 μmol/L, risk of developing ESRD at 1 year > 25%) of ESRD [Citation27]. More studies are needed on TPE in AAV, including its short-term and long-term efficacy compared with other treatment modalities, evaluation of patients’ quality of life, and cost–utility analysis.
Compared with TPE, PAIA uses Staphylococcus protein A, which can bind to antibodies, as a ligand, and then combines with a fixed carrier to make an adsorption column. It clears pathogenic antibodies while sparing other plasma proteins, resulting in rapid disease remission [Citation28]. In a prospective randomized study, PAIA was shown to be effective with TPE in improving renal function in patients with rapidly progressive glomerulonephritis, and 70% of patients could be weaned off dialysis [Citation29]. PAIA has a stronger ability to clear pathogenic antibodies than TPE because of the larger volume of plasma that can be processed at one time. A recently published retrospective study showed that in AAV patients with severe renal impairment, adding PAIA to standard immunosuppressive therapy resulted in faster remission and significantly lower ANCA titres, with death in the PAIA group rate lower than standard treatment [Citation20]. This was also confirmed by our study, which showed an increase in ANCA clearance and a significant improvement in the composite endpoint of death or ESRD in the PAIA group compared to the TPE group. Furthermore, PAIA has been reported to selectively remove immunoglobulins, especially IgG, with little effect on normal blood components such as coagulation factors or ALB. Consistent with this, our findings show that PAIA has a clear advantage over TPE in clearing IgG. Studies have shown that ANCA-IgG binding to neutrophil surface antigens leads to hyperactivation of neutrophils and the release of proteases, inflammatory mediators and oxygen free radicals, which is the key mechanism of tissue damage and aggravation of inflammatory response [Citation30,Citation31]. Therefore, the efficacy of PAIA might be closely related to the reduction of infection. Chu et al. reported that reduced infections might contribute to earlier remission in the PAIA group in the short term, while the frequency of infection in the first 12 months after treatment and adverse events during follow-up were not significantly different [Citation20]. Unfortunately, prophylactic medication was administered for severe infection in some patients in our study, it was difficult to obtain accurate data on serious infections.
Studies have found that advanced age, dialysis dependence at diagnosis, and elevated SCr are risk factors for ESRD in AAV patients [Citation4]. In addition, target organ injury (including kidney and lung, etc.) is also a risk factor for early death in AAV patients [Citation32]. In our study, we found that higher SCr and lower haemoglobin levels at admission were independent risks of ESRD. Age > 60, lower serum ALB, and failure to achieve remission were independent predictors of death. Notably, risk factors for death are largely driven by age and remission variables, with ALB having a much smaller effect. Mayo Clinic’s study showed that decreased serum ALB and haemoglobin levels were associated with severe inflammation and malnutrition, and failure to achieve remission at 6 months was predictive of ESRD or death at 18 months [Citation11]. Active intervention should be carried out for the above risk factors, and more precise and personalized treatment needs to be adopted to save the patient’s life.
The most common side effect during PAIA was hypotension. There were only two hypotensive events in our study, and both were resolved by fluid replacement. Due to the use of fresh frozen plasma as a replacement fluid, and the non-selective removal of macromolecular components in plasma, TPE increases the risk of allergic reactions, bleeding, etc. In the present study, two patients in the TPE group developed anaphylaxis and were therefore switched to double plasma exchange. PAIA does not require additional replacement fluids, thus decreasing the risk of infectious pathogens such as allergic reactions.
There are some limitations to our study. First, the data were collected at a single centre with a small number of cases, especially in the PAIA group, which may limit the generalizability of the results. For example, although the proportion SCr > 500 μmol/L or renal replacement therapy in the two groups was not statistically different at baseline, the small number of patients in the PAIA group may affect the analysis. But for rare diseases, it is difficult to have a very large sample size. Second, the adsorption capacity of PAIA for ANCA may be underestimated because the measured value of MPO-ANCA was up to 738.9 U/mL, and the true value may exceed this level. Third, few patients had renal biopsies, which precluded further analysis of which patients would benefit from PAIA from a renal pathology perspective. A prospective, multicentre and randomized controlled trial with large-sample, long follow-up duration is needed in the future. Despite these limitations, to our knowledge, this was the first study comparing the efficacy of PAIA and TPE therapy in severe AAV patients. This study suggests that PAIA may be an alternative treatment for AAV with severe renal impairment. Prospective studies with larger sample sizes are needed to guide clinical practice regarding the use of PAIA among patients with AAV with severe renal involvement.
5. Conclusions
In conclusion, this retrospective study reveals that additional treatment with PAIA may be a beneficial option for severe AAV. Higher SCr, lower serum ALB or haemoglobin levels, age > 60, and failure to achieve remission at 6 months independently predict the ESRD or death of these patients.
Author contributions
Ming Xia and Xiaojuan Liu contributed to the study design, and experiments, and draft the manuscript. Haiyang Liu and Di Liu contributed to data collection and statistical analysis/interpretation. Chengyuan Tang, Guochun Chen and Yu Liu contributed to the conception of the work and revised it critically for intellectual content. Fang Yuan contributed to the clinical technical operation and interpretation of clinical data. Hong Liu contributed to conceptualization, supervision and resources. All authors have read and approved the final manuscript.
Ethical approval
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (Grant No. 2018YFC1314000). The patients/participants provided their written informed consent to participate in this study.
Acknowledgements
The authors sincerely appreciate the time and effort of all who contributed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data based on the results of the current study were obtained, and are accessible from the corresponding authors upon reasonable request.
Additional information
Funding
References
- Geetha D, Jefferson JA. ANCA-associated vasculitis: core curriculum 2020. Am J Kidney Dis. 2020;75(1):1–10. doi: 10.1053/j.ajkd.2019.04.031.
- Kitching AR, Anders HJ, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6(1):71. doi:10.1038/s41572-020-0204-y.
- Binda V, Moroni G, Messa P. ANCA-associated vasculitis with renal involvement. J Nephrol. 2018;31(2):197–208. doi:10.1007/s40620-017-0412-z.
- Monti S, Craven A, Klersy C, et al. Association between age at disease onset of anti-neutrophil cytoplasmic antibody-associated vasculitis and clinical presentation and short-term outcomes. Rheumatology. 2021;60(2):617–628. doi:10.1093/rheumatology/keaa215.
- Mustapha N, Barra L, Carette S, et al. Efficacy of leflunomide in the treatment of vasculitis. Clin Exp Rheumatol. 2021;39(2):114–118. doi:10.55563/clinexprheumatol/ve38dj.
- Berti A, Alsawas M, Jawaid T, et al. Induction and maintenance of remission with mycophenolate mofetil in ANCA-associated vasculitis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2021;37(11):2190–2200.
- Brilland B, Boud’hors C, Copin MC, et al. Assessment of renal risk score and histopathological classification for prediction of end-stage kidney disease and factors associated with change in eGFR after ANCA-glomerulonephritis diagnosis. Front Immunol. 2022;13:834878. doi:10.3389/fimmu.2022.834878.
- Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133.
- Jayne DR, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18(7):2180–2188. doi:10.1681/ASN.2007010090.
- Yamada Y, Harada M, Hara Y, et al. Efficacy of plasma exchange for antineutrophil cytoplasmic antibody-associated systemic vasculitis: a systematic review and meta-analysis. Arthritis Res Ther. 2021;23(1):28. doi:10.1186/s13075-021-02415-z.
- Walsh M, Merkel PA, Peh CA, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(7):622–631. doi:10.1056/NEJMoa1803537.
- Fuchs K, Rummler S, Ries W, et al. Performance, clinical effectiveness, and safety of immunoadsorption in a wide range of indications. Ther Apher Dial. 2022;26(1):229–241. doi:10.1111/1744-9987.13663.
- Zöllner S, Pablik E, Druml W, et al. Fibrinogen reduction and bleeding complications in plasma exchange, immunoadsorption and a combination of the two. Blood Purif. 2014;38(2):160–166. doi:10.1159/000367682.
- Schossee N, Veit G, Gittel J, et al. Profile of the single-use, multiple-pass protein A adsorber column in immunoadsorption. Vox Sang. 2022;117(3):393–398. doi:10.1111/vox.13205.
- Oji S, Nomura K. Immunoadsorption in neurological disorders. Transfus Apher Sci. 2017;56(5):671–676. doi:10.1016/j.transci.2017.08.013.
- Yang M, Liao C, Zhu Q, et al. Meta-analysis on the efficacy and safety of immunoadsorption for systemic lupus erythematosus among Chinese population. Clin Rheumatol. 2020;39(12):3581–3592. doi:10.1007/s10067-020-05156-7.
- Schwenger V, Morath C. Immunoadsorption in nephrology and kidney transplantation. Nephrol Dial Transplant. 2010;25(8):2407–2413. doi:10.1093/ndt/gfq264.
- Palmer A, Cairns T, Dische F, et al. Treatment of rapidly progressive glomerulonephritis by extracorporeal immunoadsorption, prednisolone and cyclophosphamide. Nephrol Dial Transplant. 1991;6(8):536–542. doi:10.1093/ndt/6.8.536.
- Jin S, Wang D, Luo J, et al. Diffuse alveolar hemorrhage in a patient with anti-neutrophil cytoplasm antibody-associated vasculitis successfully treated with immunoadsorption combined with methylprednisolone. Med Clin. 2022;158(3):133–136. doi:10.1016/j.medcli.2021.08.004.
- Chu X, Hong Y, Wang Y, et al. Immunoadsorption improves remission rates of patients with antineutrophil cytoplasmic antibody-associated vasculitis and severe kidney involvement. Am J Nephrol. 2021;52(12):899–908. doi:10.1159/000519608.
- Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171–354. doi:10.1002/jca.21705.
- Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 2009;68(12):1827–1832. doi:10.1136/ard.2008.101279.
- Mukhtyar C, Flossmann O, Hellmich B, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism Systemic Vasculitis Task Force. Ann Rheum Dis. 2008;67(7):1004–1010. doi:10.1136/ard.2007.071936.
- Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70(3):488–494. doi:10.1136/ard.2010.137778.
- Lai QY, Ma TT, Li ZY, et al. Predictors for mortality in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: a study of 398 Chinese patients. J Rheumatol. 2014;41(9):1849–1855. doi:10.3899/jrheum.131426.
- Toyoda T, Yates M, Watts RA. Is there still a role of plasma exchange in the current management of ANCA-associated vasculitides? Curr Rheumatol Rep. 2022;24(4):111–117. doi:10.1007/s11926-022-01064-8.
- Zeng L, Walsh M, Guyatt GH, et al. Plasma exchange and glucocorticoid dosing for patients with ANCA-associated vasculitis: a clinical practice guideline. BMJ. 2022;376:e064597. doi:10.1136/bmj-2021-064597.
- Matic G, Bosch T, Ramlow W. Background and indications for protein A-based extracorporeal immunoadsorption. Ther Apher. 2001;5(5):394–403. doi:10.1046/j.1526-0968.2001.00370.x.
- Stegmayr BG, Almroth G, Berlin G, et al. Plasma exchange or immunoadsorption in patients with rapidly progressive crescentic glomerulonephritis. A Swedish Multi-Center Study. Int J Artif Organs. 1999;22(2):81–87. doi:10.1177/039139889902200205.
- Porges AJ, Redecha PB, Kimberly WT, et al. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994;153(3):1271–1280. doi:10.4049/jimmunol.153.3.1271.
- Sciascia S, Ponticelli C, Roccatello D. Pathogenesis-based new perspectives of management of ANCA-associated vasculitis. Autoimmun Rev. 2022;21(3):103030. doi:10.1016/j.autrev.2021.103030.
- Mirouse A, Parrot A, Audigier V, et al. Severe diffuse alveolar hemorrhage related to autoimmune disease: a multicenter study. Crit Care. 2020;24(1):231. doi:10.1186/s13054-020-02936-0.